Abstract
Mammary stem cells reside in protected tissue locales (niches), where their reproductive potency remains essentially unchanged through life. Disruption of the tissue leads to a reduced capacity of dispersed epithelial cells to recapitulate complete functional mammary structures. Previous studies demonstrate that during the reformation of mammary stem cell niches by dispersed epithelial cells in the mammary stroma, nonmammary cells of ectodermal germ origin may be sequestered and reprogrammed to perform mammary epithelial cell (MEC) functions, including those ascribed to mammary stem/progenitor cells. To test whether tissue cells from organs derived from different germ layers could respond to mammary epithelial-specific signals, we utilized fluorescence-activated cell sorting-purified Lin− and Lin−/cKit+adult male bone marrow cells to mix with MECs. Our evidence shows that the signals provided by the mammary microenvironment are capable of redirecting mesoderm-derived adult progenitor cells to produce functional MEC progeny.
Introduction
Development of the WAP-Cre/Rosa26 flox-Stop-flox-LacZ (R26R) model provided evidence for a LacZ-marked lobule-limited progenitor cells that were detected in parous mouse mammary epithelium from these mice. These β-gal-positive, parity-identified mammary epithelial cells (PI-MECs) were found to be multipotent, self-renewing, and capable of maintaining their multi-potent lobule-limited progenitor activities after serial transplantation in epithelium-free mammary fat pads [1]. During pregnancy in these hosts, the PI-MECs proliferated and gave rise to LacZ+luminal progeny that were progesterone receptor (PR) or estrogen receptor alpha (ERα)-positive and luminal progeny that were bereft of these steroid receptors. Further, in the developing secretory acini, they contributed not only secretory progeny but also LacZ-+myoepithelial cells. Originally, it was proposed that the LacZ+PI-MECs arose from de-differentiated secretory epithelial cells that had survived involution and remodeling of the mammary tissue; however, further study indicated that these cells were present in the mammary tissue of nulliparous females and that they could be detected in explant cultures after treatment of the fragments with growth factors that did not induce lactogenic differentiation [2]. These cells were shown to possess all the properties of PI-MECs, including self-renewal and multipotency. This transgenic model is a useful tool to follow progenitor cell fates in developing mammary glands.
The dominance of the mammary niche over a stem cell's autonomous phenotype has been demonstrated in several reports involving cells crossing lineage boundaries to regenerate foreign tissues. Using the R26R model, we set out to determine if cells from organs other than the mammary gland in R26R mice would be re-directed toward a multipotent MEC fate when interacted with wild-type MECs during mammary gland regeneration. We have previously demonstrated that cells isolated from the seminiferous tubules of the mature testis and adult and fetal neural stem cells, when mixed together with normal MECs, would cooperate with these cells and contribute robust numbers of epithelial progeny to normally growing mammary glands in the context of the stroma within transplanted mammary fat pads [3,4]. The cells from these previous experiments are from organs that are primarily of ectodermal germ origin, the same germ layer that MECs derive from. That led to the question of whether or not cells from other germ layers have the ability to be reprogrammed and function as MECs. Here we examine the capacity of the niche to reprogram cells from other tissues, most specifically from cells generated from tissues of mesodermal origin.
Materials and Methods
Mice
The transgenic WAP-Cre/Rosa26R (R26R) mice were engineered and typed according to Wagner et al. [5]. Female Nu/Nu mice were used as hosts for transplantation studies. All mice were housed in Association and Accreditation of Laboratory Animal Care–accredited facilities in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The National Cancer Institute Animal Care and Use Committee approved all experimental procedures.
MEC preparation
MECs were collected from primary mammary cultures after 4–7 days on plastic culture flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), insulin (1.0 μg/mL), and epidermal growth factor (10 ng/mL) (Complete Media). Fibroblasts were reduced before collection of the epithelial cells by differential trypsinization [6].
Bone marrow extraction and hematopoietic progenitor cell enrichment
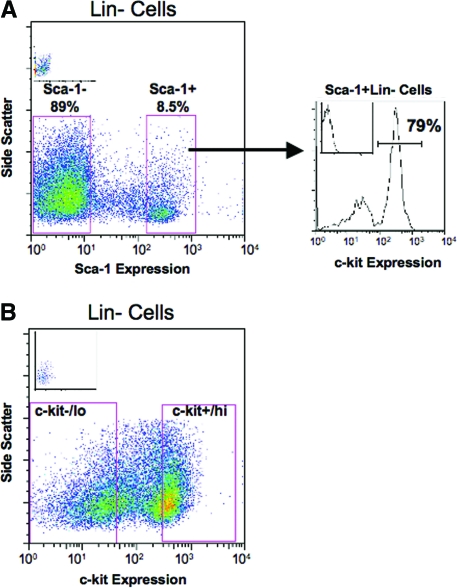
Bone marrow cells (BMCs) were flushed from the femurs, tibias, and iliac crests of male R26R transgenic mice using DMEM containing 2% FBS (Invitrogen, Carlsbad, CA). Red blood cells were lysed with Ammonium Chloride buffer and Lin− cells were purified by negative selection using EasySep™ (Stem Cell Technologies, Vancouver, BC, Canada). Hematopoietic progenitor cell (HPC)-enriched Sca-1+Lin− or c-kit+/hi Lin− cells were purified by fluorescence-activated cell sorting (FACS) using anti-murine Sca-1 (clone D7) or c-kit (clone 2B8) conjugated to PE and PeCy5.5, respectively (eBioscience, San Diego, CA) (Fig. 1).
FIG. 1.
Purification of Lin− bone marrow populations that are enriched or depleted for hematopoietic stem and progenitor cells. BMCs lacking the expression of markers for mature lymphoid, myeloid, and erythroid cells (Lin−) were isolated from R26R mice and stained with monoclonal antibodies specific to allow the purification of (A) Sca-1 or (B) c-kit expressing and nonexpressing cells by fluorescence-activated cell sorting. All cell populations were purified using the gates determined based on unstained controls (insets). (A) Sca-1+Lin− cells were subsequently analyzed for c-kit expression. (B) Lin− cells were separated based on either undetectable to low (c-kit-/lo) or high (c-kit+/hi) levels of c-kit expression. BMCs, bone marrow cells. Color images available online at www.liebertonline.com/scd
Cell and tissue transplantation
The surgical techniques used to clear the mammary epithelium from the fat pads of 3-week-old host mice and the subsequent transplantation of tissue fragments or cell suspensions have been described in detail previously [6–8]. In brief, the mice were anesthetized, and the clearing procedure was performed immediately before the insertion of transplanted fragments or cell suspensions. Cell suspensions were implanted in 10-μL volumes with a Hamilton syringe equipped with a 30-gauge needle.
The implanted females were placed with males 4–6 weeks after implantation to initiate pregnancy and secretory development. In those cases where pregnancy was not achieved the outgrowths were minced (1–2.0 mm3) and placed as explant fragments into DMEM without serum but with insulin (I-1.0 μg/mL), hydrocortisone (H-1.0 μg/mL), and prolactin (Prl-1.0 μg/mL) added [2]. The explants were incubated for 72 h in this milieu and then were fixed in 4% paraformaldehyde, permeabilized with detergent as were glandular whole mounts, and reacted with X-gal to detect the expression of beta-galactosidase. Histological sections confirmed the presence of lacZ+MECs in the ducts and random fragments were frozen viably without fixation and subsequently implanted into the epithelium-divested mammary fat pads of 3-week-old Nu/Nu females. These secondary outgrowths were allowed to develop for 8–10 weeks and were subsequently reacted in X-gal mixture after fixation and permeabilization as described earlier in Methods. LacZ+epithelial cells were also found in these secondary outgrowths produced from IHPrl-treated primary outgrowth fragments.
X-gal and immunohistochemical staining of mammary tissues
Whole mounts of the entire inguinal gland were fixed and stained as described previously [3]. In brief, the glands were spread on glass slides, fixed in paraformaldehyde (4.0%) for 1–2 h, permeabilized in 0.01% Nonidet P-40 and 0.01% sodium deoxycholate in phosphate-buffered saline overnight at 4°C, and then processed for X-gal staining as described earlier. For histological examinations, X-gal-stained whole mounts were embedded in paraffin, sectioned at 6.0 μm, and counterstained with Nuclear Fast Red. Immunohistochemistry was performed on de-paraffinized sections. Primary antibodies used were rabbit anti-ERα MC-20 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-PR (1:75; Dako, Carpinteria, CA), mouse anti-Smooth Muscle Actin 1A4 (1:100; Zymed/Invitrogen), rabbit anti-pan-keratin (1:100; Dako), and rabbit anti-casein (1:1,000) [9]. Heating sections in autoclave performed antigen retrieval at 121°C for 5 min in pH 8.0 EDTA. Immunohistochemical staining procedure was carried out using the TRU Vectastain Kit (Vector Laboratories, Burlingame, CA) per the manufacturer's protocol. For RANKL staining, biotinylated rabbit anti-Goat secondary antibody (Vector Laboratories) was used. 3,3′-Diamino-benzidine peroxidase substrate kit (Vector Laboratories) was used for staining according to manufacturer's protocol. All sections were counter-stained with nuclear fast red (Sigma-Aldrich, St. Louis, MO).
FISH analysis on paraffin sections
Slides containing multiple 5–6-μm paraffin sections were deparaffinized 3×10 min in xylenes. The tissue was then rehydrated in an ethanol series (100%, 90%, and 70%) 3×3 min each, followed by 2×3 min in 2× SSC with shaking. Slides were pepsin treated (catalog No. P6887; Sigma–Aldrich) in 0.01 M HCl at 37°C for 30 min and then washed in PBS 3×3 min. Fixation and dehydration were done simultaneously by reversing the ethanol series (70%, 90%, 100%) for 10 min each. Using an X chromosome telomere probe and a Y chromosome locus-specific BAC probe (bJKB4) [10] that were labeled by nick translation by using Spectrum Orange-dUTP (Vysis, Des Plaines, IL) and Oregon Green-dUTP (Invitrogen), respectively, analysis was performed according to a previously published protocol [11].
DNA extraction and PCR
Genomic DNA was extracted from mammary tissue according to Qiagen DNeasy kit (catalog No. 69506; Valencia, CA) protocol. PCR analysis was performed with Y6 primers from Peters et al. [12].
Results
Formation of BMC/MEC chimeric outgrowths upon transplantation
Bone marrow was isolated from the tibias of bitransgenic R26R mice as described above, and lineage-negative (Lin−) cells were further isolated through a negative selection magnetic bead prep as described. Mammary cells (50,000) mixed with Lin−, Lin−/cKit+, and Lin−/cKit+/Sca+ BMCs (Fig. 1) at a 1:1 ratio were implanted into epithelium-divested mammary fat pads to develop chimeric mammary outgrowths comprised of cells from both contributors. The cells were combined and immediately inoculated (10 μL) directly into epithelium-divested mammary fat pads of 3-week-old Nu/Nu females. Mammary ductal growth proceeded for 6–8 weeks after injection, whereupon hosts were allowed to complete a full-term pregnancy (required for activation of the Rosa26 LacZ reporter gene via WAP-Cre expression) [13]. Pups were removed after birth. Complete glandular involution was allowed. Subsequently, the mammary outgrowths were removed and stained as whole mounts for LacZ activity by X-Gal. Only mesodermal cells contained both the WAP-Cre and Rosa26 reporter transgenes. Therefore, only BMC-derived cells that survived tissue remodeling after lactation and possessed both the WAP-Cre and Rosa26 reporter transgenes during pregnancy will be LacZ+. After Cre-induced recombination, LacZ expression is constitutive and subsequently acts as a lineal marker, which can be used to trace the subsequent fate of the activated, surviving LacZ+cells and their progeny. The presence of LacZ+(blue) cells signals the occurrence of mesodermal cell progeny within the mammary outgrowth. An important feature of the experimental design is the regulation of Cre expression from the WAP promoter to mammary epithelium during secretory differentiation. This conclusion is borne out by the absence of LacZ+cells in the mammary outgrowths removed from control nulliparous hosts. When mixed with normal MECs, total Lin−, Lin−/ckit+, and Lin−/ckit+/sca1+ cellular fractions all contributed to the resulting outgrowths in 6/11, 6/8, and 5/7 positive takes, respectively, after lactation and involution (Table 1 and Fig. 2). No LacZ+cells appeared in fat pads and mammary outgrowths produced by injecting wild-type mammary epithelium alone, and no outgrowths resulted in fat pads inoculated with mesodermal-derived cells alone (Table 1).
Table 1.
Numbers of Positive Mammary Outgrowths (Takes) and Presence of Redirected Cells (Blue)
| |
TGEN 1 |
TGEN 2 |
||
|---|---|---|---|---|
| Total takes | LacZ+ | Total takes | LacZ+ | |
| Lin− alone | 0/6 | N/A | N/A | N/A |
| Lin−+MECs | 11/20 | 6/11 | 8/10 | 7/8 |
| Lin−/ckit++MECs | 8/10 | 6/8 | 6/6 | 6/6 |
| Lin−/ckit+/Sca++ MECs | 7/10 | 5/7 | ND | ND |
MECs, mammary epithelial cells; N/A, not applicable; ND, not determined.
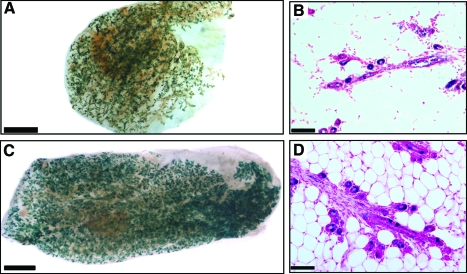
FIG. 2.
Lin− BMCs contribute to mammary gland regeneration when mixed with normal mouse MECs. (A) X-gal stained whole mounts of parous, nonpregnant chimeric mammary outgrowths resulting from inoculation of Lin− BMCs isolated from R26R mice and wild-type MECs (1:1 ratio). (B) Cross section of the same gland shown in (A). (C) X-gal stained whole mount of second-generation outgrowth resulting from transplantation of a fragment taken from Lin− BMC/MECs first-generation chimeric outgrowth. (D) Cross section of the same gland shown in (C). Scale bars: (A) 2 mm; (C) 1.5 mm; (B, D) 150 μm. MECs, mammary epithelial cells. Color images available online at www.liebertonline.com/scd
Appearance of LacZ+cells in second-generation transplants of chimeric BMC/MEC tissue
Our previous studies of WAP-Cre activated, LacZ+PI-MECs in intact primiparous, involuted chimeric mammary outgrowths indicated that they were capable of self-renewal upon transplantation. In addition, the LacZ-positive cells gave rise to epithelial cell progeny of both luminal and myoepithelial cell lineages. Second-generation transplantation of fragments from the BMC/MEC chimeric outgrowths confirmed that the LacZ+, mesoderm-derived epithelial cells were also capable of self-renewal and proliferation (Table 1). These activities are analogous to those displayed by the PI-MECs in intact R26R mouse mammary glands. The results clearly demonstrate that cells from bone marrow of adult mice interact with MECs upon inoculation into the epithelium divested mammary fat pad and proliferate to contribute cells analogous to PI-MECs in the resulting epithelial outgrowths.
Demonstration of transgene and Y chromosome-specific sequences in chimeric glands
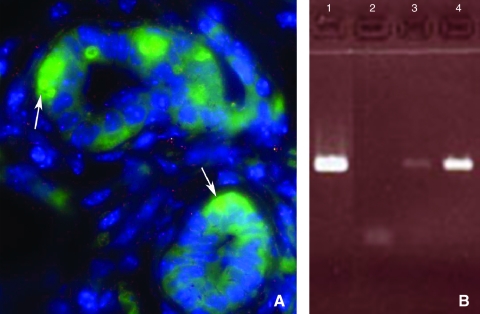
PCR analysis of the DNA isolated from the chimeric outgrowths demonstrated the presence of both transgenes and sequences specific to the Y chromosome, verifying the presence of mesodermal-derived male cell DNA. Similar results were obtained when secondary outgrowths from BMC/MEC outgrowths were sectioned and analyzed by in situ fluorescent hybridization for male Y-chromosome-associated DNA (Fig. 3).
FIG. 3.
Presence of Y chromosome in chimeric outgrowths. (A) Y chromosome FISH analysis performed on paraffin-embedded, 6 μm sections of mammary glands containing chimeric outgrowths. A mammary secretory structure is shown with the Y chromosome labeled in green (arrows), and the nuclei stained with DAPI (blue). Serial 1.0 μm slices photographed under 3-color confocal microscopy reveal the presence of male BM cell progeny juxtaposed with mammary cells in the same acinus. (B) DNA PCR of Y6 male primers. Lane 1 is male DNA control, lane 2 is negative female control, lane 3 is first-generation Lin− chimeric outgrowth, and lane 4 is DNA from a secondary chimeric outgrowth. Presence of male DNA is a result BM cell contribution. Color images available online at www.liebertonline.com/scd
Reprogrammed BMC function as multipotent PI-MECs
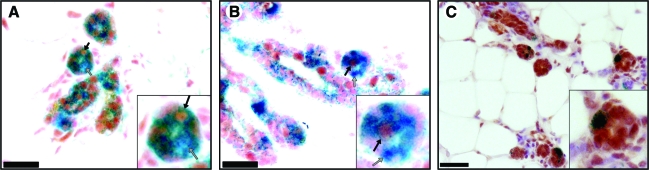
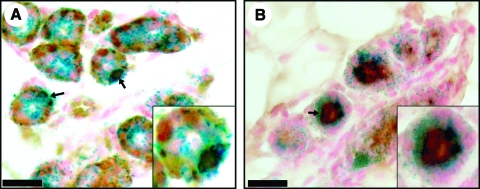
We have previously demonstrated that PI-MECs are multipotent, giving rise to ER α+and PR+, as well as epithelial cells lacking both hormonal receptors. Further, during pregnancy, these cells expand to produce both SMA+myoepithelial cells and milk-protein producing luminal cells in developing secretory lobules. To determine if reprogrammed BMC function as bona-fide PI-MECs, we stained parous nonpregnant chimeric glands for PR and ERα (Fig. 4). BMC-derived LacZ+cells were found to be both ERα+and ERα−, as well as PR+and PR−. BMC-derived LacZ+cells also expressed cytokeratins (Fig. 4C), confirming that they had adopted an epithelial cell fate. Further, during early pregnancy (4 days before de novo activation of Wap-Cre), BMC-derived LacZ+cells expanded to produce SMA+myoepithelial cells in the developing acini (Fig. 5A). In addition, LacZ+luminal cells in developing secretory lobules were found to express and secrete caseins (Fig. 5B). These results demonstrate that reprogrammed BMC-derived cells adapt a fully functional PI-MEC cellular fate.
FIG. 4.
Reprogrammed BMCs are multipotent. (A) LacZ+PI-MECs derived from Lin− BMCs (blue) produce progeny that are ERα+(black arrows) and ERα−(gray arrows). (B) LacZ+PI-MECs derived from Lin− BMCs (blue) produce progeny that are PR+(black arrows) and PR−(gray arrows). (C) Pan-keratin staining of chimeric outgrowths confirms that LacZ+PI-MECs derived from Lin− BMCs (blue) have adopted an epithelial cell fate. Insets are higher magnification of the regions of interest. Scale bars: (A, B) 50 μm; (C) 150 μm. ER, estrogen receptor; PI-MECs, parity-identified mammary epithelial cells; PR, progesterone receptor. Color images available online at www.liebertonline.com/scd
FIG. 5.
Reprogrammed BMCs give rise to SMA+myoepithelial cells and casein producing luminal cells during early pregnancy. (A) Cross section of a mammary gland taken from a 4-day pregnant mouse reveals β-Gal+. PI-MECs derived from Lin− BMCs (blue) produce SMA+myoepithelial progeny (arrows) in developing secretory acini. (B) Cross section of the same gland stained for casein. Insets represent higher magnification of the regions of interest. Scale bars: 50 μm. Color images available online at www.liebertonline.com/scd
Discussion
Bone marrow contains a small population of stem and progenitor cells that are primarily responsible for regenerating all of the different cell types that comprise the hematopoietic system [14]. In the mouse, hematopoietic stem cells and HPCs are known to be highly enriched in a population of BMCs that lack surface expression of proteins associated with erythroid, myeloid, and lymphoid cells (lineage negative; Lin−) but express the tyrosine kinase receptor, c-kit (CD117), and the Sca-1 (Ly6A/E) glycoprotein [15]. Interestingly, BMCs expressing this phenotype have also been shown to participate in the regeneration of many different cell types outside of the blood system, including skeletal muscle [16], cardiac muscle [17], and liver [18]. In some tissues, such as liver and cardiac muscle, HPCs contribute to regeneration by fusing with existing cells or secreting factors that aid in the repair process. However, in tissues such as the skeletal muscle, there has been strong evidence supporting the ability of HPCs to differentiate into nonhematopoietic cell types (ie, transdifferentiation). Collectively, these studies provide evidence that the tissue microenvironment may play an important role in governing HPC transdifferentiation.
In 2002, we reported [13] the discovery of an adjunct mammary epithelial population marked by activation of the Rosa26-LacZ reporter gene after Cre-Lox recombination as the result of WAP-Cre expression during pregnancy and lactation. This population has been named PI-MECs. These cells survive postlactation involution and tissue re-modeling of the mammary epithelium and are found primarily associated with ductal side branches in the involuted parous mammary tissue. Upon subsequent pregnancies, PI-MECs proliferate and produce epithelial progeny to form secretory acini during early pregnancy. Further study by Boulanger et al. [1] demonstrated that PI-MECs are long-lived and capable of proliferation through 4 serial transplant generations. During their expansion, PI-MECs give rise to luminal MECs (both ERα- and PR-positive and -negative cells) during ductal morphogenesis and are found within the body of active terminal end buds as well as along the subtending ducts. They did not give rise to the specialized cap cells found at the growing ends of the termini and therefore duct-associated myoepithelial cells (which arise from cap cells) were not LacZ+. Upon impregnation of the transplant-bearing host, the PI-MECs proliferated and produced both luminal and myoepithelial progeny to the developing secretory acini [1]. This property was maintained by the PI-MECs throughout 4 transplant generations. Subsequently, PI-MECs were found to exist before pregnancy in the mammary epithelium [2] and were found to exhibit the same properties as those PI-MECs originally detected in postparous glands.
Consistent with the conclusion that they represent lobule-limited progenitor cells, PI-MECs are not capable of producing a fully developed and functional mammary outgrowths without interacting with epithelial cells whose reporter had not been activated [1] although they were able to form complete lobular-only structures in limiting dilution transplant experiments. In addition, the presence of PI-MECs among implanted cells appeared to be essential for positive mammary epithelial growth [1,19]. Because PI-MECs are identified after pregnancy and survive involution, this model afforded us the possibility to determine whether cells from nonmammary tissue would interact with wild-type MECs in the context of the mammary fat pad, proliferate, differentiate to the extent that their WAP-Cre gene was activated during pregnancy, and survive involution. Then, as a result, their progeny after mammary growth and functional differentiation could be detected by activation of the Rosa26-LacZ reporter. Using this experimental model, we have demonstrated that spermatogenic cells and neural stem cells from embryonic and adult brains are redirected to mammary progenitor epithelial cell fates in vivo when interacting with wild-type MECs in vivo [3,20]. In these studies and in subsequent ones [21,22], cell fusion between the nonmammary and mammary cells has been ruled out by several criteria. Therefore, we are confident that cell–cell fusion is not responsible for the result we have described here for BM-derived cells.
Both neuronal stem cells and spermatogenic cells are, like MECs, derived from the ectodermal germ layer. In the present study, we have carried out experiments using BMCs that are derived from the mesoderm freshly isolated from mature R26R male mice. Our results show that they are capable of interacting with normal MECs and cooperate copiously in the generation of a fully differentiated functional mammary gland. Previous studies have indicated that hematopoietic stem cells circulating in the blood of parabiotic mice do not contribute progeny to brain, heart, kidney, gut, or muscle [23]. However, in our experiments, we are introducing the BMCs directly into the mammary fat pad in combination with MECs where subsequently organogenesis of a complete and functional mammary epithelial gland is completed. Therefore, our experiments do not contradict those reported by Wagers et al. [23] and are not directly comparable to the results reported by these authors. Lin− BMCs alone without co-operation from coinjected MECs failed to produce mammary epithelium. This indicates that signals, both paracrine and juxtacrine, from bona fide MECs are indispensable to their active participation in chimera formation. Once incorporated into mammary epithelial structures, BMC progeny behave similarly to PI-MECs, producing both luminal and myoepithelial progeny and actively proliferating in secondary transplants. LacZ+ BMC luminal progeny synthesize milk protein in pregnant hosts and along ducts, some express PR and ERα.
The redirected BMCs proliferated and contributed extensively to primary and secondary mammary outgrowth and produced both luminal epithelial progeny capable of synthesizing milk protein and myoepithelial cells in secretory acini. BMC progeny also included ERα- and PR-positive epithelial offspring among the chimeric epithelium. MECs with these properties have been shown to be indispensable to complete duct and secretory alveolar development in murine mammary gland [24,25]. In the chimeric mammary tissue BMC-derived mammary progeny, conditionally marked by LacZ expression due to WAP-Cre-initiated recombination, behaved in the manner exactly like the PI-MECs described earlier in intact WAP-Cre/Rosa26R female mammary glands after parity. In these experiments, we are not able to ascertain whether NSC-derived mammary epithelial progeny contributed to other aspects of mammary development because WAP-Cre activation of the reporter does not mark all mammary epithelium. Despite this, the widespread contribution of BMC-derived progeny to the mammary epithelial population before pregnancy (as determined by the uniform distribution of LacZ+ progeny along the mammary ducts in fully involuted primiparous females) suggests that BMC-derived progeny contribute robustly to the total mammary epithelial content in the chimeric outgrowths. In addition, the demonstration that a specific tissue locale comprised of stromal and epithelial factors can dictate the repertoire of bona fide Lin− BMCs to its own purpose reinforces the concept of the inductive power of the tissue microenvironment in redirecting the intrinsic nature of a tissue-specific stem cell.
We are presently engaged in studies designed to elucidate the essential characteristics of MECs comprising the mammary stem cell niche and the indispensable signals required from these cells that enables acquisition of nonmammary cells and redirection of their cell fate(s).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Boulanger CA. Wagner KU. Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 2.Booth BW. Boulanger CA. Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–736. doi: 10.1002/jcp.21071. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger CA, et al. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth BW, et al. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner KU, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 7.Kordon EC. Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 8.Smith GH, et al. Long-term in vivo expression of genes introduced by retrovirus-mediated transfer into mammary epithelial cells. J Virol. 1991;65:6365–6370. doi: 10.1128/jvi.65.11.6365-6370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GH, et al. Expression of pregnancy-specific genes in preneoplastic mouse mammary tissues from virgin mice. Cancer Res. 1984;44:3426–3437. [PubMed] [Google Scholar]
- 10.Simpson EM, et al. Novel Sxr(a) ES cell line offers hope for Y chromosome gene-targeted mice. Genesis. 2002;33:62–66. doi: 10.1002/gene.10093. [DOI] [PubMed] [Google Scholar]
- 11.Van Prooijen-Knegt AC. Van der Ploeg M. Localization of specific DNA sequences in cell nuclei and human metaphase chromosomes by fluorescence microscopy. Cell Biol Int Rep. 1982;6:653. doi: 10.1016/0309-1651(82)90128-x. [DOI] [PubMed] [Google Scholar]
- 12.Peters SO, et al. Quantitative polymerase chain reaction-based assay with fluorogenic Y-chromosome specific probes to measure bone marrow chimerism in mice. J Immunol Methods. 2002;260:109–116. doi: 10.1016/s0022-1759(01)00525-7. [DOI] [PubMed] [Google Scholar]
- 13.Wagner KU, et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 15.Morrison SJ, et al. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 16.LaBarge MA. Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 17.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 18.Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 19.Matulka LA. Triplett AA. Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Booth BW. Boulanger CA. Smith GH. Stem cells and the mammary microenvironment. Breast Dis. 2008;29:57–67. doi: 10.3233/bd-2008-29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussard KM, et al. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–6343. doi: 10.1158/0008-5472.CAN-10-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth BW, et al. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene. 2011;30:679–689. doi: 10.1038/onc.2010.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagers AJ, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;237:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 24.Chu EY, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- 25.Mallepell S, et al. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]