Abstract
Induced pluripotent stem (iPS) cells are being used increasingly to complement their embryonic counterparts to understand and develop the therapeutic potential of pluripotent cells. Our objectives were to identify an efficient cardiac differentiation protocol for human iPS cells as monolayers, and demonstrate that the resulting cardiac progenitors could provide a therapeutic benefit in a rodent model of myocardial infarction. Herein, we describe a 14-day protocol for efficient cardiac differentiation of human iPS cells as a monolayer, which routinely yielded a mixed population in which over 50% were cardiomyocytes, endothelium, or smooth muscle cells. When differentiating, cardiac progenitors from day 6 of this protocol were injected into the peri-infarct region of the rat heart; after coronary artery ligation and reperfusion, we were able to show that human iPS cell-derived cardiac progenitor cells engrafted, differentiated into cardiomyocytes and smooth muscle, and persisted for at least 10 weeks postinfarct. Hearts injected with iPS-derived cells showed a nonsignificant trend toward protection from decline in function after myocardial infarction, as assessed by magnetic resonance imaging at 10 weeks, such that the ejection fraction at 10 weeks in iPS treated hearts was 62%±4%, compared to that of control infarcted hearts at 45%±9% (P<0.2). In conclusion, we demonstrated efficient cardiac differentiation of human iPS cells that gave rise to progenitors that were retained within the infarcted rat heart, and reduced remodeling of the heart after ischemic damage.
Introduction
Mortality rates are high for ischemic heart disease and its consequences [1]. As the adult heart is unable to rapidly regenerate the large number of cardiomyocytes lost immediately after infarction, patients are left with an area of irreversibly damaged myocardium. Stem cells and their products have the potential to provide or stimulate the formation of a new blood supply and new viable cardiomyocytes within the damaged heart, thereby reducing the hemodynamic stress and left ventricular remodeling pivotal in the transition to failure.
Cellular therapies using human postnatal blood and bone marrow have been tested in clinical trials for cardiac regeneration and, although randomized clinical trials show some evidence of improvement in left ventricular ejection fraction (EF) over the short term, there is still no conclusive evidence of cardiomyocyte replacement with these cells [2–5]. Recently identified cardiac stem cells (CSCs) offer a promising cell population for autologous cell therapy or for developing drug therapeutics for endogenous repair [6,7]. However, human cardiac biopsy samples are required and this lack of readily available tissue limits studies on autologous CSCs in patients susceptible to myocardial infarction. Human embryonic stem (ES) cells remain one potential source of cells for regenerating infarcted tissue with functioning cardiomyocytes and their ability to contribute to cardiac repair after myocardial infarction has been described in detail [8–12]. However, their derivation from live embryos is considered ethically contentious and problems related to donor-recipient mismatch should not be underestimated [13].
In groundbreaking studies, Yamanaka and coworkers have identified the genetic factors that can reprogram somatic cells to an embryonic-like state [14]. These cells, termed induced pluripotent stem (iPS) cells, have been generated in vitro from adult mouse [14] and subsequently human tissue sources [15–17]. Being derived from host somatic cells, they also represent a source of autologous cells for therapy and research. Other technical advances, such as the use of nonintegrating viral vectors [18,19] or small molecules [20–22] have been reported, but the therapeutic potential of such human iPS lines for cardiac repair has yet to be confirmed.
Currently, only limited in vivo studies describe the potential of human iPS cells for cardiac repair [23], and report the effects of directly injecting undifferentiated iPS cells into infarcted hearts of immunosuppressed and immunocompetent mice. Treatment of immunocompetent hearts resulted in successful cell engraftment and an improvement in cardiac function. However, undifferentiated iPS cells administered to immunosuppressed hearts formed tumors over 2–4 weeks. Here we describe, for the first time, protocols for efficient cardiac differentiation of human iPS cells that, when injected into the infarcted rat heart, result in long term retention of enhanced green fluorescent protein (eGFP)-positive cells that stain positive for markers representative of cardiomyocytes and smooth muscle, and that contribute to reduced remodeling of the rat heart post-acute myocardial infarction (AMI), when measured at 10 weeks.
Materials and Methods
Derivation and culture of human iPS cells
iPS cells were generated essentially as described by Takahashi et al. [15] and by this laboratory elsewhere [24]. Briefly, we reprogrammed human dermal fibroblasts (Lonza), using retrovirus expressing Oct4, Sox2, and Klf4. Retrovirus was generated from pMX-based plasmids (Addgene) and PLATGP packaging cells (Cell Biolabs). Transduced human dermal normal fibroblasts (HDNF) were co-cultured with Mitomycin C–treated mouse embryonic fibroblasts in Dulbecco's modified Eagle's medium (DMEM)/F12, 20% (v/v) Knock Out Serum Replacement, 10 ng/mL basic fibroblast growth factor (bFGF), 2 mM sodium pyruvate, 1× nonessential amino acids (Invitrogen), and 1 μM beta-mercaptoethanol (Sigma-Aldrich). Cultures were maintained in 5% CO2 and 5% oxygen with half medium changes every 2 days, for up to 34 days. iPS colonies were picked and expanded before adaptation to feeder-independent culture as described by Ludwig et al. [25], while cultured routinely in 5% oxygen. iPS colonies were harvested by day 5–6, when compacted with 2 h pre-incubation in Y27632 Rho associated coil kinase inhibitor (10 μM final; Sigma Aldrich) as previously described [26]. iPS cells were washed in DMEM/F12 basal media (Invitrogen) and incubated in 1×dispase (Invitrogen) for 20 min, after which cells were harvested, dispersed mechanically into clumps of 10–20 cells, and dispersed at ratios of 1:12 onto Matrigel (ES qualified Matrigel from BD Biosciences)-coated plates with 2 mL fresh mTeSR (Stem Cell Technologies) supplemented with Y27632. Further feeds involved half medium changes every 48 h, without the need to further supplement with ROCK inhibitor. iPS cells used in this study, referred to as the C18 line, have been fully characterized for ES-like morphology, transgene silencing, ES-specific gene expression, and pluripotency in vitro with the differentiation toward neurons, hepatocytes and muscle, and in vivo as teratomas [24].
Optimized protocol for cardiac differentiation of iPS cells
We have assessed various protocols that have been described previously [10,27] with our own differentiation strategies. Only our own protocol yielded sheets of contractile tissue, and this is described herein. iPS cells were differentiated as a monolayer, in Stem Pro-34 medium was supplemented with 2mM L-glutamine, 1% (v/v) PenStrep (PAA), 400uM monothioglycerol (Sigma Aldrich) and 50 ug/mL ascorbic acid (Sigma Aldrich). Stem Pro-34 basal media was further supplemented with 50ng/ml Activin A for 4h; 10ng/mL BMP4 (R&D Systems), 5ng/mL bFGF and 5ng/mL Activin A (Invitrogen) for 44h. A full media change with fresh cytokines was made on day 2 for a further 48h. Cells were then incubated in Stem Pro 34 basal media alone, with no added cytokines, from days 4–14. Cell masses started beating after 10 days.
Characterizing cardiovascular progenitor cells
To assess whether the iPS cells contributed to the cardiac lineage and were capable of adult cardiogenesis, we cultured the C18 iPS cell line in the optimized protocol to first identify the mesoderm and cardiac progenitor phenotype. As described by Yang et al. for hES cells [27], cKit–KDRlow (Flk 1) cardiac progenitors can be isolated and developed into cardiomyocytes. Additionally, platelet derived growth factor alpha receptor (PDGFαR) is expressed during cardiovascular development [28], and thus may be considered an additional marker for the cardiac progenitor. Initially, cells were collected after dispersal in accutase (PAA) for ∼10 min during day 4–8 differentiation, pelleted at 300 g for 10 min, re-suspended in 90 μL phosphate-buffered saline (PBS) with 0.5% (w/v) bovine serum albumin (BSA), and mixed with 10 μL FcR blocking reagent (Miltenyi Biotec.) for 15 min on ice. Next, live cells were stained for KDR-PE (R&D Systems), ckit-APC (BD Biosciences), Tie2-APC (R&D Systems), and PDGFRα-PE (BD Biosciences), by applying antibodies at 1:5–1:10 dilution for 1 h at 4°C. After washing 3 times with PBS and pelleting at 1,200 rpm for 10 min, the cells were analyzed using an LSRII flow cytometer (BD Biosciences).
Demonstrating cardiac and endothelial potential of human iPS cells
Day 4–21 iPS cell differentiation cultures were fixed in 4% (w/v) formaldehyde/PBS for 15 min on ice, washed 3 times in PBS, and incubated with PBS-BSA Triton [or PBS-BT: PBS with 0.5% (w/v) BSA and 0.1% (v/v) Triton X-100] at 4°C overnight before staining with relevant antibodies. Fixed cells were stained for the cardiac phenotype using troponin I (clone EP1106Y; Abcam), troponin C (clone H-110; Santa Cruz Technologies), α actinin (clone EA-53; Sigma Aldrich), and α-smooth muscle actin (SMA) (clone SPM332; Abcam), using antibodies at 1:100–1:250 dilution for 1 h at room temperature. After washing in PBS, appropriate Alexa-conjugated secondary antibodies (Invitrogen) were applied at 1:1,000 dilution for 1 h at room temperature. Cells were also stained with antibodies CD34-FITC (clone AC136; Miltenyi Biotec.) and CD31 (clone WM59; BD Biosciences) at 1:100 for 1 h at room temperature. Cells were imaged with a Nikon TE300 inverted fluorescent microscope and images captured using Hamamatsu Photonics CCD camera and processed with SimplePCI software (Digital Pixel). To quantify percentages of different cells in this heterogeneous population, cells were analyzed by fluorescence-activated cell sorting (FACS) analysis. Following our cardiac differentiation protocol, day 14–21 iPS-derived cells were harvested using 1× accutase, pelleted at 300 g for 10 min, resuspended in 100 μL PBS, and fixed by 900 μL 4% (w/v) formaldehyde/PBS for 15 min on ice. After 3 washes with PBS, fixed cells were permeabilized using the Fixation/Permeabilization Kit (BD cytofix/Cytoperm™; BD Biosciences). The cells were stained for cardiac troponin I, troponin C, α-actinin, and α-SMA, using antibodies at 1:20–1:50 or CD34-FITC and CD31-PE at 1:10, all for 1 h on ice. After washing, appropriate Alexa-conjugated secondary antibodies were applied at 1:1,000 dilution for 1 h on ice.
Generating eGFP-positive iPS cells
C18 iPS cells were transduced with lentivirus expressing eGFP (kindly provided by Prof. Adrian Thrasher, University College London), at low moi (<1 pfu/cell). At 48 h post-transduction, eGFP-positive iPS cells were harvested and plated at low density with colonies screened by fluorescence microscopy after 7–10 days. eGFP-positive colonies were selected for further expansion, and the most highly expressing sub-clone was selected for further in vivo studies.
Rat myocardial infarction and cell administration
The left anterior descending (LAD) coronary artery of female RNU-RNU rats (180–230 g) was occluded to induce ischemia reperfusion injury. In brief, after anesthesia and thoracotamy, the pericardium was removed and a 5-0 prolene suture placed under the LAD, about 2 mm from the origin. The suture was tied around a small piece of PE tubing, occluding the LAD, and the chest closed. After 50 min, the chest was re-opened and the tubing removed to allow reperfusion. Ten minutes after reperfusion, iPS cells (2×106 in 50 μL HBSS medium; n=4) or medium alone (50 μL; n=5) were injected in the peri-infarct region. In sham animals (n=3), the thoracotamy was performed, but no stitch placed in the heart.
Cardiac cine magnetic resonance imaging
Cardiac cine magnetic resonance imaging (MRI) was performed as described [29] at 2 days and at 2, 6, and 10 weeks after infarction. Briefly, rats were anesthetized with 2.5% (v/v) isoflurane in O2, positioned supine in a purpose built cradle, and lowered into a 60 mm birdcage coil in a vertical bore 500 MHz, 11.7 T MR system with a Bruker console running Paravision 2.1.1. A stack of 7–8 contiguous 1.5-mm true short-axis ECG-gated cine images [field of view 51.2 mm2, matrix size 256×256 zero filled to 512×512 giving a voxel size of 100×100×1,500 μm, echo time/repetition time (TE/TR) 1.43/4.6 ms, 17.5° pulse, 25–35 frames per cardiac cycle] were acquired to cover the entire left ventricle. The end diastolic and end systolic volumes (ESVs) were measured for each slice using ImageJ (NIH; http://rsbinfonihgov/ij) and summed over the whole heart. Stroke volume was calculated as end diastolic volume minus ESV. The EF was calculated as the stroke volume divided by the end diastolic volume. The akinetic area of the myocardial surface was calculated as the average of the endocardial and epicardial circumferential lengths of the thinned, akinetic region of all slices, measured at diastole, multiplied by the slice thickness, and expressed as a percentage of the total myocardial surface [30].
Evaluating iPS cell engraftment at 10 weeks post-MI
Hearts were harvested 10 weeks postsurgery and fixed in 1% (w/v) formaldehyde/PBS at 4°C overnight. Fixed tissues were paraffin-embedded for histology and sectioned at a thickness of 6–7 μm, transversely across the infarct zone. Sections were deparaffinized and hydrated with deionized water. Immunofluorescence was carried out with anti-GFP (Invitrogen), anti-cardiac troponin I (clone EP1106Y; Abcam), anti-α-actinin (clone EA-53; Sigma Aldrich), and anti-α-SMA (clone SPM332; Abcam) at 1:100–1:400 dilution, and then appropriate Alexa-conjugated secondary antibodies (Invitrogen) applied at 1:1,000 dilution. Slides were then treated with 0.1% (w/v) sudan black in PBS for 10 min at room temperature with further washes in deionized water before visualization. All cells and slides were imaged with a Nikon ECLIPSE E600 microscope and images captured using a Nikon AxioCam and processed using PCISimple software as above.
Statistics
Results are presented as means±standard errors. Statistical assessment was conducted using analysis of variance at 10 weeks, with a post hoc test with Tukey correction. P values are presented for difference between infarct control group and iPS treatment group. For in vivo studies, n=5 for infarct control, n=4 for iPS-treated, and n=3 for sham-operated groups.
Results
Inducing efficient cardiac differentiation strategies from human iPS cells as monolayers
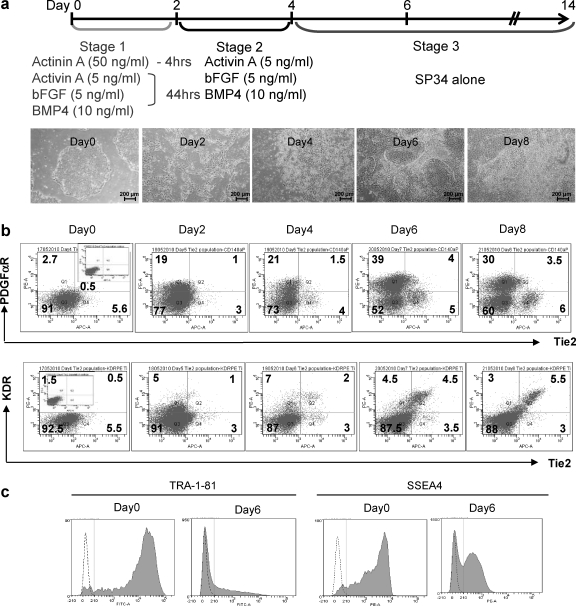
To-date, several differentiation strategies exist for the derivation of cardiovascular cells from ES and iPS cells, both for mouse and human cells [27,31]. These usually require the formation of embryoid bodies or the use of serum, although some also describe cardiac differentiation as monolayers [10,15], while others have described differentiation of specific cells types such as endothelium from pluripotent stem cells using signal inhibition [32]. To optimize cardiac differentiation as a monolayer, we considered these protocols and compared them to our own. In this novel protocol, human iPS cell monolayers were exposed to reduced activin A (50 ng/nL) in StemPro34 basal media for a short period (4 h) to prevent the extensive cell death that occurred after exposure to high doses of activin A (100 ng/mL) for extended periods of up to 24 h (data not shown). Additional modifications included continuous exposure to lower levels of activin A (5 ng/mL), bFGF (5 ng/mL), and BMP4 (10 ng/mL) for a further 92 h, followed by culture in StemPro34 for 10 days with no further supplementation. Figure 1a outlines this optimized protocol with bright-field photos of cells at each significant time point. At day 0, iPS cells were clearly evident, with large flat colonies, and cells with a large nucleus and scant cytoplasm. By 4 h incubation with Activin A in StemPro34, although there was extensive cell death, a majority of live cells remained attached (see Fig. 1a day 2) and expanded over the early stages of culture, and continued to proliferate until sheets of stromal-like cells were overlaid by cardiac progenitors. These overlying cells fused as single structures that eventually beat with other neighboring structures, often as a single sheet (see Supplementary Movie S1; Supplementary Data are available online at www.liebertonline.com/scd). When human iPS cells are differentiated as embryoid bodies using directed approaches such as those described by Yang et al., we typically see <5% of the embryoid masses beating (Lee Carpenter, unpublished data) and approaches described by Laflamme et al. [10] result in such extensive cell death that very few iPS cells survive for subsequent analysis. We therefore report this as a novel defined strategy for the efficient differentiation of human iPS cells as monolayers toward the cardiac lineage.
FIG. 1.
Optimizing directed differentiation of induced pluripotent stem (iPS) cells and assessing cardiovascular commitment. The directed differentiation protocol utilized in the study is outlined in (a) top, with bright-field images (×4) that clearly reflect the drastic loss of iPS morphology, as cells differentiate toward the cardiovascular lineage, shown below. On days 4–8, populations were assessed after harvesting, by fluorescence-activated cell sorting (FACS) analysis after staining for the expression of Tie2, c-kit, and platelet derived growth factor alpha receptor (PDGFαR) as shown in (b) (where n=3), with subsequent loss of embryonic stem cells markers TRA-1-81 and SSEA4 shown in (c).
Figure 1b shows a representative analysis of the cell surface expression of biomarkers, over days 4–8 of culture, that are associated with the cardiovascular lineage. This shows the formation of PDGFαR-positive populations at day 5, which subsequently increased in numbers over 4–8 days (Fig. 1b) and which represented up to 38.7%±8.2% of cells on day 6. This marker has previously been associated with the mesoderm [33], and more specifically the cardiac lineage [28,31,34], while it has been shown recently to be induced by factors such as Mesp1 [35], responsible for mesodermal fate specification. We also show staining for Tie2, a marker thought to identify the endothelium [36] (aswell as other cells) and which, with KDR-positive cells, represented 5.4%±2.9% of cells on day 8. This, upon co-staining for PDGFαR, appeared to be a distinct population that was Tie2+KDR+PDGFαR−. It is unclear at this time whether these cells arise from the cardiac progenitor described by Yang and colleagues [27] or from a distinct progenitor that can form independently during differentiation, such as the hemangioblast [37]. In Fig. 1c, we show consequent loss of ES-specific markers SSEA3 and TRA-1-81. Human ES and iPS cells should be dual positive for these markers (see day 0), suggesting that iPS cells do not persist at day 6 of the cardiac differentiation protocol. We also demonstrate expression of markers associated with the mesoderm by reverse transcriptase–polymerase chain reaction (RT-PCR), as iPS cells differentiated toward the cardiovascular lineage, which profiles up-regulation in expression of Goosecoid, Brachyury, Mix1, and Mesp1 (as described in Supplementary Experimental Procedures and Supplementary Fig. S1).
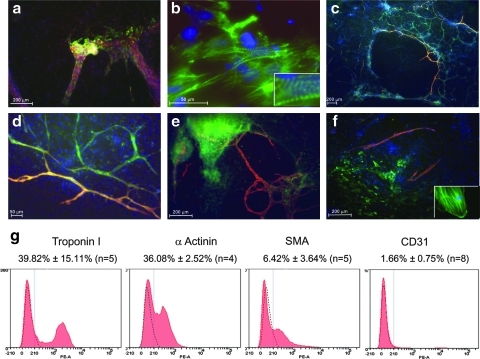
When these differentiating monolayers were assessed by immunohistochemistry (Fig. 2), they were highly positive for cardiac markers, troponin I, α-actinin, and SMA. In the case of α-actinin, staining clearly showed sarcomeric Z lines with highly distinctive striations characteristic of cardiomyocytes. Furthermore, strong co-staining was observed for CD34 and CD31, suggestive of an endothelial cell type. Although these markers are not specific to endothelium, and are expressed on hematopoietic cells and a subset of mesenchymal stem/stromal cells, our CD34+ cells were negative for CD45 and expressed low/negligible CD90. In separate studies, we have shown mesenchymal stromal cells to be very strongly positive for CD90 and negative for CD31 (unpublished data), data that support an endothelial phenotype. Interestingly, these iPS-derived endothelial cells formed tubule networks that were shown to be integrated into regions that were rich in cardiomyocytes and smooth muscle, but represented just 1.66% of the total population when assessed by FACS (see Fig. 2).
FIG. 2.
Phenotypic assessment of cardiovascular phenotypes after differentiation in vitro. After 14 days of differentiation as described previously, cultures were stained for a range of cardiovascular markers that include (a) α-actinin (green) and Troponin C (red) (×10) with (b) showing a magnified (×60) image and inset showing striations that indicate sarcomeric staining for α-actinin (in green). (c) CD34 (green) with CD31 (red) (×20) and (d) a magnified (×40) image of these double-positive networks. (e) Troponin C staining (green) with CD31 staining (red) demonstrating association of endothelial networks with cardiomyocytes (×20). (f) Smooth muscle actin (green) with CD31 (red) staining (×20) with a magnified image demonstrating smooth muscle actin (SMA) specific cytoskeletal staining in inset. (g) Quantification of these biomarkers, after harvest of cells from culture on day 14, and analysis by FACS as indicated (n=4–8 as indicated).
Upon subsequent assessment of day 14 cultures by FACS analysis, positive staining for differentiated cardiac cell markers such as troponin I, α-actinin, α-SMA, and CD34 was quantitatively assessed (Fig. 2g). About 39.8%±15.1% (n=5 independent cultures) of the population was positive for troponin I, thus representing cardiomyocytes, while cells expressing α-actinin correlated closely at 36.1%±2.5%. SMA, a marker for smooth muscle, was detected on 6.4%±3.6% of cells analyzed, and expression of the endothelial marker CD31 was 1.66%±0.75%. These data match the FACS profiling for cardiovascular markers over days 4–8, and suggest that our protocols favor the formation of cardiomyocytes and smooth muscle, with endothelium forming to a lesser extent. In Supplementary Movie S1, we also show that the high level of cardiomyocyte formation in the monolayer gave rise to extensive beating masses that resulted in sheets of contractile tissue.
We have found this to be a highly reproducible protocol that routinely produces contractile sheets with greater efficiency than previously observed by this laboratory for cardiac differentiation of iPS cells as embryoid bodies or monolayers (Lee Carpenter, unpublished data). We therefore wanted to assess the function of human iPS cell-derived cardiac differentiated cells, prepared using this protocol, in heart repair.
Functional assessment of human iPS cell-derived cardiac progenitors in a rat model of ischemia-reperfusion injury
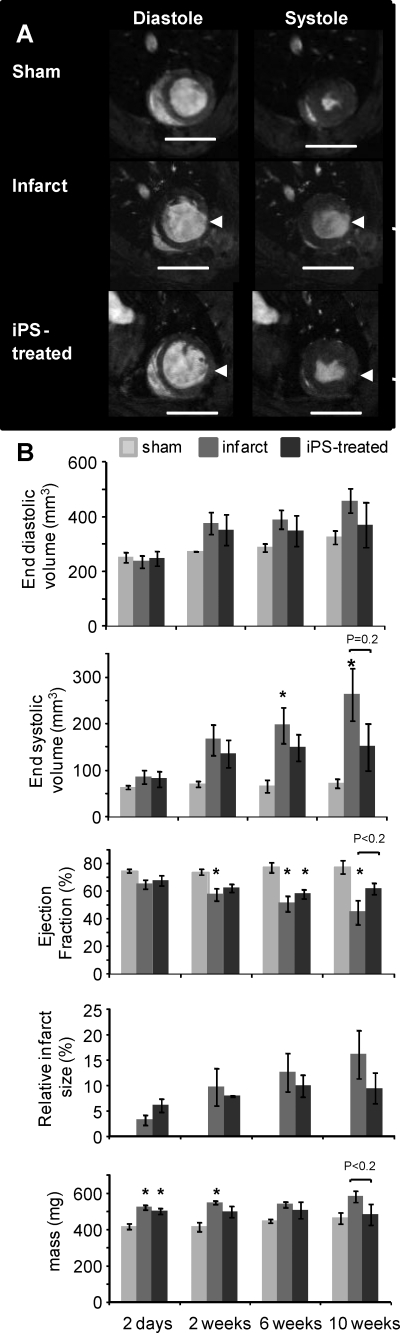
After reperfusion, 2×106 day 6 eGFP-positive, cardiac-differentiated iPS cells were administered by intra-myocardial injection. Cardiac function, measured at 2 days using in vivo cine-MRI (as shown in Fig. 3A and quantified in 3B), showed no significant difference in EF, ESV, or end diastolic volume between control-infarcted and iPS-treated rat hearts. Although there were no significant differences in cardiac morphology and function between infarcted and iPS-treated hearts at any time point, hearts injected with iPS-derived cardiac differentiated cells showed attenuated functional decline after infarction (62%±4%) compared to infarct control (45%±9%) at 10 weeks. Although the EF was not significantly different from those of the infarct control animals at 10 weeks (also see Supplementary Table S1 for full functional data), there was a nonsignificant trend (P<0.2). This was also true for end systolic volume (P=0.2) and average left ventricular mass (P<0.2), which further suggests that the protection from decline in heart function observed after myocardial infarction was conferred by introduction of iPS-derived cardiomyoctes.
FIG. 3.
Assessment of heart function for 10 weeks after myocardial infarction and administration of iPS cells. RNu rats were monitored from day 2 to week 10 after myocardial infarction using in vivo magnetic resonance imaging (MRI). (A) Representative MR images, taken at 10 weeks after myocardial infarction of sham-operated hearts (top), infarcted hearts (middle), and iPS-treated infarcted hearts (bottom) at diastole (left) and systole (right) (scale bar=10 mm; arrows indicate infarcted region). (B) Mean left ventricular end diastolic volumes, end systolic volumes (ESVs), and EFs from sham (light gray), infarcted (mid gray), and iPS-treated (black) hearts show that iPS-derived cell administration attenuated the decrease in EF at 10 weeks compared to infarct controls (P<0.2 at 10 weeks). This was also true for end systolic volume (P=0.2) and average left ventricular mass (P<0.2). Significance between infarct/iPS and sham groups is also shown where *P<0.05.
Although the protective effects from injection of the iPS-derived cardiac cells were generally modest after myocardial infarction, the functional data showed a nonsignificant trend toward an effect in the iPS group, which is not seen in other long-term functional studies [4].
Histological assessment to determine retention of eGFP-positive cells at 10 weeks post-transplant
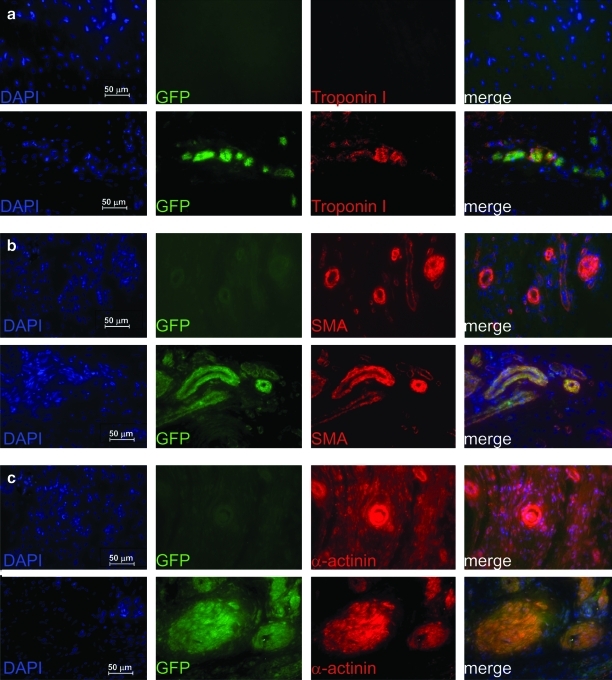
To demonstrate that protection of rat hearts from AMI resulted from the injected human iPS-derived cardiac cells, we showed that they had engrafted into the rat heart and were retained for up to 10 weeks after myocardial infarction. At this time hearts were recovered, preserved by fixation, and sectioned and stained for cardiac specific markers. Although large sheets of engrafted tissue were not evident, we did find areas around the infarct zone that retained eGFP-positive cells that were clearly of the cardiac lineage, since they were dual positive for eGFP and α-actinin, SMA, or troponin I, when assessed by immunofluorescence (Fig. 4). Some heterogeneity in signal is observed in Fig. 4a, since we experienced silencing of the eGFP transgene (∼58% of iPS cells were detectably eGFP positive, while the remaining cells were silenced; see Supplementary Fig. S2), so that, in vivo, some cells appeared positive for human-specific Troponin I only, and not GFP (hence staining red only). Conversely, not all iPS-derived cardiac differentiated cells formed cardiomyocytes and so these did not stain for Troponin I, and thus may have appeared green only. We did observe, however, a large number of cells with dual staining for both eGFP and Troponin I as expected. Similar staining patterns were observed for α-actinin (Fig. 4b) and SMA (Fig. 4c) in those hearts treated with eGFP-positive iPS cells (lower panels). eGFP-positive cells were counted to be at 22.04% (SD±3.4% n=3), while Troponin I-positive cells were counted to be 9.38% (SD±0.01%, n=3), in areas around the infarct zone.
FIG. 4.
Assessment of eGFP-iPS cell engraftment in rat heart at 10 weeks post-myocardial infarction by immunofluorescence. After 10 weeks postinfarction, rat heart tissues from iPS-injected infarcts were collected, fixed, and sectioned before demonstrating in (a) direct GFP fluorescence (green) and immunofluorescence staining for human Troponin I (red), or immunofluorescent staining for GFP (green) with (b) SMA and (c) αActinin-positive cells (in red) (all at ×40). In each case the left panel shows counter staining with 4′,6-diamidino-2-phenylindole (blue), middle left panel shows GFP expression (green), middle right shows the relevant cardiovascular stain (red), and the right panel shows a merged imaged to demonstrate co-localization. Upper panels in (a–c) are representative sections from infarct control groups, while the lower panels are from those infracted hearts injected with GFP-positive iPS cells differentiated toward the cardiac lineage. eGFP-iPS, enhanced green fluorescent protein-positive iPS cell.
Control infarct only tissue (upper panels) showed no expression of human-specific Troponin I nor eGFP; however, α-actinin and SMA expression was detected since antibodies used in these groups were not specific for the human antigen. We show that in these control panels, however, there was no eGFP expression and thus no co-localization. From these studies, we suggest that retention of a limited number of cells at 10 weeks is highly significant, since engrafted tissue is lost over this time, even in immunocompromised models. For us to observe the presence of transplanted cells at 10 weeks indicates that many more iPS-derived cardiac differentiated cells were retained and engrafted during the initial injection and recovery from infarct, resulting in the benefit in cardiac function observed with the iPS-treated group, compared to the infarct group, over subsequent weeks.
Discussion
To date, most iPS research has focused on the derivation of cardiac progenitors from mouse iPS cells [38,39] and, in general, this has been to assess differentiation capacity in vitro. While several studies have demonstrated human iPS differentiation toward the cardiac lineage [15,31], these protocols have not been extended to demonstrate long-term engraftment in animal models of AMI. Where long-term engraftment of human iPS cells has been reported in murine models of myocardial infarction [23], these studies involved the transplantation of undifferentiated human iPS cells into immunosuppressed mice resulted in formation of tumors. Here, we have been able to describe a highly efficient differentiation protocol for the production of cardiovascular cells from human iPS cells as monolayers. When cardiac differentiated cells were transplanted at day 6 of differentiation, in a rat model of myocardial infarction, we have shown them to be capable of engraftment and to persist long term, with further differentiation into cardiovascular cell types in vivo. Although the protective effects from injection of the iPS-derived cardiac cells were generally modest after myocardial infarction, there was a nonsignificant trend in decline of EF at 10 weeks (P<0.2), when compared to the control infarcts.
Optimization of the methods for cell delivery and in the type of cell populations transplanted is probably essential for improved functional benefit from such cellular approaches for repair of infarcted heart. Previously, mixed populations of differentiating human ES cultures have been used [10,11] as it is thought that contaminating cells such as fibroblasts may actually improve transplant efficiency by supporting engraftment of the cardiac progenitors [12]. It was this approach that was adopted here. Of note in this study is that even with a mixed differentiated population, no teratoma formation was observed, and there were no tumor-related mortalities. This helps to alleviate safety concerns associated with such a procedure; however, a more dedicated study over an extended period will need to be conducted before such procedures can be considered safe. Purified populations of cardiomyocytes have also been used with success to promote cardiac repair [10], and this could be considered for future studies using human iPS cells to enhance efficacy and improve safety further. Selection could be based on biomarkers such as PDGFαR as described in this study. Other considerations are to improve cell survival and engraftment once cardiac progenitors are delivered to the site of injury, as described with the administration of pro-survival factors [10,40]. Better experimental strategies are clearly necessary to demonstrate the full potential of human-derived pluripotent stem cells for cardiac repair, which include the introduction of novel methods for delivery in appropriate animal models, such as those described recently using primate ES cells, with their cardiac differentiated progeny introduced via composite sheets before implantation [41]. However, these approaches were not within the scope of the present study, which was primarily to identify efficient protocols to induce efficient human iPS cell differentiation toward the cardiac lineage and to demonstrate their engraftment and any potential functional benefit for cardiac repair in rodent models of myocardial infarction.
We were able to show, for the first time, that human iPS-derived cardiac progenitors can be derived at high efficiency using directed differentiation as monolayers. When injected as progenitors into the infarcted rat heart, they were able to engraft, differentiate into cardiomyocytes and smooth muscle, and persist long term. Furthermore, compared to control infarcted hearts, the iPS-derived cells ameliorated the reduction in EF at an extended time frame of 10 weeks. Although this effect at 10 weeks was small and nonsignificant, this does suggest a contribution from functional cardiomyocytes, as these had been shown to be contractile from day 12, with, potentially, an additional contribution from paracrine-mediated protection from remodeling over this time. Further experiments will need to consider the mechanism of action, with issues such as proliferation in vivo, functional integration, and electrophysiological parameters to be determined.
That they were produced efficiently as monolayers in a serum- and feeder-free, directed approach means we have gone some way to addressing a major limitation to the field: that is, good manufacturing practice-compliant manufacture and scale up where the availability of human clinical-grade tissue for heart repair will no longer be in short supply.
Supplementary Material
Acknowledgments
We would like to thank NHS Blood and Transplant and the Oxford Stem Cell Institute (University of Oxford) for their support. This article presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants scheme (L.C., C.T.Y., and S.M.W.). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. C.A.C., D.J.S., and K.C. were funded by grants from the British Heart Foundation.
Author Disclosure Statement
There are no conflicts of interest to declare.
References
- 1.Segers VF. Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Rendon E. Brunskill SJ. Hyde CJ. Stanworth SJ. Mathur A. Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Rendon E. Sweeney D. Lu F. Girdlestone J. Navarrete C. Watt SM. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox sanguinis. 2008;95:137–148. doi: 10.1111/j.1423-0410.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 4.Carr CA. Stuckey DJ. Tatton L. Tyler DJ. Hale SJ. Sweeney D. Schneider JE. Martin-Rendon E. Radda GK, et al. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: an in vivo cine-MRI study. Am J Physiol Heart Circ Physiol. 2008;295:H533–H542. doi: 10.1152/ajpheart.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuckey DJ. Carr CA. Martin-Rendon E. Tyler DJ. Willmott C. Cassidy PJ. Hale SJ. Schneider JE. Tatton L, et al. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells (Dayton, Ohio) 2006;24:1968–1975. doi: 10.1634/stemcells.2006-0074. [DOI] [PubMed] [Google Scholar]
- 6.Tillmanns J. Rota M. Hosoda T. Misao Y. Esposito G. Gonzalez A. Vitale S. Parolin C. Yasuzawa-Amano S, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins RW, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Laake LW. Passier R. den Ouden K. Schreurs C. Monshouwer-Kloots J. Ward-van Oostwaard D. van Echteld CJ. Doevendans PA. Mummery CL. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res. 2009;3:106–112. doi: 10.1016/j.scr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 9.van Laake LW. Passier R. Doevendans PA. Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA. Chen KY. Naumova AV. Muskheli V. Fugate JA. Dupras SK. Reinecke H. Xu C. Hassanipour M, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 11.van Laake LW. Passier R. Monshouwer-Kloots J. Verkleij AJ. Lips DJ. Freund C. den Ouden K. Ward-van Oostwaard D. Korving J, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Kolossov E. Bostani T. Roell W. Breitbach M. Pillekamp F. Nygren JM. Sasse P. Rubenchik O. Fries JW, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuasa S. Fukuda K. Recent advances in cardiovascular regenerative medicine: the induced pluripotent stem cell era. Exp Rev Cardiovasc Ther. 2008;6:803–810. doi: 10.1586/14779072.6.6.803. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Park IH. Zhao R. West JA. Yabuuchi A. Huo H. Ince TA. Lerou PH. Lensch MW. Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2007;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 17.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY) 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 18.Stadtfeld M. Nagaya M. Utikal J. Weir G. Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science (New York, NY) 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okita K. Nakagawa M. Hyenjong H. Ichisaka T. Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science (New York, NY) 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 20.Huangfu D. Osafune K. Maehr R. Guo W. Eijkelenboom A. Chen S. Muhlestein W. Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 21.Silva J. Barrandon O. Nichols J. Kawaguchi J. Theunissen TW. Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y. Zhang Q. Yin X. Yang W. Du Y. Hou P. Ge J. Liu C. Zhang W, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:1969–1982. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson TJ. Martinez-Fernandez A. Yamada S. Perez-Terzic C. Ikeda Y. Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter L. Malladi R. Yang CT. French A. Pilkington KJ. Forsey RJ. Sloane-Stanley J. Silk KM. Davies TJ, et al. Human-induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;51:4008–4011. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig TE. Bergendahl V. Levenstein ME. Yu J. Probasco MD. Thomson JA. Feeder-independent culture of human embryonic stem cells. Nature methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T. Takahashi JB. Nishikawa S. Nishikawa S. Muguruma K. Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 27.Yang L. Soonpaa MH. Adler ED. Roepke TK. Kattman SJ. Kennedy M. Henckaerts E. Bonham K. Abbott GW, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 28.French WJ. Creemers EE. Tallquist MD. Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol Cell Biol. 2008;28:5646–5657. doi: 10.1128/MCB.00441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler DJ. Lygate CA. Schneider JE. Cassidy PJ. Neubauer S. Clarke K. CINE-MR imaging of the normal and infarcted rat heart using an 11.7 T vertical bore MR system. J Cardiovasc Magn Reson. 2006;8:327–333. doi: 10.1080/10976640500451903. [DOI] [PubMed] [Google Scholar]
- 30.Nahrendorf M. Wiesmann F. Hiller KH. Hu K. Waller C. Ruff J. Lanz TE. Neubauer S. Haase A. Ertl G. Bauer WR. Serial cine-magnetic resonance imaging of left ventricular remodeling after myocardial infarction in rats. J Magn Reson Imaging. 2001;14:547–555. doi: 10.1002/jmri.1218. [DOI] [PubMed] [Google Scholar]
- 31.Kattman SJ. Witty AD. Gagliardi M. Dubois NC. Niapour M. Hotta A. Ellis J. Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Park SW. Jun Koh Y. Jeon J. Cho YH. Jang MJ. Kang Y. Kim MJ. Choi C. Sook Cho Y, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2011;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- 33.Vodyanik MA. Yu J. Zhang X. Tian S. Stewart R. Thomson JA. Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Synnergren J. Akesson K. Dahlenborg K. Vidarsson H. Ameen C. Steel D. Lindahl A. Olsson B. Sartipy P. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells (Dayton, Ohio) 2008;26:1831–1840. doi: 10.1634/stemcells.2007-1033. [DOI] [PubMed] [Google Scholar]
- 35.Lindsley RC. Gill JG. Murphy TL. Langer EM. Cai M. Mashayekhi M. Wang W. Niwa N. Nerbonne JM. Kyba M. Murphy KM. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell. 2008;3:55–68. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suri C. Jones PF. Patan S. Bartunkova S. Maisonpierre PC. Davis S. Sato TN. Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy M. D'Souza SL. Lynch-Kattman M. Schwantz S. Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narazaki G. Uosaki H. Teranishi M. Okita K. Kim B. Matsuoka S. Yamanaka S. Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 39.Mauritz C. Schwanke K. Reppel M. Neef S. Katsirntaki K. Maier LS. Nguemo F. Menke S. Haustein M, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes S. Naumova AV. Zhu WZ. Laflamme MA. Gold J. Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49:941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bel A. Planat-Bernard V. Saito A. Bonnevie L. Bellamy V. Sabbah L. Bellabas L. Brinon B. Vanneaux V, et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 122:S118–S123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.