Abstract
HIV-1 Tat-interacting protein of 110 kDa [Tip110; p110(nrb)/SART3/p110] is an RNA binding nuclear protein implicated in regulation of HIV-1 gene and host gene transcription, pre-mRNA splicing, and cancer immunology. Recently, we demonstrated a role for Tip110 in regulation of hematopoiesis. Here, we show that TIP110 is also expressed in human embryonic stem cells (hESCs) and expression was decreased with differentiation of these ESCs. TIP110 was found, through up- and down-modulation of expression of Tip110, to be important in maintaining pluripotent factor (NANOG, OCT4, and SOX2) expression in and pluripotency of hESCs, although the mechanisms involved and whether the Tip110 effects are direct remain to be determined.
Introduction
Embryonic stem cells (ESCs) are pluripotent, self-renew, and can be differentiated into cells of all 3 germ layers. Nanog, Oct4, and Sox2 form a core of the self-renewal transcription network [1,2]. Nanog expression is restricted to pluripotent cells and is downregulated upon differentiation; little is known about its regulation [3]. Oct4, a critical regulator of pluripotency, is expressed in unfertilized oocytes, the inner cell mass and epiblasts of pregastrulation embryos and primordial germ cells [4]. Expression of the OCT4 gene maintains cell pluripotency via a stringent dose-dependent regulation with OCT4 levels above or below required dosages producing cellular differentiation; thus, maintenance of a critical amount of OCT4 is necessary to prevent ESC differentiation [5–7]. Sox2, a high-mobility group domain containing transcription factor, binds to the consensus motif CATTGTT. OCT4 and Sox2 reciprocally regulate each other's transcription via the Oct4-Sox2 complex in ESCs and regulate Nanog [8–10].
We reported that Tip110 is an essential gene expressed in the earliest cells of adult bone marrow hematopoietic development. Increased TIP110 expression enhanced hematopoietic progenitor cell (HPC) numbers, survival, and cell cycling; decreased Tip110 expression manifested the opposite effect, demonstrating a role for TIP110 in regulation of hematopoiesis [11]. Herein, we demonstrate TIP110 expression in human embryonic stem cells (hESCs). Its expression is decreased with ESC differentiation, suggesting that TIP110 may play a role in ESC regulation. Our results demonstrate that TIP110 is strongly associated with and apparently necessary for maintenance of expression of NANOG, OCT4, SOX2, and for hESC pluripotency.
Materials and Methods
Human ESCs and their culture
The hESC line (H7 clone) was cultured in hESC medium which contains Dulbecco's modified Eagle's medium (DMEM):F12,4 ng/ml bFGF, 2 mM glutamine, 0.1 mM non-essential amino acids, 50 units/ml penicillin and 50 μg/ml streptomycin, 0.1 mM β-Mercaptoethanol, supplemented with 20% knockout serum replacement (KSR; Invitrogen), on feeder layer of mitotically inactivated MEF (mouse embryonic fibroblasts). ESC cultures were split using microdissection passaging for 100–150 colonies per 35-mm dish. Cells were seeded 24 h prior to transfection without feeder layers in 20% KSR hESC medium without bFGF to let cells differentiate, or in mTeSR medium (Stemcell Technologies) on Matrigel-coated dishes (BD Bioscience) to maintain cell undifferentiation [12]. Cells were transfected with pshTip110/empty vector or pCSC.TIP110.GFP/empty vector by Lipofectamine 2000 (Invitrogen), and harvested 3 to 5 days after transfection.
Immunohistochemistry
Cells were fixed with 4% (w/v) paraformaldehyde for 30 min, washed with phosphate-buffered saline (PBS), and permeabilized with 0.1% (v/v) TritonX-100 in PBS for 5 min. Cells then were blocked in 10% (v/v) goat serum for 30 min at room temperature, and incubated with primary antibodies at 4°C overnight [13]. Primary antibodies for OCT4 (sc-5279), Tuj1 (sc-58888), and AFP (sc-51506) were purchased from Santa Cruz Biotechnology, Inc.; NANOG (Cat. 4893) was purchased from Cell Signaling Technology, Inc; SMA (Cat. 04-1094) was purchased from Millipore, and used at 1:100 dilution.
RNA extraction
Total RNA was extracted using TRIzol reagent (Invitrogen) [11]. To remove traces of DNA contamination, RNA samples were treated with acid phenol:chloroform (Cat. No. AM9722; Ambion). Total RNA (20 ng) was used as a negative control for polymerase chain reaction (PCR).
Primer design, semi-quantitative reverse transcription–PCR, and real-time reverse transcription–PCR analysis
There are many OCT4 pseudogenes in the human genome that have high similarity to the OCT4 sequence. Specific reverse transcription (RT)-PCR primer design is important to identify OCT4 from its pseudogenes. We performed multiple alignment of OCT4 (NM_002701.4) and 6 other pseudogene sequences. We used the same primer sequences as others [14] to amplify OCT4A (OCT-AF and OCT-RB1). This produced PCR products of 492 bp. Specific primers for SOX2 and NANOG were SOX2-5′: atgcaccgctacgacgtga, SOX2-3′: cttttgcacccctcccattt. This produces PCR products of 436 bp. NANOG 5′-ctcgctgattaggctccaacc-3′ and 5′-ggac actggctgaatccttcc-3′. RT-PCR was performed using a One-tube Titan RT-PCR kit. The RT-PCR program consisted of 1 cycle at 50°C for 30 min and 94°C for 2 min, followed by 10 cycles at 94°C for 30 s, 60°C for 45 s, 68°C for 1 min, and 25 cycles at 94°C for 30 s, 60°C for 45 s, 68°C for 1 min plus 5 s cycle elongation for each successive cycle, and 1 cycle at 68°C for 7 min. For quantitative (q)RT-PCR, total RNA was reverse-transcribed into cDNA using Takara RT reagent kit. qPCR reactions were performed using the Agilent MX3005P qPCR system with SYBR Green mix.
Results and Discussion
Expression of TIP110 in hESCs
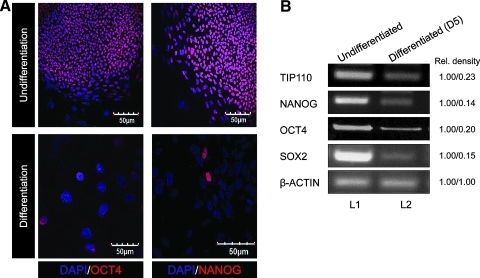
TIP110 is expressed in human CD34+ cells, and its expression is decreased with differentiation [11]. Tip110 mRNA was also expressed in phenotyped mouse marrow hematopoietic stem cells (HSCs) and HPCs [11]. This led us to assess TIP110 expression in hESCs during maintenance of pluripotency and after differentiation. ESCs, expressing NANOG and OCT4 (assessed by staining), were cultured (Fig. 1A). qRT-PCR showed that TIP110 as well as Nanog, Oct4, and Sox2 were expressed in this hESC line (Fig. 1B, L1). hESCs were removed from feeder layers and bFGF for 5 days, to allow ESC differentiation (Fig. 1A). TIP110 expression levels were dramatically reduced (by 77%); this was associated with large decreases in expression of NANOG (82%), OCT4 (80%), and SOX2 (85%; Fig. 1B, L2).
FIG. 1.
Expression of Tip110 and pluripotent factor mRNA expression in hESC line H9. ESCs were cultured with/without MEFs and bFGF (A); expression in undifferentiated (L1) and differentiated hESCs (L2) determined by semi-quantitative RT-PCR (B). Numbers to right of each bar equal relative expression levels of L1 versus L2. Data are representative of 3 reproducible experiments. hESC, human embryonic stem cell; MEF, mouse embryonic fibroblast; bFGF, basic fibroblast growth factor; RT-PCR, reverse transcription–polymerase chain reaction.
TIP110 is important for maintaining hESC pluripotency
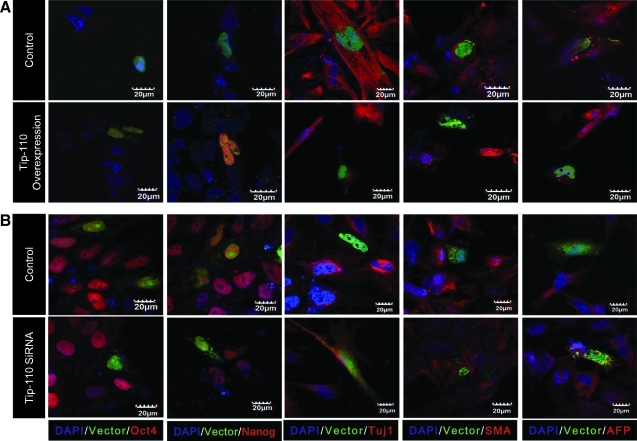
We assessed whether TIP110 might regulate hESC pluripotency. We exogenously overexpressed viral vector TIP110 in hESCs. Feeder layers and bFGF were withdrawn upon introducing the TIP110 vector and cell cultured for 5 days to test whether sustained TIP110 expression rendered ESCs less sensitive to differentiation. Compared with controls, TIP110 overexpressing cells stained positive for OCT4 and NANOG and were negative for Tuj1, SMA, and AFP (Fig. 2A), demonstrating that overexpression of TIP110 rendered ESCs less responsive to differentiation. Next, we reduced TIP110 expression by transfection of the hESCs with TIP110 siRNA. Cells were cultured in mTeSR medium on Matrigel-coated dishes for an additional 5 days in order to maintain cells under undifferentiation conditions [12]. TIP110 siRNA vector expressing cells were negative for OCT4 and NANOG, and positive for Tuj1, SMA, and AFP expression compared with control cells (Fig. 2B), demonstrating that enforced reduction of TIP110 expression in hESCs causes hESC differentiation. Together, this demonstrates that TIP110 plays an important role in maintenance of hESC pluripotency.
FIG. 2.
Characterization of hESCs with sustained expression or knockdown of TIP110 expression. hESCs with sustained TIP110 expression (A); ESCs with siRNA TIP110 (B). Immunocytochemistry for OCT4 (Alexa 448 or 546 nm), NANOG (Alexa 448 or 546 nm), ectodermal (Tuj1), mesodermal (a-SMA), and endodermal (AFP) proteins (all with Alexa 546 nm). Nuclei are stained with DAPI (4′,6-diamidino-2-phenylindole) (blue). Scale bars shown.
TIP110 regulates levels of pluripotent factors in hESCs
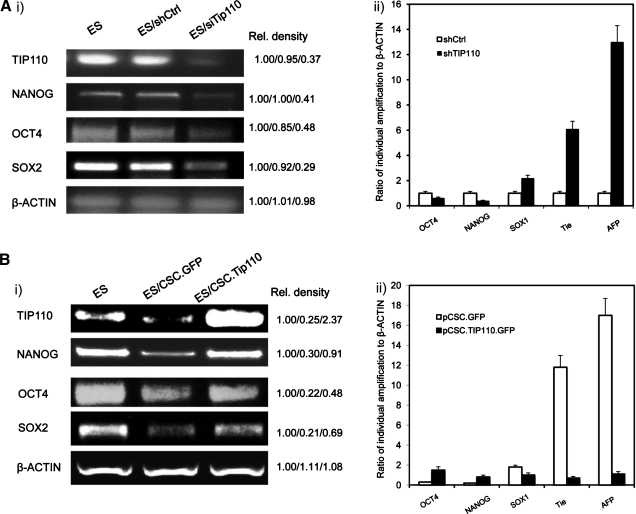
The above data demonstrated the importance of TIP110 in maintenance of ESC pluripotency. We speculated that TIP110 maintenance of hESC pluripotency might be through regulation of NANOG, OCT4, and SOX2. We silenced TIP110 expression in this hESC line by transfection with a TIP110 siRNA vector, previously shown to reduce TIP110 expression by 70% [11,15,16]. Cells were cultured in complete 20% KSR hESC medium for an additional 5 days. Expression of these transcription factors was dramatically decreased, but differentiation markers' levels increased (Fig. 3A). When we overexpressed TIP110 in hESCs and withdrew bFGF with the same culture condition for 5 days, these transcription factors were increased to some degree, and differentiation marker expression levels were also reduced (Fig. 3B). This demonstrates that TIP110 is required for maintaining NANOG, OCT4, and SOX2 levels in this hESC line. Reduction of TIP110 expression caused hESC differentiation directly or indirectly through downregulation of NANOG, OCT4, and SOX2 expression.
FIG. 3.
Reduced TIP110 expression decreases pluripotent factor expression. Semi-quantitative RT-PCR (Ai, Bi) and quantitative RT-PCR (Aii, Bii) analysis of pluripotent-related factors and/or differentiation markers. ESCs transfected with TIP110 siRNA. Cells were cultured in full medium for 5 days after transfection (A); ESCs transfected with viral vector for TIP110. Cells were cultured in the medium that lacks bFGF for 5 days (B). β-Actin was used as a loading control. The data are from 1 of at least 3 reproducible experiments.
TIP110 is preferentially expressed in the undifferentiated state in a hESC line and plays a key role in regulating OCT4, SOX2, and NANOG, factor required to maintain pluripotency. Together, our present and previous studies [11] suggest TIP110 expression as a useful marker to distinguish early from more-differentiated cells. Future studies to determine whether or not the Tip110 effects on these transcription factors are direct, and to understand mechanisms of TIP110 regulation of the transcriptional network that contributes to pluripotency of hESCs, as well as HSCs and HPCs, are warranted. Modulating TIP110 expression in a controlled fashion maybe relevant for cellular engineering and regenerative medicine.
Acknowledgments
These studies were supported by Public Health Service Grants R01 HL56416 and R01 HL67384, and by P01 DK90948 from the National Institutes of Health to Hal E. Broxmeyer.
Author Disclosure Statement
The authors have no potential conflicts of interest to disclose.
References
- 1.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loh YH. Wu Q. Chew JL. Vega VB. Zhang W. Chen X. Bourque G. George J. Leong B, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 3.Pan G. Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 4.Pesce M. Gross MK. Scholer HR. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays. 1998;20:722–732. doi: 10.1002/(SICI)1521-1878(199809)20:9<722::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Niwa H. Miyazaki J. Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 6.Hay DC. Sutherland L. Clark J. Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 7.Matin MM. Walsh JR. Gokhale PJ. Draper JS. Bahrami AR. Morton I. Moore HD. Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- 8.Chambers I. Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 9.Pesce M. Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 10.Chew JL. Loh YH. Zhang W. Chen X. Tam WL. Yeap LS. Li P. Ang YS. Lim B. Robson P. Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y. Timani K. Mantel C. Fan Y. Hangoc G. Cooper S. He JJ. Broxmeyer HE. TIP110/p110nrb/SART3/p110 regulation of hematopoiesis through CMYC. Blood. 2011;117:5643–5651. doi: 10.1182/blood-2010-12-325332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broxmeyer HE. Lee MR. Hangoc G. Cooper S. Prasain N. Kim YJ. Mallett C. Ye Z. Witting S, et al. Hematopoietic stem/progenitor cells, generating of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4733–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atlasi Y. Mowla SJ. Ziaee SA. Gokhale PJ. Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26:3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 14.Chan EM. Ratanasirintrawoot S. Park IH. Manos PD. Loh YH. Huo H. Miller JD. Hartung O. Rho J, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y. Kim BO. Kao C. Jung C. Dalton JT. He JJ. Tip110, the human immunodeficiency virus type 1 (HIV-1) Tat-interacting protein of 110 kDa as a negative regulator of androgen receptor (AR) transcriptional activation. J Biol Chem. 2004;279:21766–21773. doi: 10.1074/jbc.M314321200. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y. Li J. Kim BO. Pace BS. He JJ. HIV-1 Tat protein-mediated transactivation of the HIV-1 long terminal repeat promoter is potentiated by a novel nuclear Tat-interacting protein of 110 kDa, Tip110. J Biol Chem. 2002;277:23854–23863. doi: 10.1074/jbc.M200773200. [DOI] [PubMed] [Google Scholar]