ABSTRACT
Chitin, a polymer of N-acetylglucosamine, is an essential component of the fungal cell wall. Chitosan, a deacetylated form of chitin, is also important in maintaining cell wall integrity and is essential for Cryptococcus neoformans virulence. In their article, Gilbert et al. [N. M. Gilbert, L. G. Baker, C. A. Specht, and J. K. Lodge, mBio 3(1):e00007-12, 2012] demonstrate that the enzyme responsible for chitosan synthesis, chitin deacetylase (CDA), is differentially attached to the cell membrane and wall. Bioactivity is localized to the cell membrane, where it is covalently linked via a glycosylphosphatidylinositol (GPI) anchor. Findings from this study significantly enhance our understanding of cryptococcal cell wall biology. Besides the role of chitin in supporting structural stability, chitin and host enzymes with chitinase activity have an important role in host defense and modifying the inflammatory response. Thus, chitin appears to provide a link between the fungus and host that involves both innate and adaptive immune responses. Recently, there has been increased attention to the role of chitinases in the pathogenesis of allergic inflammation, especially asthma. We review these findings and explore the possible connection between fungal infections, the induction of chitinases, and asthma.
CHITIN AND THE FUNGAL CELL WALL
The fungal cell wall is a complex organelle that is a composite of glucan and chitin fibers held together by proteins and mannan. The second most common polysaccharide in the environment, chitin, is a polymer of N-acetylglucosamine. The content and localization of chitin vary among the fungi. Though the primary role of chitin appears to be related to its role in structural integrity (including responses to environmental changes and replication), other roles have been hypothesized, including epithelial adhesion (1, 2), linkage between the cell wall and capsule (3), and antifungal resistance (4). Chitosan is the deacetylated form of chitin and has been investigated as a vehicle for a variety of therapeutics. In recent studies, chitosan has been shown to be essential for cell wall integrity and virulence for Cryptococcus neoformans (5).
In their article, Gilbert et al. (6) explore the mechanisms by which chitin deacetylase (CDA), the enzyme responsible for chitosan production, is linked to the fungal cell wall. The authors find that CDA is present in both the cell wall and the cell membrane but that the attachment mechanism is organelle specific, so that cell membrane attachment but not cell wall attachment is dependent on covalent binding via a glycosylphosphatidylinositol (GPI) linkage. Importantly, biological activity correlates with the cell membrane-associated CDA. A noncovalent association between CDA and the cell wall is distinct from the mechanism previously elucidated for the phospholipase of C. neoformans (7) but parallels the description for an acid phosphatase of Aspergillus fumigatus (8). Findings from this study provide important new insights into chitin biology and mechanisms by which proteins are associated with the external surface of C. neoformans. The connection of chitin not to the cell, but to the host response and inflammation, has also garnered significant interest.
CHITIN AS AN IMMUNE MODULATOR
Given the importance of chitin to a variety of pathogens, it makes sense that humans have evolved mechanisms to recognize and respond to chitin exposures. However, studies attempting to elucidate the type of inflammation that chitin elicits have yielded conflicting results. Early studies highlighted the immunoadjuvant activities of chitin and indicated that chitin and chitin derivatives (partially deacetylated chitin) induced interleukin-1 (IL-1) expression and increased antibody production and antitumor activity, although the extent of these activities was affected by the chitin preparation (9, 10). Consistent with these findings of enhanced TH1 inflammation, inoculation of chitin and chitosan particles ameliorated allergic inflammation in murine models of asthma (11). In direct contrast with these findings, recent studies suggest that chitin induces TH2 inflammation by enhancing accumulation of eosinophils and basophils within the airways (12, 13). Still, some studies have suggested that chitin is proinflammatory, leading to enhanced IL-17A expression by macrophages via a TLR2-dependent mechanism (14). Finally, some studies have attributed anti-inflammatory properties to chitin, including the inhibition of T cell proliferation (15) and blockage of dectin 1-mediated inflammation (16). The basis for these conflicting findings regarding the inflammatory properties of chitin is not known but may be related to differences in the sizes of chitin particles (17) and amounts and modes of administration and to differences in polymer structures.
ANTIFUNGAL ACTIVITY OF CHITINASE
Mammalian cells do not contain chitin, but they do produce several forms of chitinases with chitinolytic activity, including chitotriosidase (CHIT1), acidic mammalian chitinase (AMCase), and other chitinases that apparently lack activity, like YKL-40. Chitinases with chitinolytic activity are thought to play a role in the innate immune response to fungal and parasitic infections. CHIT1 is produced by activated macrophages, and elevated levels of CHIT1 in serum are present in humans during infection, including but not limited to fungal infections (18). AMCase is expressed in the lungs of rats with pulmonary cryptococcosis (19), and elevated serum chitotriosidase levels were present in guinea pigs with systemic aspergillosis (20). CHIT1 inhibits fungal growth both in vitro and in vivo (21). Increased CHIT1 expression is protective in murine models of fungal infection, including cryptococcosis (22). Transgenic plants that overexpress chitinase and are resistant to fungal infections have been developed (23). Nonetheless, the relative contribution of this enzyme to the host response to fungal infection remains to be determined. It is conceivable that the role of chitinase in the host response is fungal type specific and also related to the host immune status.
CHITINASES AND ALLERGIC INFLAMMATION
Independent of their role in host defense, chitinases have been increasingly recognized for their role as mediators of allergic inflammation. Early studies in mice demonstrated that Ym1 and Ym2 (murine-specific chitinases) and AMCase are induced in an experimental model of asthma (24). In this model, AMCase is elicited by IL-13 and is an essential downstream mediator of IL-13 activity, including the induction of eosinophilia and airway hyperreactivity (25). On the other hand, AMCase, by virtue of its chitinolytic activity, has been reported to reduce allergic inflammation induced by chitin (12). Some studies, but not others, have linked AMCase polymorphisms to asthma in humans (26, 27). The nonchitinolytic chitinase YKL-40 has also been linked to allergic inflammation. Mice genetically deficient in YKL-40 exhibit less allergic inflammation than do normal mice. Additional studies in this system suggest that YKL-40 promotes inflammation by preventing the death of inflammatory cells (including eosinophils) and promoting alternative activation of macrophages (28). In humans, YKL-40 levels are elevated in the serum and bronchoalveolar lavage (BAL) fluid of asthmatics (29). Furthermore, increased YKL-40 levels correlated with asthma severity and are elevated in response to allergen challenge (30). Thus, chitinases appear to play a protective role in the innate response to fungal infection but also mediate adaptive TH2 inflammation.
BREAKING THE MOLD: EMERGING CONCEPTS IN SEVERE ASTHMA, FUNGAL INFECTION, AND CHITINASES
In concert with an increased understanding of chitinases in allergic inflammation, there has been increased attention devoted to the potential role of fungal infections in asthma. Fungal antigens are an important cause of allergen-induced asthma. Sensitization to fungal allergens is thought to occur as a result of transient, repeated exposures without invasion or colonization of host tissue. Nonetheless, it is well recognized that fungi can also elicit asthma symptoms in association with persistent colonization or superficial invasion of host tissue. Airway colonization with Aspergillus causes allergic bronchopulmonary aspergillosis (ABPA) in patients with cystic fibrosis or chronic asthma. While ABPA is most commonly associated with A. fumigatus, other Aspergillus species and fungi have been implicated (31–33). Chronic fungal infections outside the respiratory tract (including skin infections) can also exacerbate allergic symptoms (34, 35).
Together with our colleagues, we have explored the potential contribution of ongoing fungal infection to asthma and the role of the chitinase pathway by using C. neoformans as a model pathogen. Because of its association with pigeon droppings, subclinical infection with C. neoformans is common among individuals (including children) living in an urban area (36). C. neoformans is also well recognized for its tendency to cause persistent infections (37–39) and allergic inflammation in animal models (40, 41). Cryptococcal virulence has been linked to its capacity to elicit TH2 inflammation, which is mediated in part through IL-13 (42, 43). In a rat model, persistent pulmonary C. neoformans infection is associated with increased IL-13 levels and enhancement of many of the features of asthma, including allergic inflammation, airway hyperreactivity, and increased goblet cell numbers (44). Antifungal therapy ameliorates these effects.
IS THE CONNECTION BETWEEN PERSISTENT FUNGAL INFECTION AND SEVERE ASTHMA RELATED TO ACTIVATION OF THE CHITINASE PATHWAY?
While associations between fungal infection and asthma are well described in the literature, they are frequently viewed as uncommon phenomena or coincidental observations. Recent data, however, suggest that fungi may play a larger role, particularly in the context of severe disease. A newly described subtype of asthma, termed severe asthma with fungal sensitization (SAFS), highlights the expanding awareness of fungi in asthma (45). SAFS—characterized by failure of step 4 asthma therapy, elevated serum IgE levels, and sensitization to one of several environmental fungi—may represent one point on a spectrum of fungus-associated asthma. Importantly, asthma control is improved in SAFS patients using prolonged itraconazole therapy. Since the initial description of SAFS, numerous cases have been reported, but its true prevalence remains unknown. However, we recently demonstrated a high prevalence of SAFS (>40%), as well as significant differences in lung function, in a small cohort of severe asthmatics from the greater New York City area (A. G. Vicencio, M. Tyberg, M. T. Santiago, E. A. Foley, D. Bush, A. Casadevall, and D. L. Goldman, submitted for publication). In addition, we previously demonstrated increased IgA and IgG reactivity to fungal proteins in the bronchoalveolar lavage fluid of children with severe asthma (46). Hence, the contribution of fungi to the development and persistence of asthma may be underestimated.
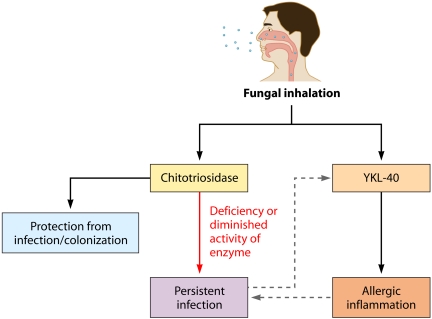
Although the precise mechanisms underlying fungus-associated asthma remain unclear, emerging evidence suggests a potential role for chitinases. We propose that fungus-associated asthma could represent an imbalance between chitinolytic and nonchitinolytic chitinases. Specifically, we hypothesize that mutations resulting in decreased activity of chitotriosidase confer increased susceptibility to fungal infection, thereby contributing to increased asthma severity, possibly via enhanced induction of YKL-40 (Fig. 1). In support of this hypothesis, we previously described 6 children who fitted modified pediatric criteria for SAFS, all of whom were heterozygous for a 24-bp duplication in CHIT1, which results in a 50% decrease in enzymatic activity (47). In further support, polymorphisms in CHIT1 have been associated with asthma exacerbations in children but are dependent on environmental fungal burden (48). Certainly, additional studies are warranted to more precisely define the complex interactions between host chitinases, fungal exposures, and subsequent inflammatory responses in the airway.
FIG 1 .
Model of hypothesized role of chitinases in fungus-associated asthma. Mutations resulting in decreased activity of chitotriosidase confer increased susceptibility to fungal infection, thereby contributing to increased asthma severity, possibly via enhanced induction of YKL-40.
SUMMARY
Chitin and its derivatives may represent a critical link between fungal pathogens and their interactions with the environment. Host responses to this important polysaccharide may depend in part on complex interactions between fungal chitin and host chitinases. Future studies focusing on not only the role of chitin in fungal pathogenesis but also the host response to the polysaccharide will hopefully strengthen the “chitin connection.”
Footnotes
Citation Goldman DL, Vicencio AG. 2012. The chitin connection. mBio 3(2):e00056-12. doi:10.1128/mBio.00056-12.
REFERENCES
- 1. Gottlieb S, Altboum Z, Savage DC, Segal E. 1991. Adhesion of Candida albicans to epithelial cells effect of polyoxin D. Mycopathologia 115:197–205 [DOI] [PubMed] [Google Scholar]
- 2. Segal E, Gottfried L, Lehrer N. 1988. Candidal vaginitis in hormone-treated mice: prevention by a chitin extract. Mycopathologia 102:157–163 [DOI] [PubMed] [Google Scholar]
- 3. Rodrigues ML, Alvarez M, Fonseca FL, Casadevall A. 2008. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell 7:602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee KK, et al. 2012. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob. Agents Chemother. 56:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker LG, Specht CA, Lodge JK. 2011. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryot. Cell 10:1264–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilbert NM, Baker LG, Specht CA, Lodge JK. 2012. A glycosylphosphatidylinositol anchor is required for membrane localization but dispensable for cell wall association of chitin deacetylase 2 in Cryptococcus neoformans. mBio 3(1):e00007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert NM, et al. 2010. KRE genes are required for beta-1,6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol. Microbiol. 76:517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latgé JP, et al. 2005. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med. Mycol. 43(Suppl. 1):S15–S22 [DOI] [PubMed] [Google Scholar]
- 9. Nishimura K, Ishihara C, Ukei S, Tokura S, Azuma I. 1986. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine 4:151–156 [DOI] [PubMed] [Google Scholar]
- 10. Nishimura K, et al. 1986. Macrophage activation with multi-porous beads prepared from partially deacetylated chitin. J. Biomed. Mater. Res. 20:1359–1372 [DOI] [PubMed] [Google Scholar]
- 11. Strong P, Clark H, Reid K. 2002. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin. Exp. Allergy 32:1794–1800 [DOI] [PubMed] [Google Scholar]
- 12. Reese TA, et al. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Dyken SJ, et al. 2011. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J. Immunol. 187:2261–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 181:4279–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner CJ, Huber S, Wirth S, Voehringer D. 2010. Chitin induces upregulation of B7-H1 on macrophages and inhibits T-cell proliferation. Eur. J. Immunol. 40:2882–2890 [DOI] [PubMed] [Google Scholar]
- 16. Mora-Montes HM, et al. 2011. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect. Immun. 79:1961–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Da Silva CA, et al. 2009. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 182:3573–3582 [DOI] [PubMed] [Google Scholar]
- 18. Labadaridis I, et al. 2005. Chitotriosidase in neonates with fungal and bacterial infections. Arch. Dis. Child. Fetal Neonatal Ed. 90:F531–F532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vicencio AG, et al. 2008. Pulmonary cryptococcosis induces chitinase in the rat. Respir. Res. 9:40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Overdijk B, Van Steijn GJ, Odds FC. 1996. Chitinase levels in guinea pig blood are increased after systemic infection with Aspergillus fumigatus. Glycobiology 6:627–634 [DOI] [PubMed] [Google Scholar]
- 21. van Eijk M, et al. 2005. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 17:1505–1512 [DOI] [PubMed] [Google Scholar]
- 22. Gordon-Thomson C, et al. 2009. Chitotriosidase and gene therapy for fungal infections. Cell. Mol. Life Sci. 66:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jach G, et al. 1995. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 8:97–109 [DOI] [PubMed] [Google Scholar]
- 24. Webb DC, McKenzie AN, Foster PS. 2001. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J. Biol. Chem. 276:41969–41976 [DOI] [PubMed] [Google Scholar]
- 25. Zhu Z, et al. 2004. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304:1678–1682 [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee R, Batra J, Das S, Sharma SK, Ghosh B. 2008. Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J. Allergy Clin. Immunol. 122:202–208, 208 [DOI] [PubMed] [Google Scholar]
- 27. Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua A. 2010. Polymorphisms of chitinases are not associated with asthma. J. Allergy Clin. Immunol. 125:754–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee CG, et al. 2009. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 206:1149–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chupp GL, et al. 2007. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 357:2016–2027 [DOI] [PubMed] [Google Scholar]
- 30. Kuepper M, Bratke K, Virchow JC. 2008. Chitinase-like protein and asthma. N. Engl. J. Med. 358:1073–1075 [DOI] [PubMed] [Google Scholar]
- 31. Hoshino H, et al. 1999. Allergic bronchopulmonary aspergillosis due to Aspergillus niger without bronchial asthma. Respiration 66:369–372 [DOI] [PubMed] [Google Scholar]
- 32. Laham MN, Allen RC, Greene JC. 1981. Allergic bronchopulmonary aspergillosis (ABPA) caused by Aspergillus terreus: specific lymphocyte sensitization and antigen-directed serum opsonic activity. Ann. Allergy 46:74–80 [PubMed] [Google Scholar]
- 33. Sandhu RS, Mehta SK, Khan ZU, Singh MM. 1979. Role of aspergillus and candida species in allergic bronchopulmonary mycoses. A comparative study. Scand. J. Respir. Dis. 60:235–242 [PubMed] [Google Scholar]
- 34. Broberg A, Faergemann J. 1995. Topical antimycotic treatment of atopic dermatitis in the head/neck area. A double-blind randomised study. Acta Derm. Venereol. 75:46–49 [DOI] [PubMed] [Google Scholar]
- 35. Woodfolk JA. 2005. Allergy and dermatophytes. Clin. Microbiol. Rev. 18:30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldman DL, et al. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66 [DOI] [PubMed] [Google Scholar]
- 37. Garcia-Hermoso D, Janbon G, Dromer F. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haugen RK, Baker RD. 1954. The pulmonary lesions in cryptococcosis with special reference to subpleural nodules. Am. J. Clin. Pathol. 24:1381–1390 [DOI] [PubMed] [Google Scholar]
- 39. Campbell GD. 1966. Primary pulmonary cryptococcosis. Am. Rev. Respir. Dis. 94:236–243 [DOI] [PubMed] [Google Scholar]
- 40. Curtis JL, et al. 1994. Experimental murine pulmonary cryptococcosis. Differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab. Invest. 71:113–126 [PubMed] [Google Scholar]
- 41. Arora S, et al. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346–6356 [DOI] [PubMed] [Google Scholar]
- 42. Abe K, et al. 2000. Th1-Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol. Immunol. 44:849–855 [DOI] [PubMed] [Google Scholar]
- 43. Müller U, et al. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179:5367–5377 [DOI] [PubMed] [Google Scholar]
- 44. Goldman DL, Davis J, Bommarito F, Shao X, Casadevall A. 2006. Enhanced allergic inflammation and airway responsiveness in rats with chronic Cryptococcus neoformans infection: potential role for fungal pulmonary infection in the pathogenesis of asthma. J. Infect. Dis. 193:1178–1186 [DOI] [PubMed] [Google Scholar]
- 45. Denning DW, et al. 2009. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am. J. Respir. Crit. Care Med. 179:11–18 [DOI] [PubMed] [Google Scholar]
- 46. Goldman DL, et al. Increased chitinase expression and fungal-specific antibodies in the bronchoalveolar lavage fluid of asthmatic children. Clin. Exp. Allergy 42(4):523–530 [DOI] [PubMed] [Google Scholar]
- 47. Vicencio AG, et al. 2010. CHIT1 mutations: genetic risk factor for severe asthma with fungal sensitization? Pediatrics 126:e982–e985 [DOI] [PubMed] [Google Scholar]
- 48. Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua AA. 2010. Fungal exposure modulates the effect of polymorphisms of chitinases on emergency department visits and hospitalizations. Am. J. Respir. Crit. Care Med. 182:884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]