Abstract
Mesenchymal stem cells (MSCs) are capable of regenerative and immunomodulatory functions in cell-based therapies in a variety of human diseases and injuries; however, their therapeutic efficacy and potential side effects remain major obstacles in clinical applications. We report here a 3D spheroid culture approach to optimize stem cell properties and therapeutic effects of human gingiva-derived mesenchymal stem cells (GMSCs) in mitigation of experimental oral mucositis. Under growth condition of ultra-low attachment, GMSCs spontaneously aggregated into 3D spheroids and exhibited distinct early stem cell phenotype characterized by elevated expression Stro-1 and CXC chemokine receptor 4 (CXCR-4) as well as OCT-4 and Nanog, 2 important transcriptional factors relevant to stem cell properties, and decreased expression of MSC-associated markers, including CD29, CD90, and CD105. Functionally, spheroid GMSCs are capable of enhanced multipotency and augmented secretion of several chemokines and cytokines relevant to cell migration, survival, and angiogenesis. More importantly, spheroid GMSCs expressed increased levels of reactive oxygen species, hypoxia-inducible factor (HIF)-1 and -2α, and manganese superoxide dismutase, which correlated with improved resistance to oxidative stress-induced apoptosis. Using an in vivo murine model of chemotherapy-induced oral mucositis, we demonstrated that spheroid-derived GMSCs possessed better therapeutic efficacy than their adherent cells in reversing body weight loss and promoting the regeneration of disrupted epithelial lining of the mucositic tongues. These findings suggest that 3D spheroid culture allows early stemness preservation and potentially precondition GMSCs for enhanced mitigation of oral mucositis.
Introduction
Mesenchymal stem cells (MSCs) derived from adult tissues represent a heterogeneous subset of stromal cells that proliferate in vitro as plastic-adherent cells, capable of colony formation, multi-lineage differentiation, immunomodulatory, and anti-inflammatory functions [1,2]. MSCs possess the ability to home and engraft at the injured site and promote tissue repair through synergistic downregulation of proinflammatory cytokines and increased production of a myriad of soluble factors with antioxidant, antiapoptotic, and proangiogenic functions [3–5]. As such, MSC-based therapy plays a promising therapeutic modality for tissue regeneration and wound repair [3–5]; despite numerous potentials, several limitations remain, including unpredictable engraftment, survival, and biological functions of MSCs at the injury sites [6]. To date, several strategies have been reported for optimizing the functions of MSCs, including genetic modifications and in vitro priming with proinflammatory cytokines (such as IFN-γ or TNF-α), or manipulation of culture conditions [6]. For instance, several studies have reported that aggregation of MSCs in a short-term 3D spheroid culture can significantly enhance their multipotent differentiation, anti-inflammatory properties, proangiogenic ability, and engraftment at the ischemic environment [7–10]. However, maximizing therapeutic efficacy and safety and simultaneously minimizing production cost still remains a great challenge for large-scale clinical application of MSC-based therapies.
Oral mucositis is one of the most debilitating side effects of common cancer therapies such as chemotherapy and radiation therapy. It affects 10%–40% of patients receiving chemotherapy for solid tumors, 60%–100% of patients undergoing myeloablative regimen for hematopoietic stem cell transplant, and up to 100% of those undergoing high dose radiation therapy for head and neck cancers [11,12]. Oral mucositis is characterized by impaired regenerative capacity of the oral and alimentary epithelium, leading to atrophy, erythema, ulceration, and, eventually, the loss of mucosal barrier functions [13,14]. Clinically, oral mucositis is manifested as mouth, throat, and abdominal pain, bloating, nausea, vomiting, and severe diarrhea, along with a loss of appetite and increased risks of infection and bleeding at mucositis sites [11–14]. These manifestations lead to a prolonged hospital stay, an increased need for antibiotics, narcotic analgesia, and parenteral nutrition, and, consequently, a huge burden in healthcare costs [13,14]. More importantly, mucositis threatens the efficacy of cancer treatment due to interruption of radio- or chemotherapy cycle and dose de-escalation, thus adversely affecting patient overall survival and quality of life [13,14]. However, most of current interventions for oral mucositis are only palliative, neither specific nor efficient at preventing or treating this complication. Even though recombinant human keratinocyte growth factor (Palifermin) is the only drug approved by FDA for the treatment of oral mucositis, its application has been greatly limited by the requirement of frequent injections, the high cost, the inconvenience, and the potential tumor-promoting effect [15–17]. Therefore, more effective approaches for the treatment of oral mucositis are urgently needed.
Recently, we and several other groups have isolated a new population of mesenchymal stromal cells from human gingiva, namely, GMSCs, which exhibit similar stem cell-like properties and immunomodulatory abilities to human bone marrow MSCs [18–21]. Using GMSCs as cell-based therapy, we demonstrated a reversal of colonic inflammation and restoration of body weight in colitis mice [18], and a rapid dermal closure in full-thickness excisional wounds [22]. In the present study, we seek to optimize stem cell properties of our regular adherent GMSCs by generating spheroid GMSCs using a low attachment culture condition and determine their therapeutic effects in ameliorating oral mucositis in chemotherapy-treated mice and the potential mechanisms.
Materials and Methods
Animals
Balb/c mice (male, 8–10 weeks old) were obtained from Jackson Laboratories (www.jax.org) and group-housed at the Animal Facility of University of Southern California (USC) under the institutional protocols approved by the Institutional Animal Care and Use Committee (IACUC) at USC.
Cell culture
The isolation of human bone marrow and GMSCs were described previously [18]. Gingival tissues were obtained as remnants of discarded tissues under the approved Institutional Review Board protocol at USC. MSCs were cultured either as adherent monolayers on regular dishes or as suspension culture in ultra-low attachment dishes at a density of 2×105/mL to allow 3D spheroid formation for up to 3 days. All cultures were maintained in complete alpha-minimum essential medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin/100 μg/mL streptomycin, 2 mM l-glutamine, 100 mM nonessential amino acid, and 550 μM 2-mercaptoethanol, at 37°C in a humidified tissue culture incubator with 5% CO2. To obtain spheroid-derived cells, spheroids were incubated with 0.25% trypsin at 37°C for 15–30 min, while pipetting every 5 min, and spheroid-dissociated single cells were collected by centrifugation.
Cell surface phenotype detection
Spheroid-derived MSCs and their adherent counterparts were resuspended at 2×106/mL in 100 μL phosphate-buffered saline (PBS) containing 2% FBS and incubated for 30 min at 4°C with specific primary antibodies for human CD45, CD29, CD73, CD90, CD105, CD146, CXC chemokine receptor 4 (CXCR-4; eBiosciences), Stro-1 and SSEA-4 (R&D System), or isotype-matched control IgGs, followed by fluorescein-conjugated secondary antibody. Cells were subjected to flow cytometric analysis using a flow cytometer [18].
Cell viability assays
Spheroid-derived cells and their adherent counterparts were incubated at 37°C overnight in the presence or absence of 200 μM H2O2. Cells were collected and cell viability was determined by flow cytometry using Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD Biosciences). Viable cells are FITC Annexin V and PI negative, and early apoptotic cells are FITC Annexin V positive and PI negative, whereas cells at end stage apoptosis and death are FITC Annexin V and PI positive.
Cell cycle analysis
Cells collected and fixed in 75% ethanol, washed, and resuspended in PBS containing 7 U/mL RNase A (Sigma). About 50 μg/mL PI (Sigma) was added and incubated at room temperature for 1 h. DNA content was measured with a flow cytometer.
Adipogenetic differentiation analysis
To induce adipogenetic differentiation, MSCs at 80%–90% confluence were cultured in adipocyte induction medium (complete culture medium containing 500 nM dexamethasone, 500 nM isobutylmethylxanthine, and 50 μM indomethacin) for 2–3 weeks with fresh medium changes every 3–4 days. The adipocytes were determined by Oil Red O staining [18].
Cytokine antibody array
Cytokine expression profiles in the supernatants were detected using RayBio Human Cytokine Antibody Array 3 (RayBiotech, Inc.; www.raybiotech.com) and semiquantified following the manufacturer's instructions. The medium alone was used as background control and arbitrarily set as 1.0. [22].
Measurement of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) was assessed using 2′,7′-dichlorofluorescein diacetate (DCFH-DA). In brief, spheroids or their adherent counterpart GMSCs were seeded in 8-well slide chamber and incubated with 10 μM DCFH-DA for 30 min at 37°C and washed with PBS. ROS generated in the cells caused oxidation of DCFH, yielding a fluorescent product (DCF), which can be detected using a fluorescence microscope.
Immunofluorescence studies
Cells were fixed with 4% paraformaldehyde; immunostained with specific primary antibodies for Oct-4, Nanog, super-oxidative dismutase (SOD) 2, hypoxia-inducible factor (HIF)-1α, HIF-2α, CXCR-4; and then incubated with FITC- and/or rhodamine-conjugated secondary antibodies (BD Biosciences). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Isotype-matched control antibodies (eBiosciences) were used as negative controls.
Western blot analysis
Cell lysates were separated on polyacrylamide–sodium dodecyl sulfate gel and electroblotted onto nitrocellulose membrane (BioRad; www.bio-rad.com). After blocking with TBS/5% nonfat dry milk, the membrane was incubated with antibodies against Oct-4, Nanog, and SOD2, followed by incubation with a horseradish peroxidase-conjugated secondary antibody, and the signals were observed by enhanced chemiluminescence detection (Pierce; www.piercenet.com). The blots were also re-probed with a specific antibody against β-actin (Sigma).
Oral mucositis model
Oral mucositis was induced in Balb/c mice by intraperitoneal (i.p.) injection with 5-fluorouracil (5-FU; 50 mg/kg) for 3 consecutive days [23]. On day 4, mice of the treatment groups (n=5) were intravenously infused with either spheroid-derived GMSCs (1×106/mice) or their adherent counterparts (1×106/mice). Placebo group was treated with PBS. On day 7, tongue samples were collected for further analysis. Epithelial thickness and disruption was observed using standard hematoxylin and eosin (H&E) staining and optical coherence tomography scanning. To track cells homed to the injury sites, MSCs were prelabeled with CM-DiI (max absorbance at 570 nm) before systemic injection into mice. Tissue sections were prepared and observed under a fluorescence microscope. For the BrdU labeling, the mice were injected with BrdU (50 mg/kg) 1 h before sacrifice for tissue harvesting.
Histological and immunohistochemical studies
Paraffin-embedded sections of mice tongue samples were prepared for H&E or immunohistochemical staining using primary antibodies specific for mice proliferating cell nuclear antigen (PCNA), CK-14, BrdU, human vimentin, and E-cadherin as described previously [18]. Isotype-matched control antibodies (eBiosciences) were used as negative controls. For semi-quantification, positive signals in at least 5 random high-power fields (HPF) were observed, counted, and expressed as percentage of total DAPI-positive cells (mean±SD).
Statistical analysis
All data are expressed as mean±SEM from at least 3 independent experiments. Differences between experimental and control groups were analyzed by 2-tailed unpaired Student's t-test using SPSS. P values less than 0.05 were considered statistically significant.
Results
Formation of 3D spheroid GMSCs in suspension culture condition
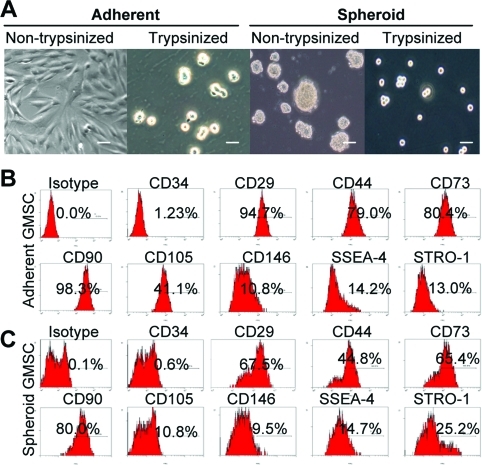
Recently, several studies have reported that aggregation of MSCs into 3D spheroids enhances MSC biological properties and therapeutic potentials [7–10]. Herein, we showed that under condition of ultra-low attachment GMSCs spontaneously aggregated into small colonies as a suspension of 3D spheroids of 20–100 μm diameter (Fig. 1A). Similar to previous studies [7], GMSCs dissociated from spheroids were relatively small, nearly half in size, and more homogeneous as compared with cells derived from adherent monolayer culture as determined by microscopy (Fig. 1A) and flow cytometric analysis (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). We next explored the phenotypic profile of spheroid-derived GMSCs using a panel of MSC-related cell surface markers. As compared with their adherent counterparts, the fraction of spheroid-derived GMSCs positively stained for CD29, CD90, and CD105 decreased significantly (P<0.05), whereas the percentage of CD44+ and CD73+-positive cells appears suppressed, however, without statistical significance (P>0.05). On the other hand, the proportion of cells positive for Stro-1 (an early lineage marker for MSCs) in spheroid GMSCs increased from 8.67.0±2.12% to 27.0±5.9% (P<0.05) (Fig. 1B, C and Supplementary Table S1). These results suggest that the GMSCs in spheroid 3D culture are more capable of maintaining early stem cell-like properties as compared with their adherent counterparts.
FIG. 1.
Characterization of cell morphology and surface phenotype of GMSCs grown in 3D spheroid cultures. (A) Representative images show the growth of GMSCs from the same donor either as standard adherent monolayer cells on a regular culture dish (left) or as suspended 3D spheroids at a density of 1×105 cells/mL on an ultra-low adherent culture dish (right). Seventy-two hours later, cells were harvested as single-cell suspensions after digestion with 0.25% trypsin. Scale bars: 100 μm. (B,C) Single cells derived from spheroid GMSCs or their adherent counterparts were immunostained with primary antibodies specific for a panel of cell surface markers, followed by incubation with FITC-conjugated secondary antibodies and then analyzed by flow cytometry. The results were representative of at least 5 independent experiments. GMSCs, gingiva-derived mesenchymal stem cells. Color images available online at www.liebertonline.com/scd
Enhanced expression of stem cell-related genes by spheroid GMSCs
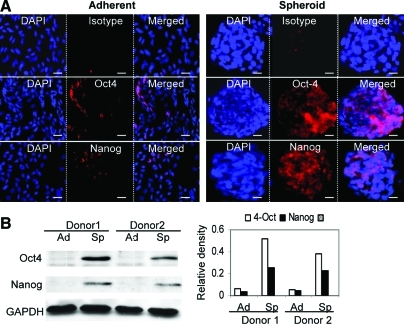
We then examined expression of Oct-4 and Nanog, 2 important transcriptional factors in the regulation of stemness and self-renewal of a various types of stem cells [24,25]. Immunofluorescence studies indicated that expression of both Oct-4 and Nanog was significantly increased in spheroid GMSCs in comparison to their adherent counterparts (Fig. 2A). Expression of both transcriptional factors was not uniform among cells in the spheroid colonies. Increased expression of Oct-4 and Nanog in spheroid GMSCs was further confirmed by western blot analysis (Fig. 2B). These results further support the notion that 3D spheroid culture can promote the stemness of GMSCs by upregulating the expression of stem cell-related transcriptional factors.
FIG. 2.
Spheroid cultures promote Oct-4 and Nanog expression in GMSCs. (A) Spheroid GMSCs or their adherent counterparts fixed in 4% paraformaldehyde were immunostained with primary antibodies specific for Oct-4 or Nanog or an isotype-matched IgG, followed by incubation with rhodamine-conjugated secondary antibodies and then observed under a fluorescence microscope. Scale bar: 100 μm. (B) Western blot analysis of Oct-4 or Nanog expression in spheroid GMSCs (Sp) or their adherent counterparts (Ad) (left panel). The right panel shows the relative densities of Oct-4 and Nanog normalized to GAPDH. The results were representative of at least 3 independent experiments. Color images available online at www.liebertonline.com/scd
Spheroid GMSCs are capable of enhanced stem-like functions
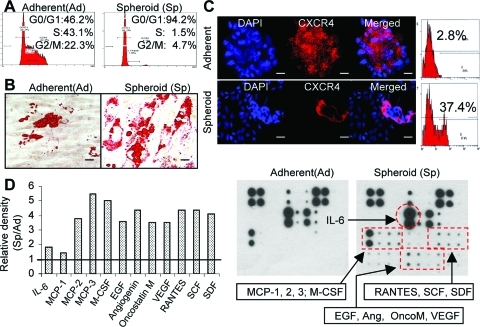
First, we studied cell cycle distribution of spheroid GMSC culture using flow cytometric analysis. As shown in Fig. 3A, spheroid culture significantly decreased the percentage of GMSCs in S-phase, with >90% of cells arrested at G0/1-phase as compared with their adherent counterparts. We next tested the multipotent differentiation of using in vitro studies. Our results showed that spheroid GMSCs displayed increased capacities to differentiate into adipocytes, suggesting an enhanced multipotency in comparison with their adherent counterparts (Fig. 3B). Meanwhile, we examined expression of CXCR-4, a receptor involved in the homing or recruitment of MSCs [6,7]. Fluorescence microscopy and flow cytometry analyses demonstrated a marked increase in expression of CXCR-4 in spheroid GMSCs as compared with cells from the adherent monolayers (Fig. 3C). MSCs exert their biological functions through the secretion of a plethora of functional soluble factors [3,5]. Therefore, antibody arrays were performed to compare the cytokine and chemokine profiles of spheroid and adherent GMSCs. Our results showed that, in comparison to the adherent GMSCs, spheroid GMSCs secrete an elevated level of a variety of cytokines and chemokines, including monocyte chemotactic protein (MCP)-2, MCP-3, RANTES, M-CSF, EGF, VEGF, SDF-1, and angiogenin (Fig. 3D), which play important role in promoting cell migration, proliferation, survival, and angiogenesis. Taken together, these results suggest that spheroid culture led to improved cellular functions of GMSCs, which might contribute to an enhanced stem-like functions and therapeutic efficacy for several clinical applications.
FIG. 3.
Three-dimensional spheroid culture improves cell plasticity of GMSCs. (A) Spheroid culture significantly reduced the percentage of S-phase cells. After culture as adherent monolayer cells or as spheroids for 72 h, cells were harvested as single cell suspensions and cell cycle distribution was determined by flow cytometry. (B) Spheroid GMSCs showed enhanced multipotent differentiation capacities. Spheroid GMSCs or their adherent counterparts were cultured in adipogenic induction medium for 3 weeks and adipocyte differentiation was determined by Oil Red O staining. (C) Increased expression of CXCR-4 by spheroid GMSCs. Both spheroid GMSCs and their adherent counterparts fixed in 4% paraformaldehyde were immunostained with primary antibodies specific for CXCR-4 or an isotype-matched IgG, followed by incubation with rhodamine-conjugated secondary antibodies and then observed under a fluorescence microscope. Scale bar: 100 μm. (D) Spheroid GMSCs displayed an enhanced secretion of various chemokines, cytokines, and growth factors. The cytokine expression profile in the conditioned media collected from spheroid-GMSCs or their adherent counterparts (1×106) were detected using the RayBio Human Cytokine Antibody Array 3. The fresh medium without cell culture was used as a background control. Right: the representative image of cytokine antibody array. Left: the graphs show the relative intensity of spots of individual protein, whereby the intensity of the medium control was arbitrarily set as 1.0. The results were representative of at least 3 independent experiments. Color images available online at www.liebertonline.com/scd
Spheroid GMSCs are more resistant to oxidative stress
The nature of the 3D spheroid structure potentially limits cells in the inner core of adequate oxygen exchange. We postulated that a hypoxic microenvironment may be present within the spheroid structure. Our studies demonstrate an increase in the production of ROS within the GMSC spheroids as determined by DCFH-DA staining (Supplementary Fig. S2A). Meanwhile, immunofluorescence studies demonstrated that spheroid GMSCs expressed a much higher level of both HIF-1α and HIF-2α, 2 important hypoxia-responsive transcriptional factors, as compared with the adherent counterparts (Supplementary Fig. S2B, S2C). These results suggest the existence of an oxidative microenvironment within GMSC spheroids.
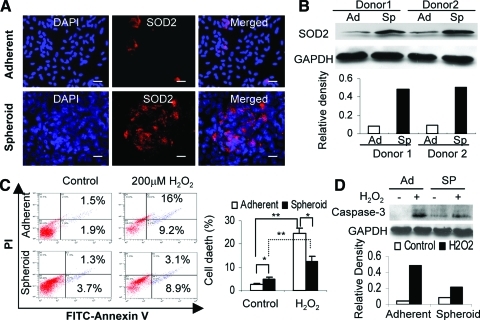
Manganese SOD (SOD2) is an antioxidant enzyme, which plays a key role in cellular resistance to oxidative stress via scavenging mitochondrial ROS (mtROS) [26]. We explored whether GMSCs expressed SOD2 in the presence of a potential oxidative and hypoxic microenvironment within the spheroid. Immunofluorescence studies demonstrated that expression of SOD2 was dramatically increased in spheroid GMSCs as compared with their adherent counterparts (Fig. 4A). Increased expression of SOD2 by spheroid GMSCs was further confirmed by western blot analysis (Fig. 4B). Next, we investigated the potential enhanced resistance of spheroid GMSCs to oxidative stress. To this end, both cells were exposed to exogenous H2O2 for 24 h, and apoptotic cells were tracked using annexin V-FITC. Interestingly, GMSCs prepared from 3-day spheroid culture without exposure to H2O2 contained a relatively high number of early and late apoptotic cells as compared with their adherent counterparts (P<0.05); however, after exposure to H2O2 for 24 h, the number of total apoptotic cells in spheroid derived GMSCs decreased to a lower level than that in monolayer-cultured cells (P<0.05) (Fig. 4C). Concomitantly, caspase-3 activities were suppressed in spheroid GMSCs as compared with those in adherent cells after exposure to H2O2 for 24 h (Fig. 4D). Taken together, these results suggest that spheroid culture conditioned GMSCs to hypoxic and oxidative stresses, mediated by the upregulation of hypoxia-adaptive gene expression (such as HIFs, VEGF, and SOD2), promotion of apoptosis resistance and survival ability, and enhanced secretion of chemokines and angiogenic factors.
FIG. 4.
Spheroid GMSCs exhibit increased resistance to oxidative stress-induced apoptosis. (A) Increased expression of SOD2 by spheroid GMSCs. Both spheroid GMSCs and their adherent counterparts fixed in 4% paraformaldehyde were immunostained with primary antibodies specific for SOD2 or an isotype-matched IgG, followed by incubation with rhodamine-conjugated secondary antibodies and then observed under a fluorescence microscope. Scale bar: 100 μm. (B) Western blot analysis of SOD2 expression in spheroid GMSCs (Sp) or their adherent counterparts (Ad) (top panel). The lower panel shows the relative densities of SOD2 normalized to GAPDH. (C) Spheroid-derived GMSCs or their adherent counterparts were exposed to 200 μM H2O2 for 24 h, followed by labeling with FITC-Annexin-V/propidium iodide and then subjected to flow cytometric analysis. (D) Western blot analysis of cleaved caspase-3 expression in spheroid GMSCs or their adherent counterparts with or without exposure to 200 μM H2O2 (top panel). The lower panel shows the relative densities of caspase-3 normalized to GAPDH. The results were representative of at least 3 independent experiments. *P<0.05; **P<0.01. SOD2, superoxide dismutase 2. Color images available online at www.liebertonline.com/scd
Spheroid GMSCs are capable of enhanced therapeutic effects on chemotherapy-induced oral mucositis
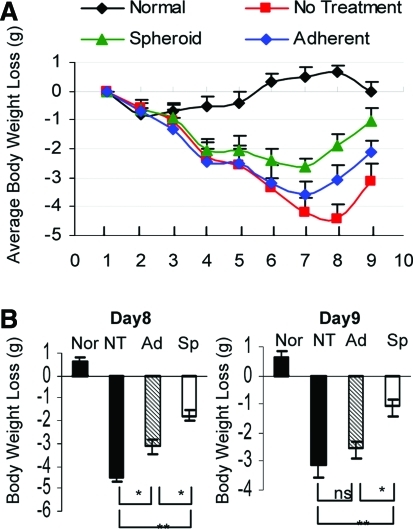
Given the findings that spheroid cultures were capable of optimizing multiple functions, we postulated that spheroid-derived GMSCs potentially possess improved therapeutic efficacy on wound healing or repair of injured tissues. Here, we employed a model of compromised wound healing using chemotherapy-induced oral mucositis model in mice. To this end, oral mucositis was induced by i.p. administration of 5-FU for 3 consecutive days. On day 4, the treatment groups (n=5) were intravenously injected with either spheroid GMSCs (1×106/mice) or their adherent counterpart (1×106/mice), whereas mice treated with PBS were used as disease control. Clinically, we observed a continued body weight loss in the disease group without cell treatment, which reached maximum on day 8 and started to recover on day 9 (Fig. 5A). Using optical coherence tomography coupled with multiphoton microscopy (MPM), we could delineate microscopic surface irregularities and loss of papillary structures on the dorsal surface of mucositic tongues (Fig. 6C). Interestingly, nearly all mice undergoing GMSC treatment demonstrated a restoration of body weight loss, but a much better effect was observed after treatment with spheroid GMSCs as compared with adherent cells (Fig. 5A, B). Meanwhile, MPM imaging delineated regeneration of topographical structures along with reorganization of filiform papilla in the tongue after spheroid GMSC treatment (Fig. 6C).
FIG. 5.
Spheroid GMSCs show enhanced therapeutic efficacy for reversing body weight loss caused by chemotherapy-induced oral mucositis. Oral mucositis was induced in Balb/c mice as described in detail in the Materials and Methods section. On day 4 after induction of oral mucositis, mice of the treatment group (n=5) were intravenously infused with either spheroid-derived GMSCs (Sp)(1×106/mice) or their adherent counterparts (Ad) (1×106/mice). Normal mice (Nor) without administration of any drug and cell treatments and mice with oral mucositis but with no cell treatment (NT) but receiving placebo treatment with phosphate-buffered saline were served as controls. (A) Body weight changes were monitored daily. (B) Representative body weight changes on day 8 and day 9, respectively. The results were representative of at least 3 independent experiments. *P<0.05; **P<0.01. Color images available online at www.liebertonline.com/scd
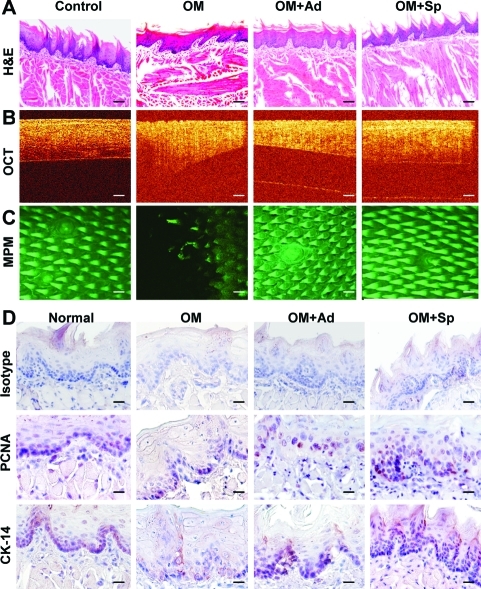
FIG. 6.
Spheroid GMSCs enhanced the recovery or regeneration of disrupted epithelial layers of mucositic tongues. On day 9, mice were sacrificed and tongue samples were harvested for further analysis. (A) H & E staining. (B) Epithelial thickness and disruptions were observed with optical coherence tomography scanning. (C) Epithelial disruptions were observed with MPM scanning. (D) Immunohistochemical studies on the expression of PCNA and CK-14. Scale bars: 50 μm. The results were representative of at least 3 independent experiments. OM, oral mucositis; Ad, adherent GMSCs; Sp, spheroid GMSCs; PCNA, proliferating cell nuclear antigen; MPM, multiphoton microscopy. Color images available online at www.liebertonline.com/scd
Histologically, H&E staining and OCT imaging showed that untreated mucositic tongues displayed a complete atrophy of epithelial surface, including loss of filliform papillae and ulceration of the mucosal lining, thinning, and disruption of the epithelium layer along with the loss of the basal membrane (Fig. 6A, B). In addition, immunostaining with specific antibodies for CK14, a marker for basal layer cells, and PCNA showed a significantly decreased number of proliferating cells along with a disruptive basal cell layer in untreated mucositic tongues (Fig. 6D). Of special interest, treatment with GMSC decreased the severity and incidence of ulceration and restored the papillae structure, the lining, and thickness of the epithelial layer as compared with those of untreated disease group (Fig. 6A, B). Immunohistochemical studies demonstrated that treatment with spheroid GMSCs led to faster regeneration of the basal layer and greater restoration of the epithelial layer compared with treatment with adherent GMSCs (Fig. 6D).
Similar to previous studies, we have also shown that administration of 5-FU lead to a reduction in intestinal crypt length and obliteration of the crypts, blunting and fusion of intestinal villi, and suppressed cell proliferation in the treated jejunum as determined by in vivo BrdU labeling and immunostainings (Supplementary Fig. S3A). Interestingly, we observed that treatment with GMSCs also promoted proliferation of mucosal epithelial cells and the regeneration of damaged crypts (Supplementary Fig. S3A, S3B). All together, these results indicate that spheroid-derived GMSCs showed improved therapeutic efficacy in reversing chemotherapy-induced oral mucositis as compared with their adherent counterparts.
Spheroid GMSCs displayed increased homing to mucositis sites and underwent mesenchymal–epithelial transdifferentiation
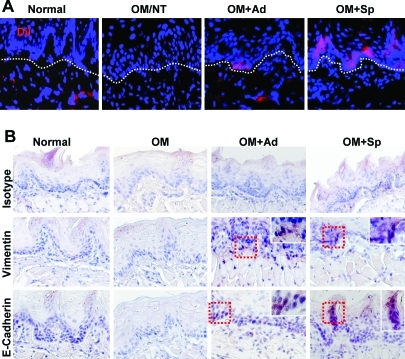
Since spheroid-derived GMSCs expressed higher level of CXCR-4 than their adherent cultures (Fig. 3C), they might be capable of increased homing or stem cell grafting at the inflammatory mucositic sites. To confirm this, spheroid and adherent GMSCs were prelabeled with CM-DiI (570 nm) and then systemically injected into mice. We observed increased homing of spheroid GMSCs to the mucositic tongue as compared with adherent cells. More interestingly, the majority of homed adherent GMSCs were located in the subepithelial layer of tongue, whereas the homed spheroid-derived GMSCs were distributed in both epithelial and subepithelial layers (Fig. 7A), suggesting that spheroid GMSCs potentially underwent mesenchymal–epithelial transition (MET) and transdifferentiated into epithelial cells (Fig. 7A). To validate these findings, we performed immunostaining with antibodies specific for human vimentin, one of the specific markers for mesenchymal cells, and human E-cadherin, a specific marker for epithelial cells. Our results displayed abundant presence of cells positively stained for human vimentin and E-cadherin in tongue sections of mice receiving spheroid GMSC treatment as compared with those of adherent GMSC-treated mice; the majority of E-cadherin-positive cells were located in the epithelial layers (Fig. 7B). These findings support the notion that GMSCs derived from 3D spheroids are capable of increased homing or engrafting functions, specifically to the injured sites, and potentially trans-differentiate into epithelial cells, in the regeneration of the mucositic lesions of mice tongue. The increased plasticity of spheroid GMSCs may contribute to their enhanced therapeutic efficacy for regeneration or repair of injured tissues in our experimental model of oral mucositis.
FIG. 7.
Spheroid GMSCs show increased homing ability and transdifferentiation potential to epithelial-like cells. (A) Spheroid-derived GMSCs and their adherent counterparts were prelabeled with DiI and then systemic injected into mice on day 4 after oral mucositis induction. On day 9, tongue samples were collected and frozen sections were prepared and observed under a fluorescence microscope. (B) Immunohistochemical studies were performed using antibodies specific for human vimentin and E-cadherin. OM, oral mucositis; NT, no cell treatment; Ad, adherent GMSCs; Sp, spheroid GMSCs. Scale bars: 50 μm. The results were representative of at least 3 independent experiments. Color images available online at www.liebertonline.com/scd
Discussion
Cancer therapy-induced oral mucositis represents a compromised oral wound characterized by atrophy, erythema, ulceration, and, eventually, loss of the mucosal barrier functions secondary to impaired regenerative capacity of the epithelium [11–14]. Using a murine model of chemotherapy-induced oral mucositis, we have demonstrated for the first time to our knowledge that spheroid-derived GMSCs are capable of enhanced therapeutic efficacy to reverse body weight loss, regenerate the epithelial lining, and recover mucosal disruption as compared with their adherent cells. The improved therapeutic benefits of spheroid-derived GMSCs for oral mucositis may be attributed to their enhanced capabilities for engraftment and survival at the injury sites, trans-differentiation into epithelial cells, and preconditioning to hypoxic and oxidative challenges existing in the 3D spheroid cultures.
Despite overwhelming benefits of MSC-based therapeutics for a variety of diseases, several obstacles clearly remain for their large-scale clinical applications. It has been recognized that progressive subculturing of MSCs potentially leads to changes in cellular phenotypes affecting their regenerative and homing abilities [5,6]. Currently, intravenous delivery of a large bolus of cells and direct tissue injection are the 2 major methodologies for preclinical studies and clinical trials of MSC-based therapy. However, intravenous delivery requires a relatively large dose of cells (from 1 to 5×106 cells/kg body weight) at significant production cost and increased risk of side effects such as pulmonary embolism and organ infarction, whereas direct tissue injection requires invasive, technique-sensitive, and precise infusion methodologies [5,6]. Therefore, how to maximize therapeutic efficacy and safety of MSC-based therapy while minimizing adverse effects, overall manufacture, and procedure costs remains a great challenge. To date, transgenic approaches have been developed to force MSCs to overexpress certain defined factors with either trophic or tropic, survival, proangiogenic, immunomodulatory, or anti-inflammatory functions [5,6]. In recent years, numerous nontransgenic approaches aiming to increase the multipotency of MSCs or to evoke the secretion of these functional factors by MSCs have attracted more and more attention due to the lack of viral and mutation risks. One of the common nontransgenic approaches for optimizing MSC functions involves priming or preconditioning MSCs in vitro with certain proinflammatory cytokines such as IFN-γ or TNF-α, which may be encountered by MSCs at the sites of inflammation or injured tissues [27]. Another strategy for optimization of MSCs involves preconditioning cells under low oxygen tension or hypoxic condition, a common physiologic or metabolic milieu of stem cell niche, that potentially play a critical role in the maintenance of an undifferentiated state of both embryonic and adult stem cells, and in the regulation of proliferation and cell fate commitment [28]. For instance, previous studies have shown that bone marrow MSCs cultured in hypoxia exhibit increased expression of Oct-4 and telomerase activity [29,30] and decreased differentiation into adipogenic and osteogenic lineages [31,32]. In addition, MSCs exposed to hypoxic conditions display a more migratory or proangiogenic phenotype, and improved survival due to an increased expression of a variety of hypoxia-responsive genes involved in angiogenesis and cell migration, such as VEGF, SDF-1α, and CXCR-4 [33–35]. Therefore, hypoxic preconditioning of MSCs is an effective methodology to optimize MSC functions, thus enhancing their global functions and therapeutic benefits after migrate to sites of inflammation and injured tissues, where they may encounter an environment of hypoxia and oxidative stresses [36,37].
Most recently, several studies have shown that 3D spheroid cultures of MSCs significantly enhance the stem cell-like properties and therapeutic effects. For example, Bartosh et al. have recently reported that aggregation of human bone marrow-derived mesenchymal stem cells (BMSC) into 3D spheroids not only enhanced their multipotent differentiation capacities, but also led to an increased expression of the TNF-α suppressing gene-6 (TSG-6), an anti-inflammatory molecule, as compared with their adherent counterparts [7]. In addition, Li et al. have shown that growing cardiac-derived progenitor cells as 3D spheroid cardiospheres led to upregulated expression of stem cell related genes and improved cell survival after exposure to oxidative stress as compared with their monolayer counterparts [8]. Most recently, Bhang et al. reported that spheroid cultures were more effective in preconditioning human adipose-derived stem cells to a hypoxic environment, leading to upregulation of hypoxia-adaptive signals and enhancing secretion of both angiogenic and antiapoptotic factors as compared with monolayer cultures [10]. Consistently, in the present study we have demonstrated that 3D spheroid culture of GMSCs not only enhanced the expression of stem cell relevant genes and their multipotent differentiation capacity but also the expression of hypoxia responsive genes such as HIF-1 and −2α, VEGF, SDF-1α, and CXCR-4, as compared with the monolayer cultures. Meanwhile, we have demonstrated that spheroid GMSCs show an increased production of ROS and superoxide dismutase-2 (SOD2) as well as improved survival under oxidative stress conditions. These findings support the notion that the 3D spheroid culture condition recapitulates hypoxic and oxidative microenvironment of the inflammatory niche at the injured or mucositic sites and potentially act as an in vitro preconditioning of GMSCs to be more resistant to apoptotic stress and optimize their therapeutic effects in reversal of oral mucositis.
Of note, the regeneration of injured or damaged epithelium-lined organs requires the coordination of multiple types of cells, the epithelial, endothelial, mesenchymal, and immune cells [38]. Recent studies have shown that mesenchymal stem cells (MSCs) participate in the regeneration and repair of a variety of diseased epithelial tissues, including injured epithelial layers in skin [39], airway [40], cornea [41], gastric and intestine [42,43], kidney [44], and oral cavity [45]. The mechanisms underlying MSC-mediated regeneration of injured epithelial tissues may involve not only the secretion of various factors with antioxidant, anti-inflammatory, antiapoptotic, or proangiogenic functions, but also the transdifferentiation of MSCs into epithelial-like cells possibly through MET [44]. In the present study, we have shown that in comparison with their adherent counterparts, spheroid-derived GMSCs displayed increased cell plasticity and abilities to home to the mucositic lesions. The relatively smaller cell sizes and increased expression of CXCR-4 by spheroid GMSCs may facilitate their faster trafficking through the lung microvasculature and more efficient distribution into tissues. Meanwhile, our findings have revealed an enhanced potential for homed spheroid-GMSCs to transdifferentiate into epithelial-like cells. This might contribute, at least in part, to their enhanced therapeutic efficacy for the regeneration or repair of mucositic lesions. However, further studies are warranted to explore the deep mechanisms.
Despite the observed therapeutic benefits of spheroid-derived GMSCs in cancer therapy-induced mucositis, their potential impact on cancer development remains to be addressed. The literature on tumor tropism, pro-, and antitumorigenicity of MSCs [46,47] is highly controversial. In some studies, MSCs have been reported to promote tumor proliferation, angiogenesis, epithelial–mesenchymal transition, as well as metastasis [46,47], whereas other studies have shown the tumor suppressive effect of MSCs in different tumor models [46]. The unique tumor tropism of MSCs renders them a unique delivery vehicle for antitumor agents [48]. Understanding the interaction between MSCs and tumor cells and their underlying mechanisms will allow development of novel therapeutic approaches to target the stromal effects on tumor growth while reversing the detrimental effect of chemotherapy-induced epithelial injuries.
Conclusion
We have demonstrated that spheroid cultures recapitulate a hypoxic microenvironment that contributes to in vitro preconditioning GMSCs to optimize their therapeutic efficacy for the treatment of oral mucositis due to their enhanced multipotency, homing, survival, and transdifferentiation capacities. These findings further support the notion that 3D spheroid cultures might provide a simple and effective strategy for optimizing MSC-based therapy for regeneration or repair of injured tissues or the treatment of other inflammation-related diseases characteristic of hypoxic and oxidative stresses.
Supplementary Material
Acknowledgments
This work was supported in part by National Institute of Health Research Grant, R01DE 019932, the Laser Microbeam and Medical Program (LAMMP, NIH), D41-RR01192, the California Institute for Regenerative Medicine, RN1-00572, USC Institutional funding, CTSI and Zumberge award, and Oral & Maxillofacial Surgery Foundation (OMSF).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.da Silva Meirelles L. Chagastelles PC. Nardi NB. Mesenchymal stem cells reside in virtually all postnatal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Tolar J. Le BK. Keating A. Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English K. French A. Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation. Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Salem HK. Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner J. Kean T. Young R. Dennis JE. Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Bartosh TJ. Ylöstalo JH. Mohammadipoor A. Bazhanov N. Coble K. Claypool K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li TS. Cheng K. Lee ST. Matsushita S. Davis D. Malliaras K, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W. Itaka K. Ohba S. Nishiyama N. Chung UI. Yamasaki Y, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Bhang SH. Cho SW. La WG. Lee TJ. Yang HS. Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Bowen JM. Keefe MK. New pathways for alimentary mucositis. J Oncol. 2008;2008:9078–9092. doi: 10.1155/2008/907892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pico JL. Avila-Garavito A. Naccache P. Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist. 1998;3:446–451. [PubMed] [Google Scholar]
- 13.Scully C. Sonis S. Diz PD. Oral mucositis. Oral Dis. 2006;12:229–241. doi: 10.1111/j.1601-0825.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 14.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 15.Zheng C. Cotrim AP. Sunshine AN. Sugito T. Liu L. Sowers A, et al. Prevention of radiation-induced oral mucositis after adenoviral vector-mediated transfer of the keratinocyte growth factor cDNA to mouse submandibular glands. Clin Cancer Res. 2009;15:4641–4648. doi: 10.1158/1078-0432.CCR-09-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brizel DM. Murphy BA. Rosenthal DI. Pandya KJ. Glück S. Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489–2496. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 17.Brake R. Starnes C. Lu J. Chen D. Yang S. Radinsky R, et al. Effects of palifermin on antitumor activity of chemotherapeutic and biological agents in human head and neck and colorectal carcinoma xenograft models. Mol Cancer Res. 2008;6:1337–1346. doi: 10.1158/1541-7786.MCR-07-2131. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q. Shi S. Liu Y. Uyanne J. Shi Y. Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomar GB. Srivastava RK. Gupta N. Barhanpurkar AP. Pote ST. Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 20.Fournier BP. Ferre FC. Couty L. Lataillade JJ. Gourven M. Naveau A, et al. Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A. 2010;16:2891–2899. doi: 10.1089/ten.TEA.2009.0796. [DOI] [PubMed] [Google Scholar]
- 21.Tang L. Li N. Xie H. Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J Cell Physiol. 2011;226:832–842. doi: 10.1002/jcp.22405. [DOI] [PubMed] [Google Scholar]
- 22.Zhang QZ. Su WR. Shi SH. Wilder-Smith P. Xiang AP. Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J. Kim KA. De Vera J. Palencia S. Wagle M. Abo A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci U S A. 2009;106:2331–2336. doi: 10.1073/pnas.0805159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greco SJ. Liu K. Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 25.Pierantozzi E. Gava B. Manini I. Roviello F. Marotta G. Chiavarelli M, et al. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 26.He T. Peterson TE. Holmuhamedov EL. Terzic A. Caplice NM. Oberley LW, et al. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 27.Heo SC. Jeon ES. Lee IH. Kim HS. Kim MB. Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 28.Mohyeldin A. Garzon-Muvdi T. Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Grayson WL. Zhao F. Bunnell B. Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CC. Chen YJ. Yew TL. Chen LL. Wang JY. Chiu CH, et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 31.Fehrer C. Brunauer R. Laschober G. Unterluggauer H. Reitinger S. Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 32.Holzwarth C. Vaegler M. Gieseke F. Pfister SM. Handgretinger R. Kerst G, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson KM. Aly A. Lerman A. Lerman LO. Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: role of oxidative stress. Life Sci. 2011;88:65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chacko SM. Ahmed S. Selvendiran K. Kuppusamy ML. Khan M. Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H. Xue W. Ge G. Luo X. Li Y. Xiang H, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 36.Tang YL. Zhu W. Chang M. Chen L. Zhang J. Sun T, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;10:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das R. Jahr H. van Osch GJ. Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 38.Stappenbeck TS. Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 39.Dai Y. Li J. Li J. Dai G. Mu H. Wu Q, et al. Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns. 2007;33:418–428. doi: 10.1016/j.burns.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Sueblinvong V. Loi R. Eisenhauer PL. Bernstein IM. Suratt BT. Spees JL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh JY. Kim MK. Shin MS. Lee HJ. Ko JH. Wee WR, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 42.Ando Y. Inaba M. Sakaguchi Y. Tsuda M. Quan GK. Omae M, et al. Subcutaneous adipose tissue-derived stem ells facilitate colonic mucosal recovery from 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Inflamm Bowel Dis. 2008;14:826–838. doi: 10.1002/ibd.20382. [DOI] [PubMed] [Google Scholar]
- 43.Okumura T. Wang SS. Takaishi S. Tu SP. Ng V. Ericksen RE, et al. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest. 2009;89:1410–1422. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeisberg M. Shah AA. Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 45.Maria OM. Khosravi R. Mezey E. Tran SD. Cells from bone marrow that evolve into oral tissues and their clinical applications. Oral Dis. 2007;13:11–16. doi: 10.1111/j.1601-0825.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 46.Klopp A. Gupta A. Spaeth E. Andreeff M. Marini F. Concise review: dissecting a discrepancy in the literature: do mesencyhmal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeck AD. Narine K. De Neve W. Mareel M. Bracke M. De Wever O. Resident and bone marrow-derived mesenchymal stem cells in head and neck squamous cell carcinoma. Oral Oncol. 2010;46:336–342. doi: 10.1016/j.oraloncology.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Kim SM. Lim JY. Park SI. Jeong CH. Oh JH. Jeong M. Oh W, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.