Abstract
To develop protective immune responses against mucosal pathogens, the delivery route and adjuvants for vaccination are important. The host, however, strives to maintain mucosal homeostasis by responding to mucosal antigens with tolerance, instead of immune activation. Thus, induction of mucosal immunity through vaccination is a rather difficult task, and potent mucosal adjuvants, vectors or other special delivery systems are often used, especially in the elderly. By taking advantage of the common mucosal immune system, the targeting of mucosal dendritic cells and microfold epithelial cells may facilitate the induction of effective mucosal immunity. Thus, novel routes of immunization and antigen delivery systems also show great potential for the development of effective and safe mucosal vaccines against various pathogens. The purpose of this review is to introduce several recent approaches to induce mucosal immunity to vaccines, with an emphasis on mucosal tissue targeting, new immunization routes and delivery systems. Defining the mechanisms of mucosal vaccines is as important as their efficacy and safety, and in this article, examples of recent approaches, which will likely accelerate progress in mucosal vaccine development, are discussed.
Keywords: delivery system, mucosal adjuvant, secretory IgA
Mucosal immune system
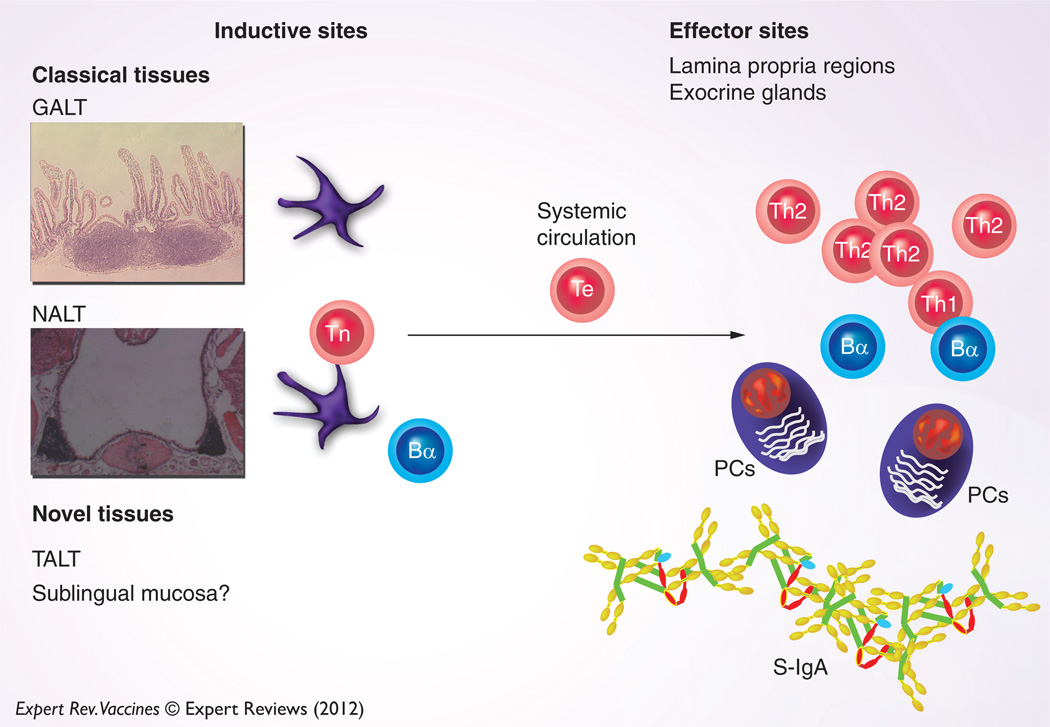
The mucosal immune system can be separated into inductive and effector sites based on the anatomical and functional properties. The migration of immune cells from mucosal inductive to effector tissues is the cellular basis for the common mucosal immune system (CMIS) (Figure 1). Thus, mucosal vaccination elicits immune responses in distant, multiple mucosal effector sites [1–5]. Mucosal inductive sites, including gut-associated lymphoreticular tissue (GALT) and nasopharyngeal-associated lymphoreticular tissue (NALT), collectively comprise a mucosa-associated lymphoreticular tissue (MALT) network for provision of a continuous source of memory B and T cells to mucosal effector sites [1,3–5]. The MALT contains T-cell zones, B cell-enriched areas containing a high frequency of surface IgA-positive (sIgA+) B cells and a subepithelial area with APCs for the initiation of specific immune responses. The MALT is covered by a follicle-associated epithelium that consists of a subset of differentiated microfold (M) epithelial cells, columnar epithelial cells and lymphoid cells, which play a central role in the initiation of mucosal immune responses. M cells take up antigens (Ags) from the lumen of the intestinal and nasal mucosa and transport them to the underlying APCs, including dendritic cells (DCs). In addition, recent studies have now identified isolated lymphoid follicles (ILFs) in the mouse small intestine. The ILFs have been identified as a part of GALT and as such are a mucosal inductive tissue [6,7]. These ILFs mainly contain B cells, DCs and M cells in the overlying epithelium. In addition, most recent studies showed that tear duct-associated lymphoreticular tissue (TALT) and conjunctiva-associated lymphoreticular tissue (CALT) play a role as mucosal inductive tissues [8,9]. Mucosal effector sites, including the lamina propria regions of the GI, the upper respiratory (UR), and reproductive tracts, secretory glandular tissues and intestinal intraepithelial lymphocytes, contain Ag-specific mucosal effector cells such as IgA-producing plasma cells and B and T cells.
Figure 1. Concept of mucosal inductive and effector sites: when mucosal immunization is initiated, Ags are taken up by mucosal inductive tissues (GALT, NALT and TALT).
This is an initial step for eliciting Ag-specific S-IgA Ab responses in mucosal effector tissues. DCs in mucosal inductive tissues play a major role as APCs for the activation of naive CD4+ T cells. In addition, ingested Ags activate IgA-committed B cells. Activated CD4+ T cells and IgA-committed B cells dispatch from mucosal inductive tissues and migrate into the mucosal effector tissues and subsequently interact for the terminal differentiation of IgA-committed B cells into IgA-producing plasma cells. In addition to the classical mucosal inductive tissues, the SL mucosa can initiate mucosal immune responses.
Bα: IgA-committed B cell; GALT: Gut-associated lymphoreticular tissue; MALT: Mucosa-associated lymphoreticular tissue; NALT: Nasopharyngeal-associated lymphoreticular tissue; PC: Plasma cell; Th1: Type 1 helper CD4+ T cell; Th2: Type 2 helper CD4+ T cell; Te: Effector CD4+ T cell; Tn: Naive CD4+ T cell.
Secretory (S)-IgA antibody (Ab) is a major player in the mucosal immune system and is locally produced in effector tissues [1,2,5,10–12]. The presence of Ag-specific S-IgA Abs at mucosal effector sites other than the inductive sites where initial Ag sampling occurred is definitive evidence for the CMIS. To this end, immunization of GALT or NALT effectively elicits Ag-specific mucosal IgA Ab responses in diverse mucosal effector tissues with some notable differences. Indeed, activated T cells in Peyer’s patches (PPs) preferentially express α4β7 and CCR9 as gut-homing receptors for their migration into the intestinal lamina propria [13–16]. In this regard, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the ligand for α4β7, mediates T-cell recruitment into the intestinal endothelium [17]. Furthermore, small intestinal epithelial cells express the CCR9 ligand, thymus-expressed chemokine. Recent studies demonstrated that retinoic acid-producing DCs in PPs and the mesenteric lymph nodes (MLNs) are key players in the enhancement of α4β7 and CCR9 expression by Ag-specific effector CD4+ T cells, which in turn guides their migration into the intestinal lamina propria [18]. In addition to mucosal T-cell homing, retinoic acid-producing DCs in PPs regulate T cell-independent IgA class switching and gut-homing receptor expression on B cells [19,20]. These findings clearly show that the CMIS exhibits distinct sites for induction and regulation of S-IgA Ab responses in mucosal effector tissues.
Although it has been shown that GALT and NALT share common features, it is also clear that a compartmentalization occurs between the oral and nasal immune systems [21–23]. Thus, oral immunization mainly elicits Ag-specific immune responses in the small intestine, in the proximal part of the large intestine, mammary and salivary glands, whereas nasal immunization induces mucosal immunity in the UR tract, nasal and oral cavities, and the cervicovaginal mucosa [21–23]. Furthermore, the organogenesis, lymphocyte trafficking and progression of immunosenescence in PPs and NALT are distinctly regulated [11,13,15,24–35]. Thus, the PPs develop between embryonic days 14 and 17 in an IL-7-IL-7Rα and LTα1β2-LTβR signaling cascade-dependent manner, whereas NALT organogenesis occurs postnatally in the absence of these cytokine cascades [28,32,34,35]. Furthermore, both Id2 and retinoic acid receptor-related orphan receptor-γt transcripts are essential for PP inducer cell development; however, NALT inducer cells require only Id2 [28,36–38]. In addition, activated T and B cells in PPs preferentially express α4β7 and CCR9 as gut-homing receptors, which help guide their migration back to the intestinal lamina propria [13,15]. In contrast, CD62L, α4β1 and CCR10 preferentially control the migration of T and B cells from NALT into the UR tract effector tissues [24,25,32,33]. The compartmentalization of GI and UR tract immune systems is also evident because distinct differences in mucosal aging occurred between the GI and UR tract immune systems [26,27,29–31]. Thus, age-associated alterations, including a reduction in number of PPs and the level of intestinal Ag-specific S-IgA Abs, occur in mice during aging [26,27]. Furthermore, mice lose oral tolerance, which represents another important mucosal immune regulatory function for maintaining systemic homeostasis to orally administered Ags during the aging process (6–12 months) [26,27,30,31]. In contrast, NALT shows a more intact immune response during aging (1-year-old mice), with signs of immunosenescence noted only in mice older than 2 years [26,27,29].
Because mucosal immunization induces not only Ag-specific mucosal S-IgA Abs but also systemic IgG Abs, developing mucosal vaccines could be used in much the same way as currently available licensed parenteral vaccines. Thus, mucosal vaccine delivery can induce systemic T-cell and Ab responses in peripheral lymphoid tissue, as is seen after parenteral vaccine delivery. However, simultaneous induction of mucosal immunity provides a dual protection against pathogens. Furthermore, mucosal adjuvants and delivery systems are essential to induce Ag-specific immune responses in both mucosal and systemic compartments by avoiding induction of systemic unresponsiveness. This review focuses on several recent approaches to induce mucosal immunity to vaccines, with emphasis on mucosal tissue targeting, new immunization routes and delivery systems that are both effective and safe. As a mucosal targeting strategy, DCs and M cells are discussed as the two major targeting cell types. Although a large number of DC-targeting components have been studied as mucosal adjuvants, CpG oligodeoxynucleotides (CpG ODN) and Flt3 ligand (FL) are selected based on their effectiveness and safety. Importantly, the cellular and molecular mechanisms for these two DC-targeting mucosal adjuvants and an M cell-targeting vaccine delivery system have been well described. In contrast, the precise mechanisms for sublingual (SL) immunization, eye drops, and rice-based and nanogel delivery systems remain to be elucidated; however, the early results are promising. In summary, these novel strategies are attractive and exhibit high potential from a practical point of view. More extensive reviews, which include additional targeting strategies, adjuvants, and delivery systems, are provided. Some specific details are essential to understand the cellular and molecular mechanisms involved in using these novel vaccine strategies.
Targeting vaccines
Mucosal DCs
DCs play a central role in bridging the innate immune system with the adaptive immune system [39–42]. Thus, DCs are found throughout the body and are especially prominent at mucosal surfaces. Immature type DCs are enriched underneath the epithelium of mucosal inductive sites and are poised to capture Ags. When Ag uptake occurs, these DCs change their phenotype by expressing higher levels of MHC class II and costimulatory molecules and move to T-cell areas of inductive sites for Ag presentation. Thus, DCs and their derived cytokines play key roles in the induction of Ag-specific effector Th-cell responses. In this regard, targeting mucosal DCs is not only an effective strategy to induce mucosal immunity but also a safe approach, especially for nasal application, because vaccines mainly initiate immune responses through DCs in the absence of central nervous system toxicity.
Because of the recent progress in the understanding of innate immunity-associated molecules, toll-like receptor (TLR) ligands are now considered to be candidates as potent mucosal adjuvants. Among these, the TLR9 ligand CpG ODN is known to target professional plasmacytoid DCs for their activation, maturation and subsequent induction of Ag-specific Th1-type responses, including cytotoxic T lymphocytes (CTLs) [43,44]. It has been demonstrated that synthetic CpG ODNs can induce innate immune responses [45–48]. In this regard, CpG ODNs as effective immunomodulators, could target malignant tumors, and reduce allergic responses [49,50]. Furthermore, CpG ODNs have been used as potent adjuvants to elicit Ag-specific Ab and cell-mediated immune responses in mice and rats against both bacterial and viral Ags [51–58]. To this end, mucosal administration of CpG ODN exhibits potent adjuvant activity (Figure 2). Mucosal immunization with CpG ODN plus formalin-inactivated influenza virus, hepatitis B virus surface Ag, or tetanus toxoid effectively elicited vaccine-specific immunity in the mucosal compartment of mice [57–59]. CpG ODN as adjuvant mainly induces Th1-type responses. In this regard, CpG ODN could even switch a predominant Th2 into a Th1-type immune response pathway [60]. Although the detailed mechanisms of adjuvant activity of CpG ODN are still unclear, it has been demonstrated that CpG ODN enhanced MAPK-mediated IL-12 production by APCs [61]. Others also clearly showed that nasal immunization with the recombinant protective Ag of the anthrax lethal toxin and CpG ODN induced protective Ag-specific plasma IgG2a and mucosal S-IgA Ab responses with in vitro neutralizing activities [62].
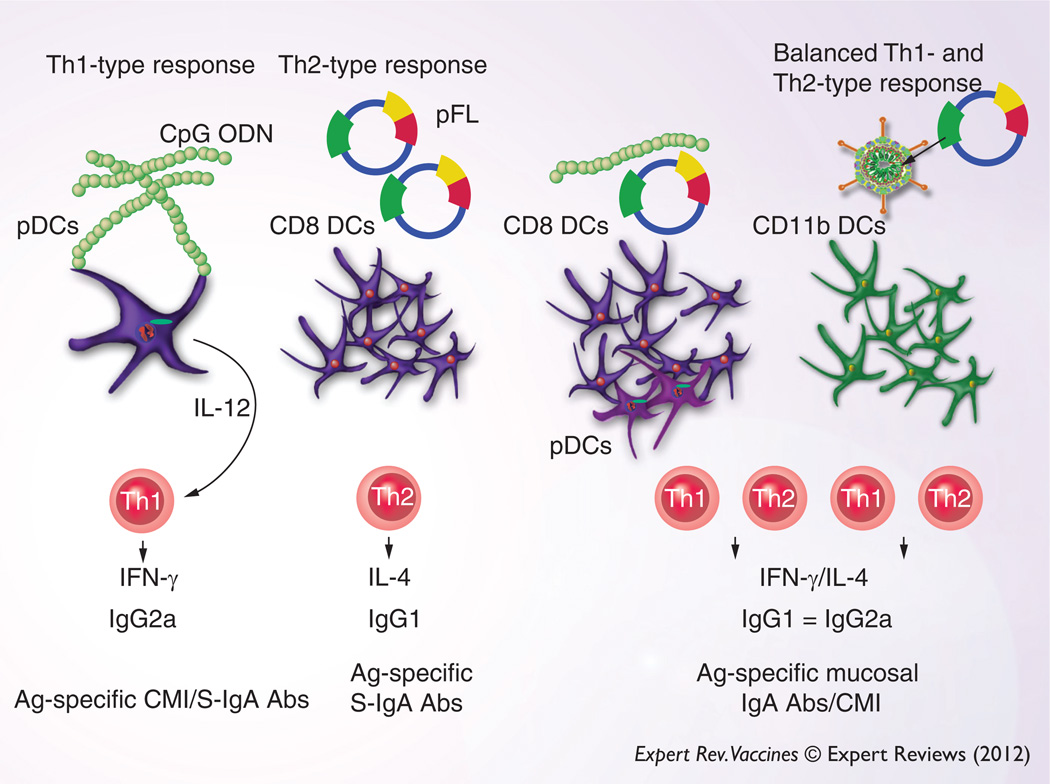
Figure 2. Nasal DC-targeting mucosal vaccines: nasal application of CpG ODN activates plasmacytoid DCs (pDC, B220+ DCs) for the induction of Th1-type cytokine responses.
Thus, CMI and cytotoxic T lymphocyte (CTL) activity can be elicited in addition to Ag-specific S-IgA Ab responses. In contrast, pFL as nasal adjuvant preferentially expands the CD8+ DC subset and subsequently elicits Th2-type cytokine-mediated Ag-specific S-IgA Ab responses. Adenovirus expressing FL (Ad-FL) or a combination of CpG ODN and pFL induces a more balanced Th1- and Th2-type immune response. Ad-FL activates CD11b+ CD11c+ DCs, whereas a combined nasal CpG ODN and pFL stimulates both CD8+ DCs and pDCs for the induction of CMI and S-IgA Ab responses.
Abs: Antibodies; Ag: Antigen; CMI: Common mucosal immune; CPG ODN: CpG oligodeoxynucleotides; DC: Dendritic cell; pDC: Plasmacytoid dendritic cell; pFL: Plasmid-expressing Flt3 ligand; S-IgA: Surface IgA.
FL is a growth factor that binds to the fms-like tyrosine kinase receptor Flt3/Flk2. In vivo FL treatment markedly upregulates the number of DCs but not their activation [63,64]. Mouse FL has been cloned and shown to be a key player in the proliferation and differentiation of early hematopoietic precursor stem cells [63,65–68]. Furthermore, it has been reported that FL could mobilize and stimulate not only DCs [64] but also natural killer cells and B cells [69]. Of interest, it was first reported that systemic FL injection facilitated oral tolerance induction because of its ability to result in significant increases in the number of DCs in several lymphoid tissues, including the intestinal lamina propria, PPs, MLNs, and spleen [70,71]. In contrast to tolerance induction, others showed that FL treatment also upregulated immune responses when delivered via mucosal [71], systemic [72], or cutaneous [73] routes. It has also been reported that when plasmid DNA encoding FL (pFL) was coadministered with plasmids encoding protein Ags or linked to the Ag itself, effective immune responses were induced [74,75]. In this regard, it has been suggested that FL possesses adjuvanticity for both humoral and cell-mediated immune responses and that the FL cDNA system may be a potential alternative approach to using the FL protein system [76–79]. To this end, pFL has been used as a mucosal DC-targeting adjuvant for the induction of Ag-specific protective mucosal immune responses (Figure 2). Nasal administration of pFL as mucosal adjuvant facilitated expansion of CD8+ DCs, which subsequently elicited IL-4-producing CD4+ T-cell- and Ag-specific S-IgA Ab responses [80]. NALT has been the major site for sampling pFL and for producing the FL protein locally, which subsequently induced the expansion and activation of DCs [80]. In this regard, pFL did not show any potential to migrate into the CNS.
Other types of FL-based NALT-DC-targeting immune modulators, including an adenovirus serotype 5 vector expressing FL (Ad-FL), were found to elicit Th1- and Th2-type responses, thereby providing both Ag-specific S-IgA Ab and cell-mediated immune responses [81]. When mice were nasally immunized with ovalbumin (OVA) and Ad-FL, high levels of Ag-specific Ab responses were elicited in both mucosal and systemic compartments. Furthermore, significantly increased levels of Ag-specific IFN-γ and IL-4 production were noted in cervical lymph nodes and spleen [81]. Because of OVA-specific Th1-type cytokine responses, Ag-specific CTL responses were upregulated in mice administered with nasal OVA and Ad-FL. Interestingly, the number of CD11b+ CD11c+ DCs was preferentially increased. This DC subset expressed high levels of costimulatory molecules and migrated from the NALT to mucosal effector tissues [81]. These findings show that nasal administration of Ad-FL facilitated the induction of mature-type CD11b+ CD11c+ DCs and Th1- and Th2-type CD4+ T cells in the NALT for Ag-specific Ab and CTL responses (Figure 2). Balanced Th1- and Th2-type responses have become key issues in mucosal vaccine development because this type of cytokine response would not only provide Ag-specific S-IgA Ab and CTL responses against viral and bacterial infections but also avoid induction of allergic (IgE) and inflammatory-type responses.
CpG ODN has been shown to induce polarized Th1-type cytokine responses in mice [62]. In contrast, pFL preferentially elicits coadministered Ag-specific Th2-type cytokine immunity [80]. To this end, one could hypothesize that an ideal but balanced Th1- and Th2-type cytokine response would be elicited by using a combination of pFL and CpG ODN as DC-targeting nasal adjuvants. Indeed, recent studies clearly showed that pFL and CpG ODN as a combined nasal adjuvant induced the activation and expansion of plasmacytoid DCs and CD8+ DCs in the nasal cavity for the development of Th1- and Th2-type cytokine-producing CD4+ T cells. Thus, these Ag-specific CD4+ T cells successfully upregulated coadministered Ag-specific immunity in both the mucosal and systemic immune compartments (Figure 2) [82,83]. Increased frequencies of mature-type DCs in NALT correlated well with induction of Ag-specific immune responses. Of significance, nasal delivery of pFL and CpG ODN successfully elicited significant levels of Ag-specific S-IgA Ab responses in 2-year-old mice [82,83]. To this end, aged mice given nasal pneumococcal surface protein A and a combination of pFL and CpG ODN showed protective immunity against nasal Streptococcus pneumoniae colonization [83]. These results suggest that nasal administration of pFL and CpG ODN as mucosal adjuvants provides an attractive possibility for the development of a vaccine against S. pneumoniae in the elderly.
M cells
As discussed earlier, GALT, including PPs, is covered by a specialized follicle-associated epithelium, 10–20% of which is composed of M cells that show a unique topical morphology (microfold/membranous) and form pockets for the inclusion of lymphoid cells, including B and T cells, DCs, and macrophages [84–89]. M cells show significantly different features compared with intestinal epithelial cells. M cells possess relatively short microvilli, small cytoplasmic vesicles and few lysosomes. Thus, M cells are able to capture and transport lumenal Ags, including viruses, bacteria, small parasites, and microspheres [86,87,89,90]. It has been suggested that M cells may also play a role as APCs because M cells express MHC class II molecules and acidic endosomal–lysosomal compartments [91]. In this regard, activation and potential MHC class II expression by M cells may depend on the nature of endocytosed Ag. M cells serve not only for transport of lumenal Ags but also for provision of an entry way for pathogens to invade the host. In particular, it has been shown that invasive but not noninvasive strains of Salmonella typhimurium enter the host through PP M cells [92]. In addition to PPs, the ILFs and NALT also contain a lymphoepithelium with M cells. Thus, Mycobacterium tuberculosis uses NALT M cells for host entry [93]. In addition, it was reported that M cells are also detected in nonlymphoid follicle-associated epithelium that covers small intestinal villi [94]. Thus, villous M cells in the small intestine were present in several PP-deficient mouse strains, including in utero LT- βR-Ig-treated, LT-α−/−, TNF/LT-α−/− and inhibition of differentiation 2 (Id2)−/− mice [94]. Importantly, these villous M cells functionally take up bacteria and induce bacterial Ag-specific immune responses [94]. Indeed, the MLNs from PP-deficient mice play a key backup role as a mucosal inductive tissue [95]. It has been suggested that MHC class II+ sIgA+ B cells and lamina propria macrophages may be able to capture Ag through endocytic pathways and process and present peptides to CD4+ Th cells. These findings clearly suggest that the intestinal lamina propria–MLN axis performs a potent mucosal inductive function in addition to the PPs.
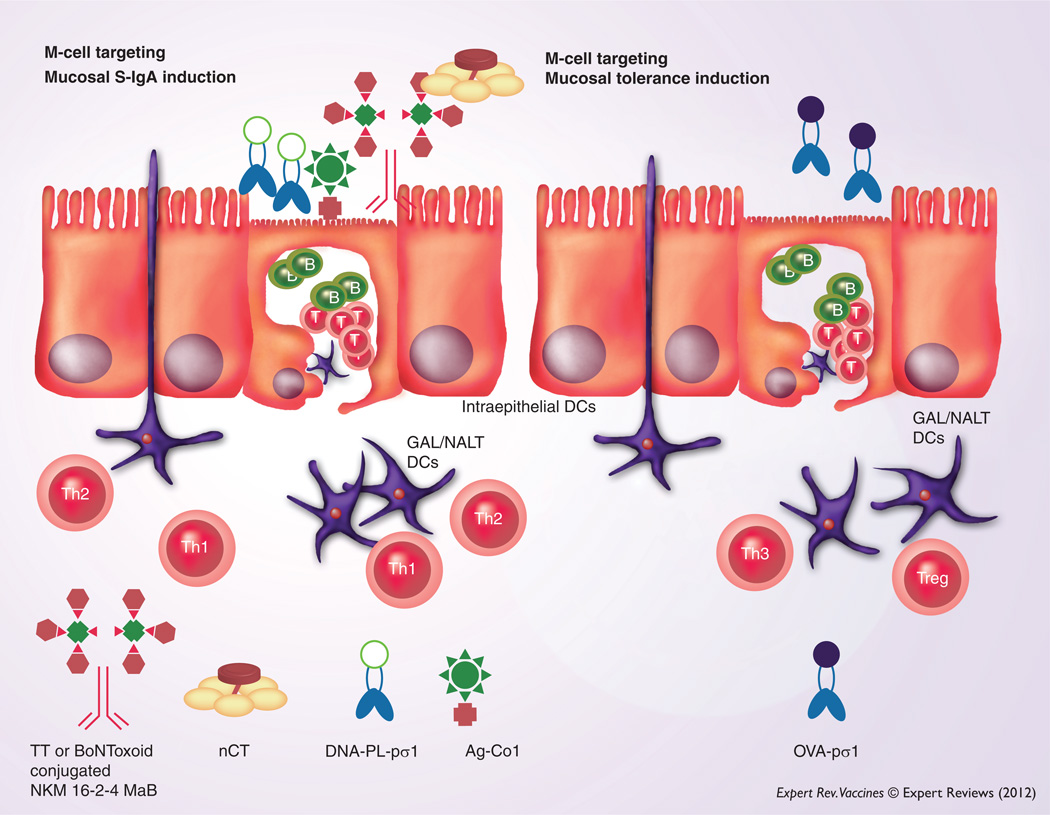
If one could identify the key molecules expressed by bacteria and viruses that are needed for their invasion or infection of M cells, it would be a great advantage for designing and constructing effective delivery systems for M-cell targeting of vaccines. Reoviruses initially infect the mouse through M cells [96], by using their surface protein sigma-1 (pσ1) [97,98]. In this regard, an M cell- targeting DNA vaccine complex consisting of plasmid DNA and the reovirus pσ1 covalently attached to poly-l-lysine induced significant mucosal S-IgA Ab responses and systemic immunity (Figure 3) [99]. Furthermore, a newly developed M cell-specific monoclonal Ab (NKM 16-2-4) was used as an M cell-targeting carrier for mucosal vaccines. Thus, oral administration of a chimeric vaccine consisting of NKM 16-2-4 and tetanus toxoid or botulinum neurotoxin type A toxoid (BoNToxoid/A), together with native cholera toxin, elicited increased levels of Ag-specific S-IgA and plasma IgG Ab responses (Figure 3) [100]. Importantly, oral immunization with BoNToxoid/A-NKM 16-2-4 provided protective immunity against lethal challenge with botulinum neurotoxin [100]. In addition, oral immunization of Ag fused with M cell-targeting peptide ligand (Co1) resulted in enhanced Ag-specific immune responses [101]. These studies show that an M cell-targeting delivery system may be of central importance in developing effective mucosal vaccines. Furthermore, it is likely that M cells are also involved in the induction of oral tolerance. In this latter regard, one must carefully consider the nature of formulation of vaccine (or inclusion of adjuvant) because both nasal and oral administration of pσ1 of reovirus genetically conjugated with OVA (OVA-pσ1) alone induced systemic unresponsiveness instead of mucosal IgA immunity (Figure 3) [102,103]. Thus, mucosally induced tolerance was achieved with doses as low as 10–50 µg of OVA-pσ1 when given by the nasal or oral routes [102,103].
Figure 3. Potential for an M cell-targeting strategy: mucosal M-cell targeting by M cell-specific monoclonal antibody or surface proteins can facilitate Ag delivery for the induction of Ag-specific S-IgA antibody responses to provide effective immunity at the entry site of pathogens.
M-cell targeting is achieved by using the protein sigma-1 (pσ1) from reovirus, the ligand for M cell-specific peptide (Co1) or M cell-specific mAb. However, mucosal administration of genetically conjugated OVA protein with pσ1 in the absence of an adjuvant elicits mucosal tolerance.
Ag: Antigen; DC: Dendritic cell; GAL: Gut-associated lymphoreticular; mAb: Monoclonal antibody; NALT: Nasopharyngeal-associated lymphoreticular tissue; S-IgA: Surface IgA; Th1: Type 1 helper CD4+ T cell; Th2: Type 2 helper CD4+ T cell; Th3: Type 3 helper CD4+ T cell; Treg: T regulatory cell.
Mucosal delivery systems
MucoRice
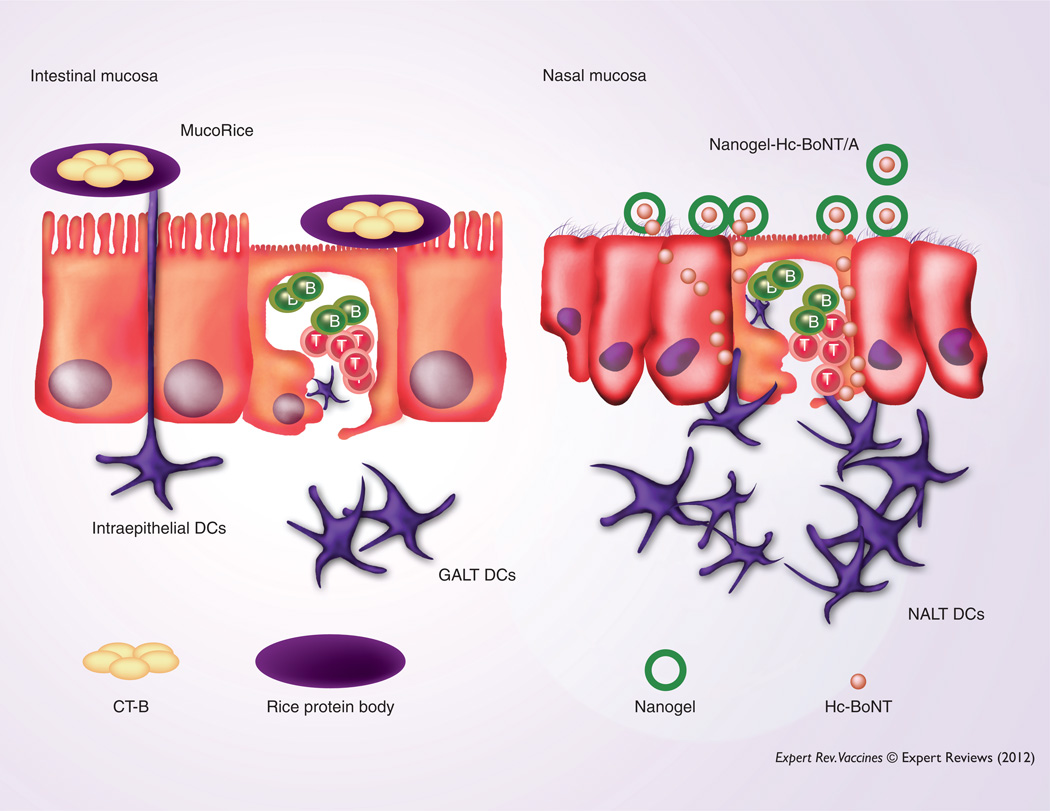
In 1997, Curtiss and Cardineau successfully filed for and received a US patent (5686079) describing tobacco leaves expressing Streptococcus mutans surface protein Ag as an initial indication of a potential plant-based mucosal vaccine. Furthermore, others have developed edible plant-based vaccines by expressing Ags from enterotoxins, hepatitis B, Norwalk virus and respiratory syncytial virus expressed in tobacco leaves or potato tubers [104–111]. Although these plant-based vaccines exhibited some functional properties in experimental systems, their practical application still remains to be elucidated. To develop practical oral vaccines for global immunization, one should consider that the vaccine must maintain effectiveness despite in vivo and ex vivo environmental changes. In this regard, several practical merits can be found in a rice-based oral vaccine compared with most traditional and other plant-based vaccines. For example, a rice-based vaccine is a rather safe approach. Because this vaccine can be given in a powder form, one could avoid potential problems by using a food-based delivery system. Although the lot-to-lot quality control of a rice-based vaccine may be challenging, stable vaccine Ag expression could be achieved by the third generation of rice-based vaccine. Furthermore, a rice-based vaccine showed stability at room temperature for 2–3 years [112,113]. Oral administration of this rice-based vaccine did not lose activity when exposed to digestive enzymes and subsequently induced protective, Ag-specific Ab responses in mice and non-human primates [112–115]. Recent studies have provided direct evidence that oral MucoRice-cholera toxin B-subunit (CT-B) induced Ag-specific S-IgA Abs that played a critical role in protection against CT-induced diarrhea (Figure 4) [113]. Importantly, cold chain-free oral MucoRice-CT-B induced long-lasting cross-protective immunity against heat-labile enterotoxin-producing enterotoxigenic Escherichia coli in addition to CT-producing Vibrio cholerae [113]. These results demonstrate that oral administration of a rice-based vaccine provides a potent practical global strategy for the development of cold chain- and needle-free vaccines that protect from gastrointestinal infection.
Figure 4. Novel mucosal delivery systems: a plant-based MucoRice-CT-B vaccine effectively induces CT-B-specific protective immunity when orally administered.
Because CT-B can be delivered to the small intestine in the rice protein body, MucoRice-CT-B effectively induced CT-B-specific Ab responses in the absence of the CT-A subunit or other potential adjuvants. The cationic nanogel-Hc-BoNT/A is retained for a longer period at the nasal epithelium for slow release of Ag when nasally administered. Thus, nasal APCs, including DCs, can more effectively take up Hc-BoNT/A to initiate Ag-specific immune responses.
DC: Dendritic cell; GALT: Gut-associated lymphoreticular tissue; NALT: Nasopharyngeal-associated lymphoreticular tissue.
Nanogels
The application of biomaterials, such as encapsulating Ags in polymer nanoparticles, microparticles, virosomes and liposomes, shows significant potential in the development of vaccines and immunotherapy [116–123]. Although use of liposomes can enhance Ag delivery across mucosal surfaces, they are rapidly cleared and do not allow for long-term Ag release at the mucosal surface [124–126]. In this regard, it is possible that using a bioadhesive gel one could upregulate the residence time and enhance Ag release and retention onto the epithelial cells themselves. Indeed, it has been shown that surface modifications or coadministration with bioadhesive materials, that is, chitosan, resulted in influenza-specific S-IgA Ab responses in nasal washes [127]. A nanometer-sized (<100 nm) bioadhesive polymer hydrogel (nanogel) system has been developed and used as an attractive drug delivery system [128]. Cholesteryl group-bearing pullulan (CHP) form self-assembly of associating polymers as physically crosslinked nanogels in water [129,130]. In general, hydrophobic interactions between CHP and various proteins revealed a CHP nanogel containing the protein inside. When CHP nanogels capture the proteins inside, they form a hydrated nanogel polymer network (nanomatrix) without aggregation. In this regard, trapped proteins maintained their native form and were slowly released [130]. On the basis of these advantages, a CHP nanogel strategy has been used for the development of adjuvant-free nasal vaccines. It was recently shown that nasal administration of a cationic type of CHP nanogel (cCHP nanogel) containing the C-terminus of the H chain (Hc) of botulinum neurotoxin-type A (BoNT/A; nanogel-Hc-BoNT/A) allowed adherence to the nasal epithelium for a longer period compared with naked Hc-BoNT/A (Figure 4) [131]. In this regard, gradually released Hc-BoNT/A was effectively taken up by mucosal APCs and subsequently elicited protective Ag-specific S-IgA Ab responses against BoNT/A intoxication [131]. In summary, this cCHP nanogel system could represent an ideal and effective mucosal delivery system to enhance pathogen-specific mucosal immune responses at the mucosal surface. Because vaccine Ags are retained for a longer period at mucosal surfaces, it is essential to consider the potential side effects of this delivery system in future applications.
Mucosal immunization routes
SL immunization
Oral and nasal routes have been the preferred ones to induce protective immunity in different mucosal compartments [1,5,21]. However, it has been demonstrated that rectal, vaginal or paramucosal (iliac and inguinal lymph nodes) immunization are also effective strategies for the induction of protective immunity against sexually transmitted infectious diseases, including HIV [132–135]. In addition to these mucosal immunization routes, SL administration of Ags has been used to treat allergic, autoimmune or infection-induced pathologic reactions [21,136], by taking advantage of the induction of oral tolerance [137–140]. It is well known that nasal immunization effectively elicits Ag-specific immunity in both mucosal and systemic compartments; however, one must consider that some nasal immunization strategies risk Ag trafficking into olfactory tissues and the CNS [141–145]. To obviate this potential problem, SL immunization may be an ideal mucosal Ag delivery system that avoids CNS involvement. SL administration is also a noninvasive route that has the advantage of requiring lower doses of Ag than the oral route because of the reduced exposure to proteolytic enzymes and lower pH of the stomach encountered after oral immunization. Furthermore, vaccine uptake may be more efficient based on the number of APCs present at the SL site [138]. Recently, studies have used the SL route for vaccine delivery [146–151]. When plasmid DNA encoding hepatitis B surface Ag was sublingually administered to mice, comparable levels of Ag-specific humoral and CD8+ CTL responses were induced as seen after intradermal injection [147]. The SL delivery of a soluble Ag 2,4-dinitrophenyl bovine serum albumin in starch microparticles in combination with a penetration enhancer resulted in good salivary IgA Ab responses [148]. Finally, SL delivery of lipopeptides induced increased serum Abs and T-cell responses in the spleen and inguinal lymph nodes of mice [146]. Compared with subcutaneous administration of the same vaccine preparation, SL application preferentially induced IFN-γ-producing T cells and IgG2a Ab responses, whereas subcutaneous injection elicited IL-4 and IgG1 Ab responses [146]. More recently, SL immunization with influenza virus successfully elicited influenza-specific immunity and provided protection against lethal viral infection [150]. Furthermore, SL immunization with the outer membrane protein of Porphyromonas gingivalis plus the plasmid expressing FL cDNA (pFL) elicited increased frequencies of DCs in submandibular lymph nodes and protective immunity in the oral cavity [151]. In addition, CCR7-expressing DCs in cervical lymph nodes were the key players in the induction of Ag-specific immune responses [149]. These findings show that by using the appropriate quantity and form of Ag with a targeted delivery system, the SL route could be the preferred one for inducing both mucosal and systemic immunity, without induction of T-cell unresponsiveness.
Eye drops
The ocular surface leading to the lacrimal sac and nasolacrimal duct also forms an interface with the outside environment. In fact, it has been proposed that CALT, together with TALT, organizes eye-associated lymphoreticular tissue to create mucosal surveillance and a barrier in the eye region of humans [152,153]. Although TALT develops in human tear ducts, little information was available on mouse TALT until recently. Thus, it was reported that TALT is located in the murine lacrimal sac covered by an epithelium with M cells for Ag uptake [8]. The administration of Ags using eye drops induced Ag-specific S-IgA Ab responses in both ocular and nasal cavities in addition to serum IgG Abs because of the presence of TALT in the conjunctival sac, located in the tear duct, which bridges the ocular and nasal cavities [8,152–154]. Ocular administered Ags migrate to tear ducts and then to the nasal cavity and thus are taken up by TALT and NALT M cells for the induction of Ag-specific immune responses. Past investigations tended to emphasize the identification and characterization of CALT [153,155–158]. Unlike other mammals (e.g., cat, dog, and human), mice and rats do not possess CALT [155]. However, recent findings showed that eye drop administration of Ag induced CALT development in mice with increased numbers of M cell-like cells [9]. Although it remains unclear whether eye drop immunization induces potential adverse effects, including inflammatory responses, it was reported that the administered Ag did not migrate into the CNS [9]. Taken together, these findings clearly showed that eye drop administration of vaccine would be a novel strategy for the induction of Ag-specific mucosal immune responses, if inflammatory responses could be avoided.
Expert commentary
The CMIS provides both an essential concept and a practical means for the development of mucosal vaccines. Thus, it is essential to effectively activate mucosal inductive tissues or MALT for effective mucosal immunity. For targeting MALT, different routes of mucosal immunization have been developed and shown to successfully elicit protective mucosal immunity against several pathogens. However, one could easily fail to elicit protective mucosal immunity without a better understanding of the cellular and molecular mechanisms that regulate the mucosal immune system. Thus, one must carefully consider the route of immunization, the adjuvant and method of Ag delivery to elicit appropriate and desired mucosal immune responses to a particular pathogen. For example, oral vaccination may have fewer side effects and be the most preferred immunization route from a practical point of view; however, oral vaccines require that one maintain their original quality and efficacy until they reach the GALT, because the GI tract represents a harsh environment. In this regard, the MucoRice delivery system could be potentially beneficial for oral vaccine development. Thus, it is important to test whether this system can be easily adapted to other types of vaccine Ags. Nasal vaccines must be safe and not be taken up by the CNS because the nasal immunization route has an advantage for the induction of Ag-specific S-IgA Ab responses in the elderly. Indeed, targeting DCs or M cells in the MALT not only facilitates Ag uptake but also avoids potential CNS toxicity. Furthermore, SL and eye drop immunization successfully elicit mucosal immunity without serious toxicity or side effects so far. Novel delivery systems significantly enhance Ag uptake by MALT for the induction of Ag- or pathogen-specific mucosal immunity. However, the precise cellular and molecular mechanisms for these immunization systems in the induction of mucosal immunity still remain to be elucidated. Nevertheless, it is possible that a strategy that uses the appropriate combination of mucosal adjuvants and delivery systems and optimizes the immunization schedule by repeating and combining different routes of mucosal immunization as a primary and boosting strategy could lead to development of a new generation of safe and effective mucosal vaccines.
Five-year view
Mucosal vaccination is a needle- and medical waste-free vaccine strategy that provides protective immunity against pathogenic bacteria and viruses in both mucosal and systemic compartments. However, mucosal vaccines must overcome two major hurdles (effectiveness and safety), which are both relatively difficult tasks compared with systemic vaccine development because of the uniqueness of the mucosal environment. Future global warming could introduce unexpected pathogens, such as the malaria parasite, into new areas where they have never been seen causing pandemic infectious diseases. Furthermore, some of the currently available vaccines, including nasal FLuMist, are less effective in the immunocompromized population such as young children and the elderly. These facts indicate that the development of novel mucosal vaccines have the potential to provide a better quality of life. According to current knowledge of mucosal vaccines, an appropriate combination of several mucosal vaccine strategies could facilitate the development of practical vaccines over the next 5 years. However, one must realize that developing licensed products is a time-consuming and difficult task from the point of view of a promising outcome. Furthermore, more intensive vaccine development studies need to be performed using novel approaches such as SL immunization, eye drop delivery, nanomatrix and plant-based delivery systems because recent evidence supports both their effectiveness and safety.
Key issues.
The concept of a common mucosal immune system with specialized compartments is required for the development of effective mucosal vaccines.
Mucosal vaccines elicit immune defense in both mucosal and systemic tissue compartments.
Mucosal adjuvants and delivery systems are needed for the induction of more effective mucosal immune responses.
Targeting mucosal dendritic cells is an effective and safe strategy for inducing antigen-specific immunity.
New routes of mucosal immunization and antigen delivery systems should facilitate mucosal vaccine development.
A combination of appropriate mucosal vaccine strategies is essential for future mucosal vaccine development.
Acknowledgement
The authors thank Ms Sheila D Turner for preparation of the manuscript.
Parts of research described in this manuscript were supported by US NIH grants AG 025873 and DE 012242 and the Japan Society of the Promotion of Science program entitled “Young Researcher Overseas Visits Program for Vitalizing Brain Circulation”, Global Center of Excellence Program “Center of Education and Research for the Advanced Genome-based Medicine – for personalized medicine, the control of worldwide infectious diseases – MEXT,” Japan, a grant from the Global Center of Excellence and “Academic Frontier” Project for Private Universities Matching Fund Subsidy from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Research for Promoting Technological Seeds from Japan Science and Technology Agency (13-043), the Japan Foundation for Pediatric Research and Houjinkai fellowship award of the Department of Pediatrics at Osaka City University Graduate School of Medicine, and The Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was used in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1. Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–5484. doi: 10.1016/j.vaccine.2006.12.001. • Describes the features of the mucosal immune system, including the concept of the common mucosal immune system (CMIS).
- 2.Lamm ME. Current concepts in mucosal immunity IV. How epithelial transport of IgA antibodies relates to host defense. Am. J. Physiol. 1998;274(4 Pt 1):G614–G617. doi: 10.1152/ajpgi.1998.274.4.g614. [DOI] [PubMed] [Google Scholar]
- 3.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4(6):667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 4.Fujihashi K, Boyaka PN, McGhee JR. Host defenses at mucosal surfaces. In: Rich RT, et al., editors. Clinical Immunology. PA, USA: Mosby Elsevier; 2008. pp. 287–304. [Google Scholar]
- 5.Kiyono H, Kunisawa J, McGhee JR, Mestecky J. The mucosal imune system. In: Paul WE, editor. Fundamental Immunology. PA, USA: Lippincott Williams & Wilkins; 2008. pp. 983–1030. [Google Scholar]
- 6.Hamada H, Hiroi T, Nishiyama Y, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 2002;168(1):57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J. Immunol. 2003;170(11):5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 8.Nagatake T, Fukuyama S, Kim DY, et al. Id2-, RORγt-, and LTβR-independent initiation of lymphoid organogenesis in ocular immunity. J. Exp. Med. 2009;206(11):2351–2364. doi: 10.1084/jem.20091436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo KY, Han SJ, Cha HR, et al. Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. J. Immunol. 2010;185(6):3610–3619. doi: 10.4049/jimmunol.1000680. [DOI] [PubMed] [Google Scholar]
- 10.Lamm ME, Phillips-Quagliata JM. Origin and homing of intestinal IgA antibody-secreting cells. J. Exp. Med. 2002;195(2):F5–F8. doi: 10.1084/jem.20011910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 12.Mestecky J, Lue C, Russell MW. Selective transport of IgA. Cellular and molecular aspects. Gastroenterol. Clin. North Am. 1991;20(3):441–471. [PubMed] [Google Scholar]
- 13.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 2002;195(1):135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 1994;152(7):3282–3293. [PubMed] [Google Scholar]
- 15.Kantele A, Zivny J, Hakkinen M, Elson CO, Mestecky J. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 1999;162(9):5173–5177. [PubMed] [Google Scholar]
- 16.Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 2002;110(8):1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 18.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.McGhee JR, Kunisawa J, Kiyono H. Gut lymphocyte migration, we are halfway ‘home’. Trends Immunol. 2007;28(4):150–153. doi: 10.1016/j.it.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11 Suppl. 4:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 22.Kantele A, Hakkinen M, Moldoveanu Z, et al. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 1998;66(12):5630–5635. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu HY, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 1997;16(2):187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 24.Campbell JJ, Brightling CE, Symon FA, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J. Immunol. 2001;166(4):2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 25.Csencsits KL, Walters N, Pascual DW. Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: α(E) versus L-selectin. J. Immunol. 2001;167(5):2441–2445. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- 26. Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30(7):334–343. doi: 10.1016/j.it.2009.04.004. • Reviews mucosal vaccine development and compartmentalization of the mucosal immune system.
- 27.Fujihashi K, McGhee JR. Mucosal immunity and tolerance in the elderly. Mech. Ageing Dev. 2004;125(12):889–898. doi: 10.1016/j.mad.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Fukuyama S, Hiroi T, Yokota Y, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3− CD4+CD45+ cells. Immunity. 2002;17(1):31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 29.Hagiwara Y, McGhee JR, Fujihashi K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 2003;170(4):1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Fujihashi K, Kato R, et al. Lack of oral tolerance in aging is due to sequential loss of Peyer’s patch cell interactions. Int. Immunol. 2003;15(2):145–158. doi: 10.1093/intimm/dxg011. [DOI] [PubMed] [Google Scholar]
- 31.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J. Immunol. 2000;165:5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- 32.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29(11):505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Pascual DW, Riccardi C, Csencsits-Smith K. Distal IgA immunity can be sustained by αEβ7+ B cells in L-selectin−/− mice following oral immunization. Mucosal Immunol. 2008;1(1):68–77. doi: 10.1038/mi.2007.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 1996;184(5):1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Honda K, Shinkura R, et al. IL-7 receptor α+ CD3(−) cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int. Immunol. 1999;11(5):643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 36.Kurebayashi S, Ueda E, Sakaue M, et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA. 2000;97(18):10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Z, Unutmaz D, Zou YR, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 38.Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397(6721):702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 39.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 40.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 2001;166(9):5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22(1):41–47. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 42.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 43.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 44.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 45.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl Acad. Sci. USA. 1996;93(7):2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 1992;148(12):4072–4076. [PubMed] [Google Scholar]
- 48.Zimmermann S, Egeter O, Hausmann S, et al. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 1998;160(8):3627–3630. [PubMed] [Google Scholar]
- 49.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr. Opin. Investig. Drugs. 2003;4(6):686–690. [PubMed] [Google Scholar]
- 50.Kline JN, Waldschmidt TJ, Businga TR, et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 1998;160(6):2555–2559. [PubMed] [Google Scholar]
- 51.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl Acad. Sci. USA. 1998;95(26):15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 1998;160(2):870–876. [PubMed] [Google Scholar]
- 53.Eastcott JW, Holmberg CJ, Dewhirst FE, Esch TR, Smith DJ, Taubman MA. Oligonucleotide containing CpG motifs enhances immune response to mucosally or systemically administered tetanus toxoid. Vaccine. 2001;19(13–14):1636–1642. doi: 10.1016/s0264-410x(00)00422-9. [DOI] [PubMed] [Google Scholar]
- 54.Klinman DM. Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998;8(2):181–184. doi: 10.1089/oli.1.1998.8.181. [DOI] [PubMed] [Google Scholar]
- 55.Klinman DM, Barnhart KM, Conover J. CpG motifs as immune adjuvants. Vaccine. 1999;17(1):19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 56.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J. Immunol. 1999;162(3):1611–1617. [PubMed] [Google Scholar]
- 57.McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 1998;161(9):4463–4466. [PubMed] [Google Scholar]
- 58.Moldoveanu Z, Love-Homan L, Huang WQ, Krieg AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16(11–12):1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 59.McCluskie MJ, Weeratna RD, Krieg AM, Davis HL. CpG DNA is an effective oral adjuvant to protein antigens in mice. Vaccine. 2000;19(7–8):950–957. doi: 10.1016/s0264-410x(00)00215-2. [DOI] [PubMed] [Google Scholar]
- 60.Weeratna RD, Brazolot Millan CL, McCluskie MJ, Davis HL. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol. Med. Microbiol. 2001;32(1):65–71. doi: 10.1111/j.1574-695X.2001.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 61.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J. Immunol. 2002;168(9):4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]
- 62.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 2003;170(11):5636–5643. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 63.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 64.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83(10):2795–2801. [PubMed] [Google Scholar]
- 66.Pulendran B, Banchereau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165(1):566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 67.Robinson S, Mosley RL, Parajuli P, et al. Comparison of the hematopoietic activity of flt-3 ligand and granulocyte-macrophage colony-stimulating factor acting alone or in combination. J. Hematother. Stem Cell Res. 2000;9(5):711–720. doi: 10.1089/15258160050196759. [DOI] [PubMed] [Google Scholar]
- 68.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc. Natl Acad. Sci. USA. 1994;91(2):459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 70.Viney JL, Mowat AM, O’Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J. Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 71.Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 1999;163:3668–3675. [PubMed] [Google Scholar]
- 72.Pisarev VM, Parajuli P, Mosley RL, et al. Flt3 ligand enhances the immunogenicity of a gag-based HIV-1 vaccine. Int. J. Immunopharmacol. 2000;22(11):865–876. doi: 10.1016/s0192-0561(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 73.Baca-Estrada ME, Ewen C, Mahony D, Babiuk LA, Wilkie D, Foldvari M. The haemopoietic growth factor, Flt3L, alters the immune response induced by transcutaneous immunization. Immunology. 2002;107:69–76. doi: 10.1046/j.1365-2567.2002.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hung CF, Hsu KF, Cheng WF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 2001;61:1080–1088. [PubMed] [Google Scholar]
- 75.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 2002;76:243–250. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esche C, Subbotin VM, Maliszewski C, Lotze MT, Shurin MR. FLT3 ligand administration inhibits tumor growth in murine melanoma and lymphoma. Cancer Res. 1998;58(3):380–383. [PubMed] [Google Scholar]
- 77.Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat. Med. 1997;3(6):625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 78.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl Acad. Sci. USA. 1999;96(3):1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vollstedt S, Franchini M, Hefti HP, et al. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 2003;197(5):575–584. doi: 10.1084/jem.20021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 2004;172(6):3612–3619. doi: 10.4049/jimmunol.172.6.3612. [DOI] [PubMed] [Google Scholar]
- 81.Sekine S, Kataoka K, Fukuyama Y, et al. A novel adenovirus expressing flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J. Immunol. 2008;180(12):8126–8134. doi: 10.4049/jimmunol.180.12.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukuiwa T, Sekine S, Kobayashi R, et al. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. 2008;26(37):4849–4859. doi: 10.1016/j.vaccine.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukuyama Y, King JD, Kataoka K, et al. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J. Immunol. 2011;186(4):2454–2461. doi: 10.4049/jimmunol.1002837. [DOI] [PubMed] [Google Scholar]
- 84.Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am. J. Anat. 1973;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 85.Farstad IN, Halstensen TS, Fausa O, Brandtzaeg P. Heterogeneity of M-cell-associated B and T cells in human Peyer’s patches. Immunology. 1994;83(3):457–464. [PMC free article] [PubMed] [Google Scholar]
- 86.Gebert A, Rothkotter HJ, Pabst R. M cells in Peyer’s patches of the intestine. Int. Rev. Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- 87. Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells, gateways for mucosal infection and immunization. Cell. 1996;86(3):345–348. doi: 10.1016/s0092-8674(00)80106-3. • Focuses on M cells in the mucosal immune system.
- 88.Owen RL, Jones AL. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66(2):189–203. [PubMed] [Google Scholar]
- 89. Wolf JL, Bye WA. The membranous epithelial (M) cell and the mucosal immune system. Annu. Rev. Med. 1984;35:95–112. doi: 10.1146/annurev.me.35.020184.000523. • Focuses on M cells in the mucosal immune system.
- 90.Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J. Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer’s patch M cells. Cell Tissue Res. 1995;279(2):433–436. doi: 10.1007/BF00318501. [DOI] [PubMed] [Google Scholar]
- 91.Allan CH, Mendrick DL, Trier JS. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology. 1993;104(3):698–708. doi: 10.1016/0016-5085(93)91004-2. [DOI] [PubMed] [Google Scholar]
- 92.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 1994;180(1):15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teitelbaum R, Schubert W, Gunther L, et al. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10(6):641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 94.Jang MH, Kweon MN, Iwatani K, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl Acad. Sci. USA. 2004;101(16):6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto M, Rennert P, McGhee JR, et al. Alternate mucosal immune system: organized Peyer’s patches are not required for IgA responses in the gastrointestinal tract. J. Immunol. 2000;164(10):5184–5189. doi: 10.4049/jimmunol.164.10.5184. [DOI] [PubMed] [Google Scholar]
- 96.Wolf JL, Rubin DH, Finberg R, et al. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212(4493):471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 97.Nibert ML, Furlong DB, Fields BN. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J. Clin. Invest. 1991;88(3):727–734. doi: 10.1172/JCI115369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y, Boysun MJ, Csencsits KL, Pascual DW. Gene transfer facilitated by a cellular targeting molecule, reovirus protein σ1. Gene Ther. 2000;7(1):61–69. doi: 10.1038/sj.gt.3301046. [DOI] [PubMed] [Google Scholar]
- 99.Wu Y, Wang X, Csencsits KL, Haddad A, Walters N, Pascual DW. M cell-targeted DNA vaccination. Proc. Natl Acad. Sci. USA. 2001;98(16):9318–9323. doi: 10.1073/pnas.161204098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nochi T, Yuki Y, Matsumura A, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 2007;204(12):2789–2796. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim SH, Seo KW, Kim J, Lee KY, Jang YS. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J. Immunol. 2010;185(10):5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 102.Rynda A, Maddaloni M, Mierzejewska D, et al. Low-dose tolerance is mediated by the microfold cell ligand, reovirus protein σ1. J. Immunol. 2008;180(8):5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki H, Sekine S, Kataoka K, et al. Ovalbumin-protein σ1 M-cell targeting facilitates oral tolerance with reduction of antigen-specific CD4+ T cells. Gastroenterology. 2008;135(3):917–925. doi: 10.1053/j.gastro.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arakawa T, Chong DK, Langridge WH. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 1998;16(3):292–297. doi: 10.1038/nbt0398-292. [DOI] [PubMed] [Google Scholar]
- 105.Haq TA, Mason HS, Clements JD, Arntzen CJ. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 1995;268:714–716. doi: 10.1126/science.7732379. [DOI] [PubMed] [Google Scholar]
- 106.Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl Acad. Sci. USA. 1996;93(11):5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mason HS, Haq TA, Clements JD, Arntzen CJ. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16(13):1336–1343. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 108.Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000;18(11):1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- 109.Sandhu JS, Krasnyanski SF, Domier LL, Korban SS, Osadjan MD, Buetow DE. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9(2):127–135. doi: 10.1023/a:1008979525909. [DOI] [PubMed] [Google Scholar]
- 110.Streatfield SJ, Jilka JM, Hood EE, et al. Plant-based vaccines: unique advantages. Vaccine. 2001;19(17–19):2742–2748. doi: 10.1016/S0264-410X(00)00512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998;4(5):607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 112.Nochi T, Takagi H, Yuki Y, et al. Ricebased mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl Acad. Sci. USA. 2007;104(26):10986–10991. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tokuhara D, Yuki Y, Nochi T, et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl Acad. Sci. USA. 2010;107(19):8794–8799. doi: 10.1073/pnas.0914121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nochi T, Yuki Y, Katakai Y, et al. A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence preexisting intestinal immunity. J. Immunol. 2009;183(10):6538–6544. doi: 10.4049/jimmunol.0901480. [DOI] [PubMed] [Google Scholar]
- 115.Yuki Y, Tokuhara D, Nochi T, et al. Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine. 2009;27(43):5982–5988. doi: 10.1016/j.vaccine.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 116.de Haan A, Geerligs HJ, Huchshorn JP, van Scharrenburg GJ, Palache AM, Wilschut J. Mucosal immunoadjuvant activity of liposomes, induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995;13(2):155–162. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- 117.de Haan A, Tomee JF, Huchshorn JP, Wilschut J. Liposomes as an immunoadjuvant system for stimulation of mucosal and systemic antibody responses against inactivated measles virus administered intranasally to mice. Vaccine. 1995;13(14):1320–1324. doi: 10.1016/0264-410x(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 118.Ernst WA, Kim HJ, Tumpey TM, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24(24):5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 119.Hasegawa H, Ichinohe T, Strong P, et al. Protection against influenza virus infection by intranasal administration of hemagglutinin vaccine with chitin microparticles as an adjuvant. J. Med. Virol. 2005;75(1):130–136. doi: 10.1002/jmv.20247. [DOI] [PubMed] [Google Scholar]
- 120.Marinaro M, Boyaka PN, Finkelman FD, et al. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. J. Exp. Med. 1997;185(3):415–427. doi: 10.1084/jem.185.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials, developing the next generation of vaccines. Trends Immunol. 2006;27(12):573–579. doi: 10.1016/j.it.2006.10.005. • Describes general features of dendritic cells in the innate and acquired immune systems.
- 122.Sharma S, Mukkur TK, Benson HA, Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J. Pharm. Sci. 2009;98(3):812–843. doi: 10.1002/jps.21493. [DOI] [PubMed] [Google Scholar]
- 123.Zurbriggen R, Gluck R. Immunogenicity of IRIV- versus alum-adjuvanted diphtheria and tetanus toxoid vaccines in influenza primed mice. Vaccine. 1999;17(11–12):1301–1305. doi: 10.1016/s0264-410x(98)00361-2. [DOI] [PubMed] [Google Scholar]
- 124.Alving CR. Immunologic aspects of liposomes: presentation and processing of liposomal protein and phospholipid antigens. Biochim. Biophys. Acta. 1992;1113(3–4):307–322. doi: 10.1016/0304-4157(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 125.Brochu H, Polidori A, Pucci B, Vermette P. Drug delivery systems using immobilized intact liposomes: a comparative and critical review. Curr. Drug Deliv. 2004;1(3):299–312. doi: 10.2174/1567201043334678. [DOI] [PubMed] [Google Scholar]
- 126.Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol. Today. 1990;11(3):89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- 127.Amidi M, Romeijn SG, Verhoef JC, et al. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25(1):144–153. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 128.Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J. Neuroimmune Pharmacol. 2008;3(2):83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kageyama S, Kitano S, Hirayama M, et al. Humoral immune responses in patients vaccinated with 1–146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008;99(3):601–607. doi: 10.1111/j.1349-7006.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nomura Y, Ikeda M, Yamaguchi N, Aoyama Y, Akiyoshi K. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 2003;553(3):271–276. doi: 10.1016/s0014-5793(03)01028-7. [DOI] [PubMed] [Google Scholar]
- 131.Nochi T, Yuki Y, Takahashi H, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010;9(7):572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 132.Lehner T. Innate and adaptive mucosal immunity in protection against HIV infection. Vaccine. 2003;21 Suppl. 2:S68–S76. doi: 10.1016/s0264-410x(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 133.Lehner T, Wang Y, Whittall T, Seidl T. Innate immunity and HIV-1 infection. Adv. Dent. Res. 2011;23(1):19–22. doi: 10.1177/0022034511399081. [DOI] [PubMed] [Google Scholar]
- 134.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 2006;80(11):5283–5291. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tengvall S, O’Hagan D, Harandi AM. Rectal immunization generates protective immunity in the female genital tract against herpes simplex virus type 2 infection: relative importance of myeloid differentiation factor 88. Antiviral Res. 2008;78(3):202–214. doi: 10.1016/j.antiviral.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 136.Mowat AM, Faria AM, Weiner HL. Oral tolerance: physiological basis and clinical applications. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. CA, USA: Elsevier/Academic Press; 2004. pp. 487–537. [Google Scholar]
- 137.Frati F, Moingeon P, Marcucci F, et al. Mucosal immunization application to allergic disease: sublingual immunotherapy. Allergy Asthma Proc. 2007;28(1):35–39. doi: 10.2500/aap.2007.28.2919. [DOI] [PubMed] [Google Scholar]
- 138.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61(2):151–165. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 139.Sun JB, Czerkinsky C, Holmgren J. Sublingual ‘oral tolerance’ induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand. J. Immunol. 2007;66(2–3):278–286. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 140.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3−CD25− CD4+ regulatory T cells. J. Immunol. 2006;177(11):7634–7644. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 141.Hagiwara Y, Kawamura YI, Kataoka K, et al. A second generation of double mutant cholera toxin adjuvants: enhanced immunity without intracellular trafficking. J. Immunol. 2006;177(5):3045–3054. doi: 10.4049/jimmunol.177.5.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Ginkel FW, Jackson RJ, Yoshino N, et al. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 2005;73(10):6892–6902. doi: 10.1128/IAI.73.10.6892-6902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 2000;165(9):4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 144.van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA, Briles DE. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc. Natl Acad. Sci. USA. 2003;100(24):14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoshino N, Lu FX, Fujihashi K, et al. A novel adjuvant for mucosal immunity to HIV-1 gp120 in nonhuman primates. J. Immunol. 2004;173(11):6850–6857. doi: 10.4049/jimmunol.173.11.6850. [DOI] [PubMed] [Google Scholar]
- 146.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 2002;32(8):2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 147.McCluskie MJ, Brazolot Millan CL, Gramzinski RA, et al. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 1999;5(5):287–300. [PMC free article] [PubMed] [Google Scholar]
- 148.Montgomery PC, Rafferty DE. Induction of secretory and serum antibody responses following oral administration of antigen with bioadhesive degradable starch microparticles. Oral Microbiol. Immunol. 1998;13(3):139–149. doi: 10.1111/j.1399-302x.1998.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 149.Song JH, Kim JI, Kwon HJ, et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J. Immunol. 2009;182(11):6851–6860. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- 150.Song JH, Nguyen HH, Cuburu N, et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl Acad. Sci. USA. 2008;105(5):1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang T, Hashizume T, Kurita-Ochiai T, Yamamoto M. Sublingual vaccination with outer membrane protein of Porphyromonas gingivalis and Flt3 ligand elicits protective immunity in the oral cavity. Biochem. Biophys. Res. Commun. 2009;390(3):937–941. doi: 10.1016/j.bbrc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 152.Knop E, Knop N. Lacrimal drainage-associated lymphoid tissue (LDALT): a part of the human mucosal immune system. Invest. Ophthalmol. Vis. Sci. 2001;42(3):566–574. [PubMed] [Google Scholar]
- 153.Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1270–1209. [PubMed] [Google Scholar]
- 154.Cain C, Phillips TE. Developmental changes in conjunctiva-associated lymphoid tissue of the rabbit. Invest. Ophthalmol. Vis. Sci. 2008;49(2):644–649. doi: 10.1167/iovs.07-0856. [DOI] [PubMed] [Google Scholar]
- 155.Chodosh J, Nordquist RE, Kennedy RC. Comparative anatomy of mammalian conjunctival lymphoid tissue: a putative mucosal immune site. Dev. Comp. Immunol. 1998;22(5–6):621–630. doi: 10.1016/s0145-305x(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 156.Giuliano EA, Moore CP, Phillips TE. Morphological evidence of M cells in healthy canine conjunctiva-associated lymphoid tissue. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240(3):220–226. doi: 10.1007/s00417-002-0429-3. [DOI] [PubMed] [Google Scholar]
- 157.Gomes JA, Jindal VK, Gormley PD, Dua HS. Phenotypic analysis of resident lymphoid cells in the conjunctiva and adnexal tissues of rat. Exp. Eye Res. 1997;64(6):991–997. doi: 10.1006/exer.1997.0297. [DOI] [PubMed] [Google Scholar]
- 158.Knop N, Knop E. Ultrastructural anatomy of CALT follicles in the rabbit reveals characteristics of M-cells, germinal centres and high endothelial venules. J. Anat. 2005;207(4):409–426. doi: 10.1111/j.1469-7580.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]