 Trypanosoma cruzi trans-sialidase is a versatile catalyst for enzymatic α-2,3-sialylation reactions.
Trypanosoma cruzi trans-sialidase is a versatile catalyst for enzymatic α-2,3-sialylation reactions.
Abstract
Systematically modified octyl galactosides and octyl N-acetyllactosamines were assessed as inhibitors of, and substrates for, T. cruzi trans-sialidase (TcTS) in the context of exploring its acceptor substrate binding site. These studies show that TcTS, which catalyses the α-(2→3)-sialylation of non-reducing terminal β-galactose residues, is largely intolerant of substitution of the galactose 2 and 4 positions whereas substitution of the galactose 6 position is well tolerated. Further studies show that even the addition of a bulky sugar residue (glucose, galactose) does not impact negatively on TcTS binding and turnover, which highlights the potential of ‘internal’ 6-substituted galactose residues to serve as TcTS acceptor substrates. Results from screening a 93-membered thiogalactoside library highlight a number of structural features (notably imidazoles and indoles) that are worthy of further investigation in the context of TcTS inhibitor development.
Introduction
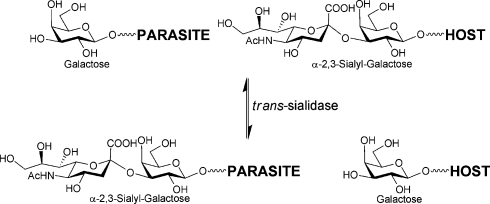
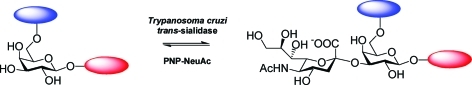
It is widely accepted that carbohydrates play a crucial role in a diverse array of biological recognition events1 and that glycobiology offers many new and specific targets for therapeutic intervention.2 A number of potential ‘glyco’ targets present themselves in relation to infection,2d,2e with parasitic diseases, for instance, offering numerous opportunities relating to cell surface glycan biosynthesis and recognition.3 In the case of the blood-borne protozoan parasite Trypanosoma cruzi, which causes Chagas' disease in South and Central America, very few effective drugs are currently available.4 T. cruzi is an obligate intracellular parasite that must bind to and invade host cells to complete its life cycle; once in the blood stream it must evade the host immune response. For both events, sialic acid-containing mucin glycoprotein structures are key players. The parasite is not itself capable of synthesising sialic acid and instead employs a cell surface trans-sialidase5 in order to scavenge this sugar from host glycoconjugates and transfer it to the parasite’s own mucin coat (Fig. 1).6
Fig. 1. trans-Sialidase-catalysed sialylation of parasite cell surface mucin with sialylated host glycoprotein.
Understanding ligand–substrate binding by TcTS is key to its exploitation as a drug target and as a catalyst for glycoconjugate synthesis. Beyond X-ray crystallography7 and in silico approaches,8 a number of groups have used NMR spectroscopy,9 enzyme kinetic analysis10 or inhibition studies11 to address this point. However, the availability of sialylated glyconjugates to probe TcTS function is hampered by issues associated with the preparation of sialylated glycans. While advances have been made in chemical synthesis approaches,12 there is still room for improvement. Enzymatic methods based on sialyltransferases,13 sialidases14 (operating ‘in reverse’) and TcTS15 have been reported. TcTS, in particular, has a relaxed acceptor specificity16 which has proved to be a useful tool with which to generate α-(2→3)-sialylated glycans for the study of carbohydrate recognition by E-selectin,17 Toxoplasma gondii microneme protein 1,18 and the Siglec family member MAG (myelin-associated glycoprotein).19 It has also been exploited for the sialylation of (synthetic) T. cruzi mucin glycans20 and for the resialylation of desialylated sheep erythrocytes, for instance.21 Herein we report studies aimed at exploring the acceptor substrate binding site of recombinant T. cruzi trans-sialidase22 using a series of small libraries of systematically modified glycans and a thiogalactoside-based combinatorial library.
Results and discussion
Since the compounds assessed in this study are acceptor substrate analogues, they were assessed as both potential TcTS inhibitors and acceptor substrates. In the former case, a well established radiochemical assay was employed:23 compounds (1 mM) were incubated in the presence of α-(2→3)-sialyl-lactose (1 mM) and [14C]-lactose (7 μM; 750 Bq) for 30 min at 37 °C, with formation of α-(2→3)-sialyl [14C]-lactose monitored following anion-exchange separation. Where compounds were screened as acceptor substrates (1 mM concentration, with incubation for 16 h at 37 °C in the presence of 5 mM PNP-sialic acid as donor substrate), analytical scale reactions were monitored by TLC, with key reactions also analysed by ES-MS.
Octyl galactosides as TcTS inhibitors
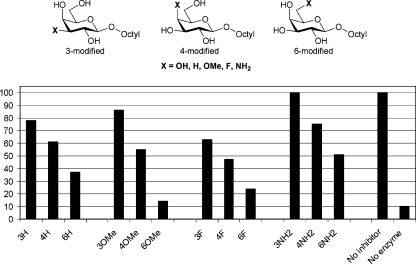
Initially a set of systematically modified octyl galactosides was investigated (Fig. 2).24 These compounds have previously been assessed as potential acceptor analogue inhibitors of the blood group H gene-specified α-(1→2)-fucosyltransferase that is involved in O blood-group antigen biosynthesis.24 Fig. 2 clearly shows that 3-modified octyl galactosides (i.e. modified at the prospective site of TcTS action) have very limited inhibitory effect on TcTS. This is in contrast to the situation with, for instance, some glycosyltransferases where the corresponding -OH to -NH2 substitution in acceptor substrates results in inhibition.25 4-Modified octyl galactosides show weak inhibition, irrespective of the 4-substituent, whilst 6-modified octyl galactosides show much stronger inhibition, with the 6-OMe compound producing stronger inhibition than the parent alcohol. Overall, these data suggest that while modification of the 3-position of a galactoside acceptor abolishes binding to TcTS, minor modification at C4 may be tolerated. Substitution of the C-6 primary alcohol has no negative impact, and on occasion may have a positive effect on ligand binding (compare 16% activity remaining for the 6-OMe compound with ∼25% activity remaining for the parent 6-OH compound).
Fig. 2. Systematically modified octyl galactosides as potential inhibitors of TcTS-catalysed trans-sialylation. The plot shows % enzyme activity remaining, as judged by radiochemical assay, as a function of inhibitor modification. The unsubstituted parent octyl galactoside resulted in 25% activity remaining.
Octyl LacNAcs as TcTS inhibitors
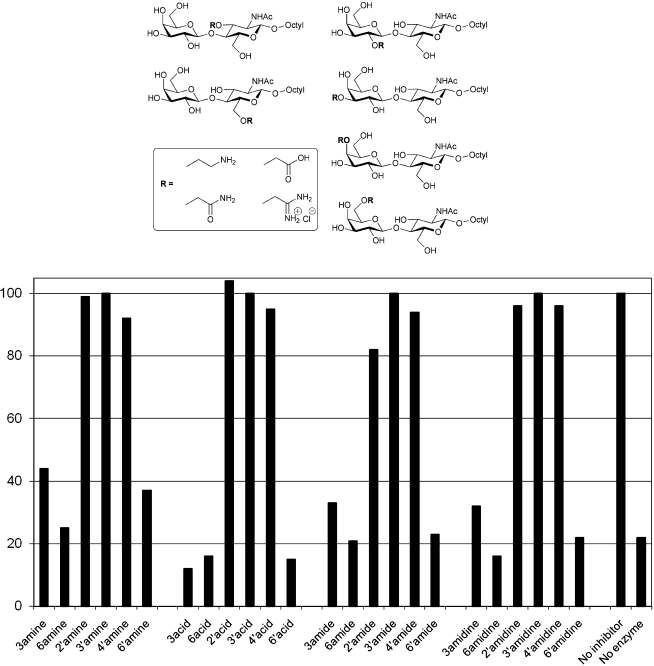
We next moved to a library of disaccharide derivatives, the synthesis of which was based on hydroxyl group functionalisation as O-cyanomethyl ethers, which provides a versatile route to the preparation of sugar amines, carboxylic acids, amides and amidine salts.26 This approach has previously been used to generate a library of 24 derivatives of N-acetyl-lactosamine (LacNAc), which was used to map acceptor hydroxyl group interactions with bovine α-(1→3)-galactosyltransferase, identifying key polar groups that are essential for glycosyl transfer.27 Here the same set of compounds were assessed in the radiochemical TcTS inhibition assay (Fig. 3).
Fig. 3. Systematically modified octyl LacNAcs (1mM) as potential inhibitors of TcTS-catalysed trans-sialylation. The plot shows % enzyme activity remaining, as judged by radiochemical assay, as a function of inhibitor modification. The unsubstituted parent octyl LacNAc resulted in 15% activity remaining.
With this series of 24 octyl LacNAc derivatives, inhibition of TcTS action is only dependent on the site of substitution, but not on the nature of the substituent (Fig. 3). Modification of either the 3 or 6 positions on the N-acetylglucosamine residue, or the 6 position of the galactose residue of N-acetyllactosamine, are readily tolerated by TcTS, with all such compounds assessed demonstrating strong inhibition in the radiochemical trans-sialylation assay. In contrast, modification of the galactose 2, 3 or 4 positions in N-acetyllactosamine essentially abolished TcTS inhibition. While the data for the 3′-substituted octyl LacNAc compounds is in keeping with that for the 3-substituted octyl galactosides (i.e. inhibition abolished), the larger substituents (Fig. 3) in the octyl LacNAc series prevent TcTS inhibition when present at the 2′ or 4′ positions while the more modest OH to H/OMe/F/NH2 substitutions in the octyl galactosides are tolerated, to varying degrees.
Octyl galactosides and octyl LacNAcs as TcTS substrates
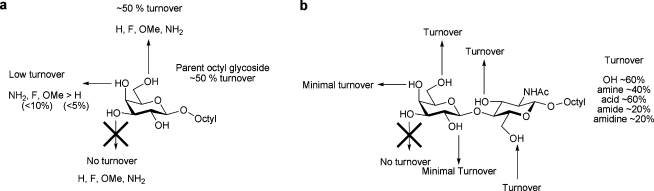
The systematically modified octyl galactosides and octyl LacNAcs discussed above were also assessed as potential acceptor substrates (1 mM) for TcTS in the presence of excess PNP sialic acid‡ as a donor substrate (5 mM). Turnover was assessed by TLC and confirmed by ES-MS (summarised in Fig. 4).
Fig. 4. TcTS-catalysed sialylation of systematically modified a octyl galactosides and b octyl LacNAc derivatives in the presence of PNP-sialic acid.
Overall, it is clear that substitution of the 2 or 4 position of galactose in either octyl galactoside or octyl LacNAc greatly diminishes or even prevents TcTS-catalysed α-(2→3)-sialylation. In contrast, in both series of compounds modification of the 6 position of galactose is well tolerated, with all such compounds assessed giving rise to sialylated product. For the octyl LacNAc derivatives where reaction was observed (i.e. the 3, 6 and 6′ modified compounds), the relative turnover was substituent-dependent, but independent of the site of substitution (Fig. 4 b).
Thiogalactosides as TcTS inhibitors
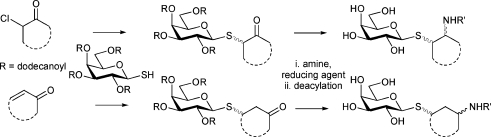
With a view to further exploring chemical space, and taking into account the established but weak inhibition of TcTS by β-galactosides,11a,11e we went on to consider the molecular diversity evident in a 1-thio-β-d-galactopyranoside library. This set of compounds emanates from a solution phase library synthesis that relies on solid-phase extraction on C18 silica as a purification strategy.28 Parallel solution S-alkylation of tetra-O-dodecanoyl-1-thio-β-d-galactopyranose with Michael acceptors and α-chloroketones, followed by ketone reductions, reductive aminations, and de-O-acylation provide access to a library of 1-thio-β-d-galactopyranosides carrying small and diverse polar-neutral, hydrophobic, aromatic, cationic, or anionic non-carbohydrate aglycone structures (Scheme 1). This library has previously given rise to novel inhibitors of toxin A from Abrus precatorius and of β-galactosidase from E. coli.28
Scheme 1. Generation of a library of diastereomeric thiogalactosides.28 For details of α-haloketones (two), α,β-unsaturated ketones (eight) and amines (nine) on which the library is based see the ESI.† .
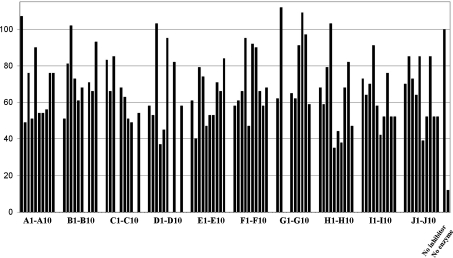
In the current study, a library of 93 compounds, each a mixture of 4 diastereoisomers, was assessed for inhibition of TcTS (Fig. 5). Of the 93 compounds assayed, 25 gave 20% inhibition or less, whilst at the opposite end of the inhibitory spectrum, 5 samples gave greater than 60% inhibition in the TcTS radiochemical assay.
Fig. 5. Assessment of a β-thio-galactopyranoside library (250 μg ml–1; ca 0.5–1.0 mM) for of TcTS-catalysed trans-sialylation. The plot shows % enzyme activity remaining, as judged by radiochemical assay. The following compounds were not assayed: B7, C4, C9, D7, D9, G3, G4.
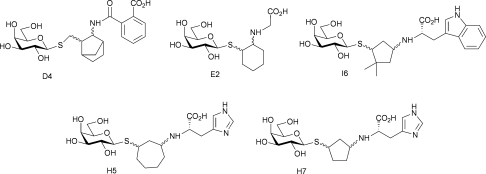
None of the compounds in this library is a potent TcTS inhibitor (e.g. low micromolar IC50) but the vast majority of compounds analysed show some inhibition at around 0.5–1.0 mM concentration with the more effective inhibitors (Fig. 6) having IC50's in the high micromolar/low millimolar range. No strong SAR is evident in the data, although a preference for a cyclic ketone building block, and a carboxyl group and weakly basic substituents is clear (H5, H6, H7, I6). There does not appear to be a strong preference for the nature of the ketone building block, with five of the ten used in library generation appearing amongst the top five TcTS inhibitors. Overall, these data suggest that the acceptor binding site of TcTS is remarkably tolerant of both physical shape and functional groups - important factors when considering TcTS as a potential catalyst for α-(2→3)-sialylation reactions. In addition, these data suggest that there is potential in exploring imidazoles and indoles as fragments for TcTS inhibitor development.
Fig. 6. Top five TcTS inhibitors from the β-thiogalactopyranoside library.
Isomeric mono- and disaccharides as TcTS inhibitors and substrates
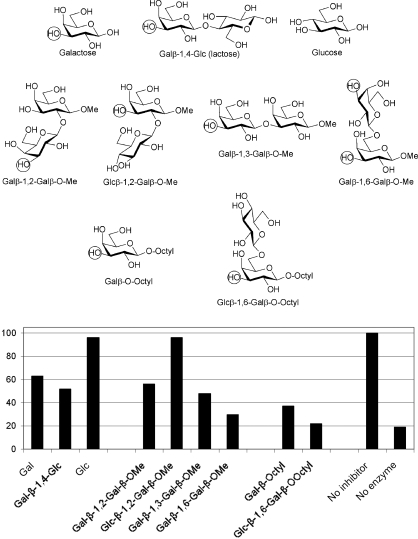
It is evident from the literature15,16 that, as highlighted by the library screens above, TcTS is rather promiscuous with respect to acceptor substrates. Its natural acceptors are branched mucin O-linked glycans,6 often presenting several non-reducing terminal as well as internal galactose residues. Previatio and colleagues29 investigated the kinetics of in vitro sialylation of these O-linked oligosaccharides by the T. cruzi trans-sialidase and showed that incorporation of one molecule of sialic acid hinders the introduction of a second molecule when two potential acceptor sites are present. Later work from de Lederkremer and colleagues20d demonstrated that while the major pentasaccharide of T. cruzi G strain mucin presents two terminal β-linked galactose residues for possible sialylation by TcTS, preparative sialylation in vitro results in the addition of only a single sialic acid residue. Nonetheless, we have shown20a that double sialylation of a synthetic branched T. cruzi Y strain mucin O-glycan can be achieved in vitro with TcTS. In addition, Crout and colleagues30 have demonstrated the double sialylation of the disaccharide β-Galp-(1→6)-Galp, confirming that two sialic acid residues be added to the same glycan chain. However, the clear preference in this work, and in accord with the observations of Previato, was for single sialylation. The Crout work30 also showed that α-Galp-(1→6)-Galp-β-OMe was susceptible to sialylation on the reducing terminal β-galactose residue, indicating that β-galactose does not need to be located at the non-reducing terminus of a glycan for it to be susceptible to TcTS-catalysed sialylation. In the current study, we were therefore drawn to investigate a series of isomeric disaccharides (Fig. 7) as potential inhibitors/acceptor of TcTS.
Fig. 7. Isomeric saccharides as potential inhibitors of TcTS-catalysed trans-sialylation. Circles indicate the potential sites of sialic acid attachment. The plot shows % activity remaining as a function of inhibitor modification.
Initial experiments with reducing galactose, lactose and glucose confirm that acceptor recognition by TcTS is dominated by the non-reducing terminal galactose residue, with lactose providing little additional inhibition with respect to galactose (Fig. 7). The importance of C4 stereochemistry is also evident: while reasonable inhibition is obtained with galactose, little or no inhibition is obtained with the C4 epimer glucose. This is in keeping with data presented earlier for octyl galactosides and octyl LacNAcs derivatives, and confirms the very limited TcTS tolerance for acceptor C4 modification.
Comparing (1→2)-, (1→3)- and (1→6)-linked β-Galp-Galp disaccharides as TcTS inhibitors, the (1→6)-isomer is a somewhat stronger inhibitor (30% activity remaining) than the other two (48% and 58% activity remaining, respectively), which are closer in activity to galactose and lactose (Fig. 7). This highlights that while the (1→2)- and (1→3)-linked compounds contain two galactose residues, only a single TcTS acceptor site is available in both cases and presumably only the non-reducing terminal galactose residue is able to bind productively to the TcTS acceptor binding site. Exploiting the fact that glucose is not a TcTS substrate, β-Glcp-(1→2)-Galp-OMe (96% activity remaining) was assessed as an analogue of β-Galp-(1→2)-Galp-OMe (56% activity remaining). As expected, 2-substitution of the galactose residues blocks its ability to interact with TcTS, which is consistent with the octyl LacNAc data presented earlier. In contrast, the (1→6)-linked Galp-Galp disaccharide possesses two potential sites for TcTS catalysed sialylation, as demonstrated by Crout and colleagues,31 and inhibits TcTS more strongly than the (1→2)- and (1→3)-linked isomers. This suggests that there are likely TcTS binding contributions from both galactose residues in this case.
With octyl glycosides, TcTS inhibition is more pronounced than with the methyl glycosides and reducing sugars (38% activity remaining for octyl galactoside vs. 62% remaining for reducing galactose, for instance)(Fig. 7). Again employing glucose as a non-substrate residue for TcTS, β-Glcp-(1→6)-Galp-octyl, which contains only a single potential site for TcTS action, was assessed as an acceptor substrate: TLC and ES-MS data demonstrate the exclusive formation of a singly sialylated compound upon incubation with TcTS and PNP-sialic acid (ES-MS: observed [M – H]– 744.5). These data suggest that β-Glcp-(1→6)-Galp-octyl binding to TcTS benefits from the positive impact of both the octyl group and the glucose substituent at the 6 position, which gives rise to strong TcTS inhibition (22% activity remaining).
Conclusions
This study has assessed the use of glycoside libraries as a means of exploring the acceptor substrate binding site of T. cruzi trans-sialidase. This enzyme catalyses the α-(2→3)-sialylation of non-reducing terminal β-linked galactose residues, but it is largely intolerant of substitution of the galactose 2 and 4 positions. In contrast, substitution of the galactose 6 position is well tolerated, with not even the addition of a bulky sugar residue (glucose, galactose) impacting negatively on TcTS binding and turnover. Data reported herein, coupled with the observations of Crout and colleagues,30 highlight the potential of ‘internal’ 6-substituted galactose residues to serve as acceptor sites for TcTS-catalysed trans-sialylation§ . In addition, the library screening approach adopted has highlighted a number of structural features (notably imidazoles and indoles) that are worthy of more thorough investigation in the context of TcTS inhibitor development. In summary, this study expands upon the demonstrated versatility of T. cruzi trans -sialidase as a catalyst for α-(2→3)-sialyl-glycoconjugate synthesis, highlighting the wide range of structures and functionalities that it can accommodate.
Experimental
The trans-sialidase used in this study was a His-tagged 70 kDa recombinant material truncated to remove C-terminal repeats but retaining the catalytic N-terminal domain of the enzyme.22 The preparation of the compound libraries screened in this study have been reported previously.24,26,28 Similarly, the disaccharides screened have either been reported in the literature or were synthesised using published methods.31
TcTS inhibition assay
TcTS was assayed using a literature radiochemical method.23 Briefly, prospective inhibitors were incubated (50 μl, 20 mM HEPES, pH 7.0) for 30 min at 37 °C in the presence of α-(2→3)-sialyl-lactose (1 mM) and [14C]-lactose (7 μM; 750 Bq). The reaction was then terminated by the addition of water (1 ml), followed by passage of the quenched mixture through QAE Sephadex A25 (0.5 ml) equilibrated in water. Unreacted [14C]-lactose was eluted with water (3 × 0.5 ml) and [14C]-sialyl-lactose product was eluted with 1 M ammonium formate (3 × 0.5 ml). Potential inhibitors were screened at 1 mM concentration in the case of octyl galactosides, octyl LacNAcs and other O-glycosides, and 250 μg ml–1 for thiogalactosides. The inhibition data, reported as bar charts, are accurate to ± 10%.
TcTS acceptor substrate assay
Prospective TcTS acceptor substrates (1 mM) were incubated (50 μl, 20 mM HEPES, pH 7.0) for 16 h at 37 C in the presence of PNP sialic acid (5 mM) and turnover was assessed by TLC (CHCl3–MeOH–H2O, 10 : 10 : 3) with detection using orcinol/sulfuric acid in ethanol. Where turnover was evident from TLC analysis, protein was precipitated from reactions with ethanol on ice and the resulting soluble material was directly analysed by ES-MS.
Acknowledgments
These studies were supported by the BBSRC and the Wellcome Trust (Grant refs: 042472 and 040331).
Footnotes
‡PNP sialic acid was used in preference to α-(2→3)-sialyl-lactose since the former can easily be obtained in hundreds of mg to gram quantities for preparative scale biotransformations.
References
- (a) Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dwek R. A. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]; (c) Essentials of Glycobiology, 2nd edition. ed. A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. M. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, Cold Spring Harbor Laboratory Press, New York, 2009 [PubMed] [Google Scholar]
- (a) Koeller K. M., Wong C.-H. Nat. Biotechnol. 2000;18:835–841. doi: 10.1038/78435. [DOI] [PubMed] [Google Scholar]; (b) Brown J. R., Crawford B. E., Esko J. D. Crit. Rev. Biochem. Mol. Biol. 2007;42:481–515. doi: 10.1080/10409230701751611. [DOI] [PubMed] [Google Scholar]; (c) Turnbull J. E., Field R. A. Nat. Chem. Biol. 2007;3:74–77. doi: 10.1038/nchembio0207-74. [DOI] [PubMed] [Google Scholar]; (d) von Itzstein M. Curr. Opin. Struct. Biol. 2008;18:558–566. doi: 10.1016/j.sbi.2008.07.006. [DOI] [PubMed] [Google Scholar]; (e) Ernst B., Magnani J. L. Nat. Rev. Drug Discovery. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Ferguson M. A. J., Brimacombe J. S., Brown J. R., Crossman A., Dix A., Field R. A., Güther M. L. S., Milne K. G., Sharma D. K., Smith T. K. Biochim. Biophys. Acta. 1999;1455:327–340. doi: 10.1016/s0925-4439(99)00058-7. [DOI] [PubMed] [Google Scholar]; (b) Mendonca-Previato L., Todeschini A. R., Heise N., Agrellos O. A., Dias W. B., Previato J. O. Curr. Org. Chem. 2008;12:926–939. [Google Scholar]; (c) Ghoshal A., Bandyopadhyay S. M. N., Mandal C. Mini-Rev. Med. Chem. 2008;8:358–369. doi: 10.2174/138955708783955980. [DOI] [PubMed] [Google Scholar]; (d) Richards M. R., Lowary T. L. ChemBioChem. 2009;10:1920–1938. doi: 10.1002/cbic.200900208. [DOI] [PubMed] [Google Scholar]; (e) de Lederkremer R. M., Agusti R. Adv. Carbohydr. Chem. Biochem. 2009;62:311–66. doi: 10.1016/S0065-2318(09)00007-9. [DOI] [PubMed] [Google Scholar]
- Moreira D. R. M., Leite A. C. L., Ribeiro, dos Santos R., Soares M. B. P. Curr. Drug Targets. 2009;10:212–231. doi: 10.2174/138945009787581140. [DOI] [PubMed] [Google Scholar]
- (a) Previato J. O., Andrade A. F. B., Pessolani M. C. V., Mendonca-Previato L. Mol. Biochem. Parasitol. 1985;16:85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]; (b) Zingales B., Carniol C., de Lederkremer R. M., Colli W. Mol. Biochem. Parasitol. 1987;26:135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]; (c) Schenkman S., Jiang M.-S., Hart G. W., Nussenzweig V. Cell. 1991;65:1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]; (d) Cross G. M., Takle G. B. Annu. Rev. Microbiol. 1993;47:385–411. doi: 10.1146/annurev.mi.47.100193.002125. [DOI] [PubMed] [Google Scholar]
- (a) Hicks S. J., Theodoropoulos G., Carrington S. D., Corfield A. P. Parasitol. Today. 2000;16:476–481. doi: 10.1016/s0169-4758(00)01773-7. [DOI] [PubMed] [Google Scholar]; (b) Buscaglia C. A., Campo V. A., Frasch A. C. C., Di Noia J. M. Nat. Rev. Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- (a) Buschiazzo A., Tavares G. A., Campetella O., Spinelli S., Cremona M. L., Paris G., Amaya M. F., Frasch A. C. C, Alzari P. M. EMBO J. 2000;19:16–24. doi: 10.1093/emboj/19.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buschiazzo A., Amaya M. F., Cremona M. L., Frasch A. C. C., Alzari P. M. Mol. Cell. 2002;10:757–768. doi: 10.1016/s1097-2765(02)00680-9. [DOI] [PubMed] [Google Scholar]; (c) Amaya M. F., Watts A. G., Damager I., Wehenkel A., Nguyen T., Buschiazzo A., Paris G., Frasch A. C. C., Withers S. G., Alzari P. M. Structure. 2004;12:775–784. doi: 10.1016/j.str.2004.02.036. [DOI] [PubMed] [Google Scholar]; (d) Buschiazzo A., Alzari P. M. Curr. Opin. Chem. Biol. 2008;12:565–572. doi: 10.1016/j.cbpa.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Neres J., Brewer M. L., Ratier L., Botti H., Buschiazzo A., Edwards P. N., Mortenson P. N., Charlton M. H., Alzari P. M., Frasch A. C., Bryce R. A., Douglas K. T. Bioorg. Med. Chem. Lett. 2009;19:589–596. doi: 10.1016/j.bmcl.2008.12.065. [DOI] [PubMed] [Google Scholar]
- (a) Haslehorst T., Wilson J. C., Liakatos A., Kiefel M. J., von Itzstein M. Glycobiology. 2004;14:895–907. doi: 10.1093/glycob/cwh108. [DOI] [PubMed] [Google Scholar]; (b) Blume A., Neubacher B., Thiem J., Peters T. Carbohydr. Res. 2007;342:1904–1909. doi: 10.1016/j.carres.2007.05.037. [DOI] [PubMed] [Google Scholar]
- (a) Watts A. G., Damager I., Amaya M. L., Buschiazzo A., Alzari P., Frasch A. C., Withers S. G. J. Am. Chem. Soc. 2003;125:7532–7533. doi: 10.1021/ja0344967. [DOI] [PubMed] [Google Scholar]; (b) Damager I., Buchini S., Amaya M. F., Buschiazzo A., Alzari P., Frasch A. C., Watts A. G., Withers S. G. Biochemistry. 2008;47:3507–3512. doi: 10.1021/bi7024832. [DOI] [PubMed] [Google Scholar]
- (a) Agusti R., Paris G., Ratier L., Frasch A. C. C., de Lederkremer R. M. Glycobiology. 2004;14:659–670. doi: 10.1093/glycob/cwh079. [DOI] [PubMed] [Google Scholar]; (b) Reviewed in: Neres J., Bryce R. A., Douglas K. T., Drug Discovery Today, 2008, 13 , 110 –117 . [DOI] [PubMed] [Google Scholar]; (c) Buchini S., Buschiazzo A., Withers S. G. Angew. Chem., Int. Ed. 2008;47:2700–2703. doi: 10.1002/anie.200705435. [DOI] [PubMed] [Google Scholar]; (d) Kim J. H., Ryu H. W., Shim J. H., Park K. H., Withers S. G. ChemBioChem. 2009;10:2475–2479. doi: 10.1002/cbic.200900108. [DOI] [PubMed] [Google Scholar]; (e) Carvalho I., Andrade P., Campo V. L., Guedes P. M. M., Sesti-Costa R., Silva J. S., Schenkman S., Dedola S., Hill L., Rejzek M., Nepogodiev S. A., Field R. A. Bioorg. Med. Chem. 2010;18:2412–2427. doi: 10.1016/j.bmc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- (a) Demchenko A. V., Boons G.-J. Chem. Rev. 2000;100:4539–4565. doi: 10.1021/cr990313g. [DOI] [PubMed] [Google Scholar]; (b) Ress D. K., Linhardt R. J. Curr. Org. Chem. 2004;1:31–46. [Google Scholar]
- Chokhawala H. A. and Chen X., Enzymatic Approaches to O-Glycoside Introduction: Glycosyltransferases, pp 415-451, in Comprehensive Glycoscience, Volume 1, Eds G.-J. Boons, Y. C. Lee, A. Suzuki, N. Taniguchi and A. G. J. Voragen, Elsevier, Oxford, UK, 2007 [Google Scholar]
- Bojarova-Fialova P. and Kren V., Enzymatic Approaches to O-Glycoside Introduction: Glycosidases, pp 453-487, in Comprehensive Glycoscience, Volume 1, Eds G.-J. Boons, Y. C. Lee, A. Suzuki, N. Taniguchi and A. G. J. Voragen, Elsevier, Oxford, UK, 2007 [Google Scholar]
- For selected examples see: ; (a) Bew S. P., Brimage R. A., L'Hermite N., Sharma S. V. Org. Lett. 2007;9:3713–3716. doi: 10.1021/ol071047t. [DOI] [PubMed] [Google Scholar]; (b) Agusti R., Giorgi M. E., de Lederkremer R. M. Carbohydr. Res. 2007;342:2465–2469. doi: 10.1016/j.carres.2007.07.018. [DOI] [PubMed] [Google Scholar]; (c) Scudlo A., Thiem J. Adv. Synth. Catal. 2006;348:1217–1227. [Google Scholar]
- For instance: ; (a) von der Kerckhove F., Schenkman S., de Carvalho L. P., Tomlinson S., Kiso M., Yoshida M., Hasegawa A., Nussenzweig V. Glycobiology. 1992;2:541–548. doi: 10.1093/glycob/2.6.541. [DOI] [PubMed] [Google Scholar]; (b) Scudder P., Doom J. P., Chuenkova M., Manger I. D., Pereira M. E. A. J. Biol. Chem. 1993;268:9886–9891. [PubMed] [Google Scholar]
- (a) Probert M. A., Milton M. J., Harris R., Schenkman S., Brown J. M., Homans S. W., Field R. A. Tetrahedron Lett. 1997;38:5861–5864. [Google Scholar]; (b) Milton M. J., Harris R., Probert M. A., Field R. A., Homans S. W. Glycobiology. 1998;8:147–153. doi: 10.1093/glycob/8.2.147. [DOI] [PubMed] [Google Scholar]; (c) Harris R., Kiddle G. R., Field R. A., Milton M. J., Ernst B., Magnani J. L., Homans S. W. J. Am. Chem. Soc. 1999;121:2546–2551. [Google Scholar]
- (a) Garnett J. A., Liu Y., Leon E., Allman S. A., Friedrich N., Saouros S., Curry S., Soldati-Favre D., Davis B. G., Feizi T., Matthews S. Protein Sci. 2009;18:1935–1947. doi: 10.1002/pro.204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Allman S. A., Jensen H. H., Vijayakrishnan B., Garnett J. A., Leon E., Liu Y., Anthony D. C., Sibson N. R., Feizi T., Matthews S., Davis B. G. ChemBioChem. 2009;10:2522–2529. doi: 10.1002/cbic.200900425. [DOI] [PubMed] [Google Scholar]
- Neubacher B., Svenja S., Kelm S., Frasch A. C., Meyer B., Thiem J. ChemBioChem. 2006;7:896–899. doi: 10.1002/cbic.200500543. [DOI] [PubMed] [Google Scholar]
- For instance: ; (a) van Well R. M., Collet B. Y. M., Field R. A. Synlett. 2008:2175–2177. [Google Scholar]; (b) Campo V. L., Carvalho I., Allman S., Davis B. G., Field R. A. Org. Biomol. Chem. 2007;5:2645–2657. doi: 10.1039/b707772f. [DOI] [PubMed] [Google Scholar]; (c) Agusti R., Giorgi M. E., Mendoza V. M., Gallo-Rodriguez C., de Lederkremer R. M. Bioorg. Med. Chem. 2007;15:2611–2616. doi: 10.1016/j.bmc.2007.01.045. [DOI] [PubMed] [Google Scholar]; (d) Mendoza V. M., Agusti R., Gallo-Rodriquez C., de Lederkremer R. M. Carbohydr. Res. 2006;341:1488–1497. doi: 10.1016/j.carres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Tomlinson S., de Carvalho L. P., van der Kerckhove F., Nussenzweig V. Glycobiology. 1992;2:549–551. doi: 10.1093/glycob/2.6.549. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Chaves L. B., Pontes L. C., Eichinger D. J. Biol. Chem. 1994;269:7970–7975. [PubMed] [Google Scholar]
- Schenkman S., Pontes, de Carvalho L., Nussenzweig V. J. Exp. Med. 1992;175:567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary T. L., Swiedler S. J., Hindsgaul O. Carbohydr. Res. 1994;256:257–273. doi: 10.1016/0008-6215(94)84212-4. [DOI] [PubMed] [Google Scholar]
- (a) Ralton J. E., Milne K. G., Güther M. L. S., Field R. A., Ferguson M. A. J. J. Biol. Chem. 1993;268:24183–24189. [PubMed] [Google Scholar]; (b) Field R. A., Neville D. C. A., Smith R. W., Ferguson M. A. J. Bioorg. Med. Chem. Lett. 1994;4:391–394. [Google Scholar]; (c) Lowary T. L., Hindsgaul O. Carbohydr. Res. 1994;251:33–67. doi: 10.1016/0008-6215(94)84275-2. [DOI] [PubMed] [Google Scholar]; (d) Helland A. C., Hindsgaul O., Palcic M. M., Stults C. L. M., Macher B. A. Carbohydr. Res. 1995;276:91–98. doi: 10.1016/0008-6215(95)00165-p. [DOI] [PubMed] [Google Scholar]
- (a) Malet C., Hindsgaul O. J. Org. Chem. 1996;61:4649–4654. doi: 10.1021/jo960284z. [DOI] [PubMed] [Google Scholar]; (b) Malet C., Hindsgaul O. Carbohydr. Res. 1997;303:51–65. doi: 10.1016/s0008-6215(97)00268-1. [DOI] [PubMed] [Google Scholar]
- Sujino K., Malet C., Hindsgaul O., Palcic M. M. Carbohydr. Res. 1997;305:483–489. doi: 10.1016/s0008-6215(97)00268-1. [DOI] [PubMed] [Google Scholar]
- (a) Nilsson U. J., Fournier E. J. L., Hindsgaul O. Bioorg. Med. Chem. 1998;6:1563–1575. doi: 10.1016/s0968-0896(98)00087-x. [DOI] [PubMed] [Google Scholar]; (b) Nilsson U. J., Fournier E. J. L., Fryz E. J., Hindsgaul O. Combi. Chem. High Throughput Screen. 1999;2:335–352. [PubMed] [Google Scholar]
- Previato J. P., Jones C., Xavier M. T., Wait R., Travassos L. R., Parodi A. J., Mendonca-Previato L. J. Biol. Chem. 1995;270:7241–7250. doi: 10.1074/jbc.270.13.7241. [DOI] [PubMed] [Google Scholar]
- Singh S., Scigelova M., Hallberg M. L., Howarth O. W., Schenkman S., Crout D. H. G. Chem. Commun. 2000:1013–1014. [Google Scholar]
- (a) Kartha K. P. R., Aloui M., Field R. A. Tetrahedron Lett. 1996;37:5175–5178. [Google Scholar]; (b) Kartha K. P. R., Aloui M., Field R. A. Tetrahedron Lett. 1996;37:8807–8810. [Google Scholar]; (c) Kartha K. P. R., Field R. A. Tetrahedron Lett. 1997;38:8233–8237. [Google Scholar]
- Campo V. L., Carvalho I., da Silva C. H. T. P., Schenkman S., Hill L., Nepogodiev S. A., Field R. A. Chem. Sci. 2010;1:507–514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.