SHIP1 regulates PtdIns(3,4,5)P3 production in response to cell adhesion. Loss of SHIP1 leads to elevated PtdIns(3,4,5)P3 and Akt activation upon adhesion. SHIP1−/− neutrophils lose polarity upon cell adhesion. They are extremely adherent, which impairs chemotaxis. Chemotaxis in SHIP1−/− neutrophils can be rescued by reducing cell adhesion.
Abstract
The second messenger phosphatidylinositol(3,4,5)P3 (PtdIns(3,4,5)P3) is formed by stimulation of various receptors, including G protein–coupled receptors and integrins. The lipid phosphatases PTEN and SHIP1 are critical in regulating the level of PtdIns(3,4,5)P3 during chemotaxis. Observations that loss of PTEN had minor and loss of SHIP1 resulted in a severe chemotaxis defect in neutrophils led to the belief that SHIP1 rather than PTEN acts as a predominant phospholipid phosphatase in establishing a PtdIns(3,4,5)P3 compass. In this study, we show that SHIP1 regulates PtdIns(3,4,5)P3 production in response to cell adhesion and plays a limited role when cells are in suspension. SHIP1−/− neutrophils lose their polarity upon cell adhesion and are extremely adherent, which impairs chemotaxis. However, chemotaxis can be restored by reducing adhesion. Loss of SHIP1 elevates Akt activation following cell adhesion due to increased PtdIns(3,4,5)P3 production. From our observations, we conclude that SHIP1 prevents formation of top-down PtdIns(3,4,5)P3 polarity to facilitate proper cell attachment and detachment during chemotaxis.
INTRODUCTION
Neutrophils are responsible for controlling pathogen invasion and are therefore an important component of the innate immune system. Neutrophils are the most abundant cell type among circulating white blood cells and are normally quiescent as they travel within blood vessels (Borregaard, 2010). Neutrophils migrate into the infected tissue by responding to a variety of chemokines (e.g., interleukin-8 [IL-8]), cytokines (e.g., tumor necrosis factor α [TNFα]), leukotrienes (e.g., leukotriene B4 [LTB4]), complement peptides (e.g., C5a, C3a), and chemicals released by bacteria directly, such as peptides bearing the N-formyl group (N-formyl-methionine-leucine-phenylalanine [fMLP]), by a process known as chemotaxis. During chemotaxis, the cell becomes morphologically polarized, with the front of the cell (pseudopod) constantly protruding and retracting motile membranous structures toward the direction of the chemoattractant gradient. This movement is largely driven by the outward extension of actin filaments (Pollard and Borisy, 2003). Actin filaments can also be associated through clutch-like complexes of proteins to adhesion complexes at the base of the cell, against which actin-polymerization–mediated protrusive forces can operate (Hu et al., 2007; Hoffman et al., 2011). Studies on the mechanisms of chemotaxis have shown that phosphatidylinositol(3,4,5)P3 (PtdIns(3,4,5)P3) plays a pivotal role in establishing cell polarity (Stephens et al., 2002; Weiner, 2002). Phosphoinositide 3-kinases (PI3Ks) are evolutionary conserved lipid kinases that convert phosphatidylinositol 4,5-bisphosphate (PtIns(4,5)P2) to PtdIns(3,4,5)P3. PI3Kα, β, and δ (class 1a) isoforms are activated by tyrosine kinases, whereas PI3Kγ (class 1b) is activated by G protein–coupled receptors (GPCRs). In neutrophils that sense a chemoattractant gradient like fMLP, GPCRs and G proteins (Gαi/o) downstream of these receptors are activated, leading to formation of PtdIns(3,4,5)P3 at the leading edge, predominantly by the activity of PI3Kγ (Ferguson et al., 2007; Stephens et al., 2008). However, class 1a PI3Kδ activity is also believed to be involved in fMLP-mediated responses (Condliffe et al., 2005). In addition, cell adhesion resulting from the interaction of integrins (αLβ2 and αMβ2) with extracellular matrix proteins, such as fibronectin and ICAM-1, can also lead to activation of PI3Kδ and production of PtdIns(3,4,5)P3 through activation of the Src family of tyrosine kinases (Axelsson et al., 2000; Puri et al., 2004). Inhibition of PI3Kδ using isoform-specific inhibitors demonstrated that PI3Kδ activity is required for neutrophil spreading and polarization on a fibrinogen-coated surface (Sadhu et al., 2003). Activation of GPCRs also leads to activation of integrins by an inside-out mechanism (Ley et al., 2007). In the social amoeba Dictyostelium discoideum, GPCR-mediated signaling and formation of a PtdIns(3,4,5)P3 gradient result in chemotaxis toward cAMP.
PtdIns(3,4,5)P3 is known to exert its function by recruiting proteins that contain pleckstrin homology (PH) domains to the membrane (e.g., Btk, PKB/Akt, PLC-γ, Gab1, P-Rex1, PDK1, and Grp1; Lemmon, 2007). PtdIns(3,4,5)P3 effectors lead to activation of Rac GTPases and F-actin polymerization at the leading edge (Xu et al., 2003). Although PtdIns(3,4,5)P3 is synthesized by PI3K, its level can also be regulated by two phosphatases—PTEN, a 3-phosphatase that converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2; and SHIP1, a 5-phosphatase that converts PtdIns(3,4,5)P3 to PtdIns(3,4)P2. Loss of PTEN in Dictyostelium results in prolonged PtdIns(3,4,5)P3 production and F-actin polymerization. As a result, the frequency of lateral pseudopodia was increased and chemotaxis was inefficient. PTEN localizes to the rear of a migrating Dictyostelium cell. Thus PTEN is proposed to be a primary driving factor in maintaining an anterior–posterior PtdIns(3,4,5)P3 gradient, which acts as an internal cellular compass necessary for determining the directionality of the cells (Iijima and Devreotes, 2002; Kriebel et al., 2003). It is surprising that genetic disruption of PTEN in neutrophils resulted in only minor defects in cell migration with slightly enhanced responsiveness to chemokines and up-regulation of neutrophil functions (Heit et al., 2008; Subramanian et al., 2007; Li et al., 2009; Schabbauer et al., 2010). However, biochemical studies of neutrophil lysates show that a large amount of the PtdIns(3,4,5)P3 phosphatase activity is also contributed by 5-phosphatases (Stephens et al., 1991). Therefore SHIP1 may be an important regulator of PtdIns(3,4,5)P3-mediated neutrophil function.
In neutrophils, it is reported that SHIP1 is essential for chemoattractant-mediated neutrophil migration and is believed to be the primary inositol phosphatase responsible for generating a PtdIns(3,4,5)P3 gradient. Disruption of SHIP1 resulted in the accumulation of green fluorescent protein (GFP)–PH-Akt (a PtdIns(3,4,5)P3 probe) and F-actin polymerization across the cell membrane. Consequently, these neutrophils are extremely flattened and display improper polarization and dramatically slower cell migration (Nishio et al., 2007). Although PtdIns(3,4,5)P3 polarization is pivotal during chemotaxis, neutrophils that lack the PtdIns(3,4,5)P3-metabolizing enzyme PI3Kγ, PTEN, or SHIP1 or have depletion of PtdIns(3,4,5)P3 by wortmannin were able to sense direction during chemotaxis (Ferguson et al., 2007; Nishio et al., 2007).
SHIP1 was identified as a 5-phosphatase that dephosphorylates PtdIns(3,4,5)P3 to PtdIns(3,4)P2 and Ins(1,3,4,5)P4 to Ins(1,3,4)P3 (Damen et al., 1996). SHIP1 contains several interacting domains, including a Src homology domain (SH2), an inositide lipid phosphatase domain, two NPXY motifs that can be tyrosine phosphorylated, and a polyproline region in the C-terminus. SHIP1 has been established as a key negative regulator of the immune system. SHIP1 is known to negatively regulate various cellular processes, such as phagocytosis, cell migration, degranulation, cell survival, proliferation, differentiation, and sensitivity to chemokines (Huber et al., 1998; Sly et al., 2007; Leung et al., 2009; Parry et al., 2010). In addition, SHIP1 is crucial for regulation of chemotaxis, but it has not been established how SHIP1 is regulated by chemoattractants and which receptors are involved in the process. Cell adhesion is a critical component of chemotaxis and also results in local PtdIns(3,4,5)P3 formation upon engagement of integrins. We hypothesized that if SHIP1 is the primary inositol phosphatase involved in the generation of a PtdIns(3,4,5)P3 gradient, then loss of SHIP1 would result in an elevation of PtdIns(3,4,5)P3 functions in both suspension and adherent cells stimulated with fMLP.
In this study, we show that SHIP1 regulates PtdIns(3,4,5)P3 production following cell adhesion on a substrate. When the cells are stimulated with fMLP in suspension, SHIP1 plays a rather insignificant role. SHIP1−/− neutrophils are extremely adherent, which results in impaired cell migration. Reduction in cell adhesion can rescue the defect in cell migration in SHIP1−/− neutrophils. We also show that SHIP1 is localized to the membrane and is tyrosine phosphorylated during cell adhesion. In addition, cell adhesion results in excessive Akt activation in SHIP1−/− but not PTEN−/− neutrophils due to increased PtdIns(3,4,5)P3 production. From our observations, we conclude that during cell migration SHIP1 acts a negative regulator of PtdIns(3,4,5)P3 formation at the cell–substratum interface, preventing the formation of top-down PtdIns(3,4,5)P3 polarity and facilitating proper cell attachment and detachment during chemotaxis.
RESULTS
Cell adhesion causes altered cell polarity in SHIP1−/− neutrophils
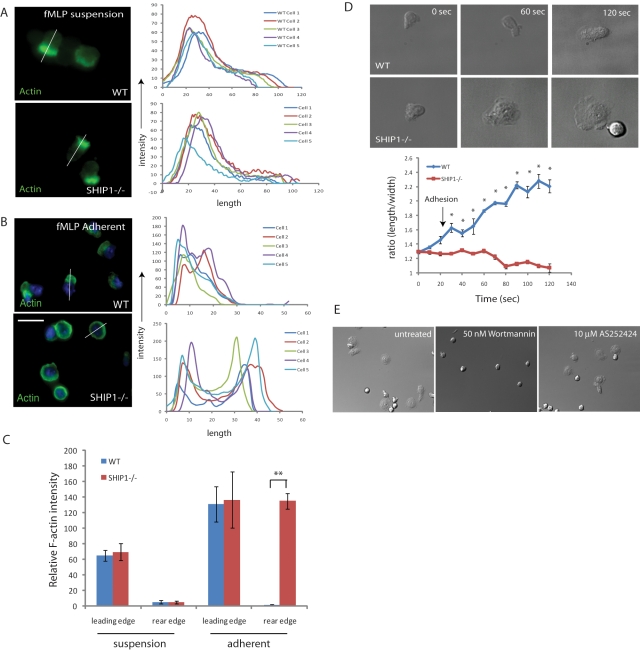
The inositol phosphatase SHIP1 has been shown to be important in regulating cell polarity. SHIP1−/− neutrophils have decreased polarization during cell migration toward a chemoattractant source with a defect in spatially restricted F-actin polymerization (Nishio et al., 2007). For this reason, we compared polarity of wild-type and SHIP1−/− neutrophils in suspension and upon cell–substratum adhesion. We stimulated wild-type and SHIP1−/− neutrophils in suspension with fMLP and fixed them with formaldehyde. fMLP stimulation causes F-actin polymerization at the leading edge, which can be detected by using fluorescein isothiocyanate (FITC)–labeled phalloidin. Analysis revealed that in suspension, both wild-type and SHIP1−/− neutrophils can polarize and have F-actin enrichment at the leading edge (Figure 1, A and C). Conversely, when neutrophils are stimulated with fMLP and allowed to adhere on a fibronectin-coated surface, wild-type neutrophils form a leading edge with polymerized F-actin upon adhesion, but SHIP1−/− neutrophils do not, and F-actin remains enriched throughout the cortex (Figure 1, B and C). Therefore we infer that adhesion results in loss of polarity in SHIP1−/− neutrophils.
FIGURE 1:
Cell adhesion causes altered cell polarity in SHIP1−/− neutrophils. (A) Wild-type and SHIP1−/− neutrophils were stimulated with fMLP in suspension and fixed with 3% paraformaldehyde (PFA), permeabilized, and stained with FITC-labeled phalloidin (stains F-actin). (B) Neutrophils were plated on fibronectin-coated coverslips (10 μg/ml) and allowed to adhere for 5 min prior to stimulation with 1 μM fMLP for 5 min. Cells were fixed using 3% PFA, permeabilized, and then stained with FITC–phalloidin. Images were captured using a fluorescence microscope. Images were analyzed using the ImageJ Plot Profile menu to quantify fluorescence intensities across the cell body. Analysis of five representative cells is shown. Bar, 10 μm. (C) Values of FITC–phalloidin fluorescence intensities for wild-type and SHIP1−/− cells at the leading and rear edges for cells in suspension and upon adhesion. n = 5; **p < 0.0005. (D) Neutrophils were stimulated in a tube (1 μM fMLP) and then plated on fibronectin-coated coverslips. Images were recorded at every 10 s for 5 min, and the process of cell adhesion and polarization was recorded. Using ImageJ, polarity index (length/width of cells) was plotted against time. Data are presented as mean ± SD, and statistical significance was assessed by two-tailed, paired Student's t test (n = 7; *p < 0.01). (E) SHIP1−/− neutrophils were allowed to adhere to a fibronectin-coated surface and treated with 50 nM wortmannin and 10 μM AS-252424.
To test this further, we analyzed the process of adhesion in fMLP-stimulated neutrophils on a coverslip coated with fibronectin. Images were captured, and relative polarity (ratio of length/width) was analyzed for each frame (Supplemental Videos S1 and S2). We found that both wild-type and SHIP1−/− neutrophils were polarized when in suspension (relative polarity ∼1.3). However, upon adhesion, wild-type neutrophils became polarized further with a relative polarity of >2.0, whereas, SHIP1−/− neutrophils lost polarity, became flattened, and were surrounded by a well-developed lamellipodia. Accordingly, the relative polarity was reduced to ∼1.0 in SHIP1−/− neutrophils (Figure 1D). These results indicate that SHIP1−/− neutrophils behave similar to wild-type neutrophils when in suspension, but upon adhesion, polarity is lost. The broad, flattened appearance of SHIP1−/− neutrophils was lost upon treatment with the pan-PI3K inhibitor wortmannin, but no effect was observed upon treatment with the PI3Kγ-specific inhibitor AS-252424. This indicates that the defect in cell polarity is not mediated by PI3Kγ (class 1B PI3K), which signals through a GPCR, but possibly through PI3Kδ (or another class 1a PI3K), which is activated by integrin-mediated signaling (Figure 1E).
Loss of SHIP1 enhances cell adhesion
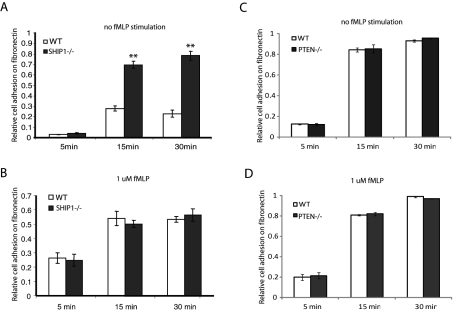
Because we observed that SHIP1−/− neutrophils lose cell polarity upon adhesion, we investigated the adhesive properties of SHIP1−/− neutrophils. Neutrophils were either unstimulated or stimulated with 1 μM fMLP for 2 min and allowed to adhere on a fibronectin-coated surface for 5, 15, or 30 min. Nonadherent cells were washed off, and the remaining adhered cells were lysed and quantified using peroxidases activity in cell lysates, using 3,3′,5,5′-tetramethylbenzidine (TMB) as substrate. Analysis revealed that under unstimulated conditions, SHIP1−/− neutrophils are more adherent than wild-type neutrophils (Figure 2A), but upon stimulation with 1 μM fMLP, both wild-type and SHIP1−/− neutrophils adhere with similar efficiency (Figure 2B). We then performed cell adhesion assays under similar conditions using PTEN−/− neutrophils. In contrast to SHIP1−/− neutrophils, adhesion in PTEN−/− neutrophils was similar to that in wild-type neutrophils under both unstimulated and fMLP-stimulated conditions (Figure 2, C and D). This indicates that the 5-PtdIns(3,4,5)P3 phosphatase SHIP1 acts as a negative regulator of cell adhesion, and loss of SHIP1 leads to enhanced cell adhesion. Conversely, the 3-PtdIns(3,4,5)P3 phosphatase PTEN does not regulate cell adhesion.
FIGURE 2:
Loss of SHIP1 enhances cell adhesion. Neutrophils were either unstimulated or stimulated with 1 μM fMLP and allowed to adhere to a fibronectin-coated surface for 5, 15, or 30 min. Nonadherent cells were removed by washing with PBS. Adherent cells were lysed using 0.5% CTAB and quantified by determining peroxidase activity using TMB as the substrate. The reaction was stopped, and absorbance at 450 nm was measured. Total cells added was taken as a positive control and was used to measure the relative cell adhesion. Cell adhesion of (A) unstimulated and (B) fMLP stimulated wild-type and SHIP1−/− neutrophils. Cell adhesion of (C) unstimulated and (D) fMLP stimulated wild type and PTEN−/− neutrophils.
SHIP1 is localized to the membrane and is tyrosine phosphorylated upon cell adhesion
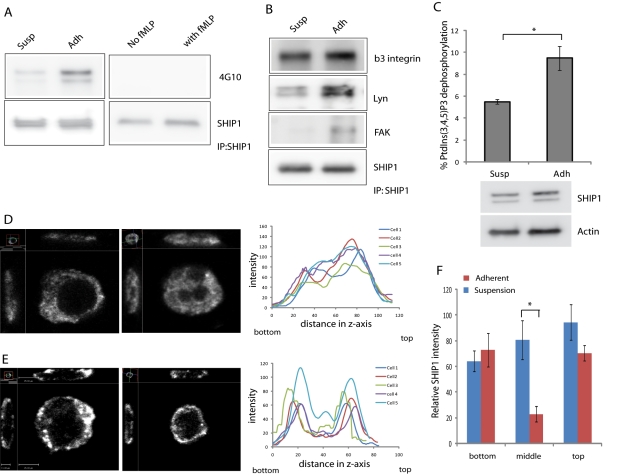
PtdIns(3,4,5)P3, the substrate for SHIP1, is restricted to the plasma membrane. Although SHIP1 is believed to be enzymatically active while present in the cytosol, its activity is determined by its membrane localization (Phee et al., 2000). Recruitment of SHIP1 to the plasma membrane is regulated by its association with adapter proteins (e.g., Shc, Grb2, Dok3), scaffolding proteins (e.g., Gab1/2), and direct association with tyrosine-phosphorylated receptors through its SH2 domain. These interactions require tyrosine phosphorylation of SHIP1 in the NPXY motif (Lamkin et al., 1997; Zhang et al., 1999; Lemay et al., 2000; Tu et al., 2001). We examined whether neutrophil adhesion on fibronectin or stimulation with fMLP in suspension caused phosphorylation of SHIP1. For adhesion, wild-type neutrophils were allowed to adhere on a fibronectin-coated surface for 30 min and lysed in IP lysis buffer (see Materials and Methods). For fMLP stimulation, neutrophils were treated with 1 μM fMLP for 2 min. Cells in suspension were used as control in both cases. Cell lysates were immunoprecipitated using SHIP1 antibody (clone P1C1; Santa Cruz Biotechnology), and immunoprecipitates were analyzed using SHIP1 and phospho-Tyr antibodies (clone 4G10; Millipore). We observed that cell adhesion led to tyrosine phosphorylation of SHIP1, but fMLP stimulation did not lead to any apparent increase in tyrosine phosphorylation of SHIP1 (Figure 3A). We also observed that SHIP1 can interact with FAK and Lyn upon cell adhesion as well as with β3 integrin in both suspension and upon cell adhesion (Figure 3B). This indicates that adhesion results in the recruitment of SHIP1 to the membrane, where it can act on PtdIns(3,4,5)P3 produced during cell adhesion. It was shown in platelets that Lyn, a Src family tyrosine kinase, regulates SHIP1 phosphorylation in integrin αIIbβ3–mediated adhesion and signaling (Maxwell et al., 2004).
FIGURE 3:
SHIP1 is localized to the membrane and is tyrosine phosphorylated upon adhesion. (A) Wild-type neutrophils either in suspension or adhered to a fibronectin-coated surface or unstimulated or stimulated with 1 μM fMLP in suspension were lysed using IP lysis buffer. The lysate was immunoprecipitated using a SHIP1 antibody (clone P1C1). Pull-down eluates were analyzed using SHIP1 and phospho-Tyr (clone 4G10) antibodies. (B) Lysates from wild-type neutrophils either in suspension or adhered to a fibronectin-coated surface were immunoprecipitated using a SHIP1 antibody (clone P1C1). Immunoprecipitates were analyzed using SHIP1, FAK, Lyn, and β3 integrin. (C) Lysates from wild-type neutrophils either in suspension or adhered to a fibronectin-coated surface were immunoprecipitated using a SHIP1 antibody. Immunoprecipitated SHIP1 was used to measure SHIP1 phosphatase activity by incubating with diC8 PtdIns(3,4,5)P3 and generation of free phosphate analyzed by malachite green. Data are presented as mean ± SD, from three independent experiments; p < 0.05. Cell lysates were analyzed for total amounts of SHIP1 and actin as input controls. Differentiated HL-60 cells in suspension (D) or adhered on a fibronectin-coated surface (E) were fixed and stained for SHIP1. Images were taken by confocal microscopy, and side-view projection cross-section images were reconstructed using Volocity software. Fluorescence intensity across the z-axis was analyzed by ImageJ. Analysis of five representative cells is shown. (F) Values of fluorescence intensities from side-view projection cross-section images of SHIP1 staining of HL-60 cells in suspension or upon adhesion analyzed by ImageJ were plotted according to the bottom surface touching the substratum, middle of the cell, and top part of the adhered cell. n = 5; *p < 0.001.
We then analyzed the relationship between SHIP1 phosphorylation and activity. We immunoprecipitated SHIP1 from cell lysates of neutrophils either in suspension or adhered to fibronectin-coated surface and incubated with diC8 PtdIns(3,4,5)P3. The free phosphate generated was then analyzed as a measure for SHIP1 phosphatase activity by malachite green. From this analysis we observed that SHIP1 from lysates of suspended and adherent cells showed considerable phosphatase activity, but lysates from adherent cells showed significantly higher phosphatase activity than lysates from suspended cells (Figure 3C). This indicates that tyrosine phosphorylation of SHIP1 may contribute to increase in SHIP1 activity during cell adhesion.
To analyze the localization of SHIP1 in suspension or upon cell adhesion, we used differentiated HL-60 cells in suspension or allowed them to adhere on fibronectin-coated glass coverslips. Cells were fixed and stained for SHIP1 (clone P290; Cell Signaling Technology) and analyzed using high-resolution sectioning confocal microscopy. Suspended cells showed SHIP1 localization throughout the cytosol (Figure 3, D and F). Adherent cells showed large accumulation of SHIP1 throughout the cell cortex. Of interest, cross-section projection images revealed that upon adhesion, SHIP1 is located throughout the plasma membrane both at the cell–substratum interface and at the top (Figure 3, E and F). This suggests that SHIP1 localizes to the membrane upon cell adhesion, where the SHIP1 in the basal cell–substratum interface is responsible for dephosphorylating PtdIns(3,4,5)P3 formed during cell adhesion.
Adhesion-mediated PtdIns(3,4,5)P3 signaling is enhanced in SHIP1−/− neutrophils
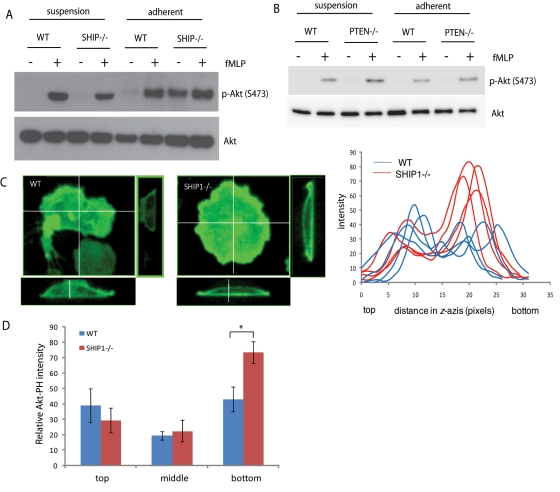
To study what causes SHIP1−/− neutrophils to behave differently when in suspension than when adhered on a surface, we first compared phospho-Akt levels (as a marker of PtdIns(3,4,5)P3 level) in neutrophils in suspension and upon adhesion. Akt activation in suspension was studied by stimulating cells with fMLP, which induces Akt activation through a GPCR-mediated pathway. Alternatively, Akt activation following cell adhesion was studied upon adhesion to a fibronectin-coated surface, which induces Akt activation through an integrin-mediated pathway. Activation of neutrophils by fMLP in suspension showed that Akt activation in wild-type and SHIP1−/− neutrophils was similar. In contrast, when neutrophils were allowed to adhere on a fibronectin-coated surface for 15 min, the adhesion resulted in a substantial increase in Akt phosphorylation in SHIP1−/− neutrophils. When adherent neutrophils were treated with fMLP, the level of Akt phosphorylation was similar in wild-type and SHIP1−/− neutrophils (Figure 4A). These results indicate that SHIP1 regulates adhesion-mediated (or integrin-mediated) Akt activation and plays no role in fMLP-mediated Akt activation in suspension. Therefore SHIP1 activity is required in order to limit excessive PtdIns(3,4,5)P3 formation and Akt activation upon cell adhesion. We then performed similar experiments in PTEN-depleted neutrophils. PTEN−/− neutrophils, unlike SHIP1−/− neutrophils, showed a greater increase in Akt phosphorylation upon fMLP stimulation in suspension as compared with wild-type neutrophils. However, adhesion on a fibronectin-coated surface did not lead to a dramatic increase in Akt phosphorylation in PTEN−/− neutrophils (Figure 4B). This further emphasizes the fact that PTEN is involved in chemoattractant signaling mediated by GPCR activation, whereas SHIP1 regulates integrin-mediated adhesive responses.
FIGURE 4:
Adhesion-mediated PtdIns(3,4,5)P3 signaling is enhanced in SHIP1−/− neutrophils. (A) Wild-type or SHIP1−/− or (B) wild-type or PTEN−/− neutrophils were either unstimulated or stimulated with 1 μM fMLP in suspension or were first allowed to adhere to a fibronectin-coated surface for 15 min, and nonadherent cells were removed and then stimulated with fMLP for 2 min. The levels of phospho-Akt were analyzed in the cell lysates. Total Akt was used as a loading control. (C) Wild-type or SHIP1−/− neutrophils were transfected with Akt-PH-EGFP and allowed to adhere on a fibronectin-coated surface. Cells were fixed and analyzed using confocal microscopy, and side-view projection cross-section images were reconstructed showing strong enrichment of Akt-PH-EGFP at the cell–substratum interface in SHIP1−/− neutrophils. Fluorescence intensity across the z-axis was analyzed by ImageJ. Analysis of four representative cells for each genotype is shown. (D) Values of fluorescence intensities from side-view projection cross-section images of wild-type and SHIP1−/− cells expressing Akt-PH-EGFP analyzed by ImageJ were plotted according to the bottom surface touching the substratum, middle of the cell, and top part of the adhered cell. n = 5; *p < 0.05.
To investigate whether increased PtdIns(3,4,5)P3 is the reason for increased Akt phosphorylation in SHIP1−/− neutrophils upon cell adhesion, we transfected Akt-PH-EGFP into neutrophils in order to visualize PtdIns(3,4,5)P3–PtdIns(3,4)P2 in wild-type and SHIP1−/− neutrophils. Cells were allowed to adhere on a fibronectin-coated surface. Cells were fixed and analyzed using high-resolution sectioning confocal microscopy. SHIP1−/− neutrophils had a large accumulation of Akt-PH-EGFP throughout the cell cortex. Of interest, side-view projection cross-section images revealed that upon adhesion, Akt-PH-EGFP is located throughout the plasma membrane in wild-type neutrophils, but in SHIP1−/− neutrophils, there is a strong enrichment at the cell–substratum interface (Figure 4, C and D). Because loss of SHIP1 would inhibit formation of PtdIns(3,4)P2 from PtdIns(3,4,5)P3, the Akt-PH-EGFP enrichment is primarily due to a higher level of PtdIns(3,4,5)P3 formed at the site of adhesion. Elevated PtdIns(3,4,5)P3 levels are indeed the driving factor for enhanced cell adhesion and Akt activation upon cell adhesion in SHIP1−/− neutrophils. These results also suggest that during the process of cell migration that involves cell adhesion, signals from integrin engagement would lead to formation of PtdIns(3,4,5)P3 at sites of attachment. Generation of excessive PtdIns(3,4,5)P3 at the bottom of a migrating cell could interfere with generation of the anterior–posterior PtdIns(3,4,5)P3 gradient required for cell migration. Our observations indicate that SHIP1 plays a predominant role in metabolizing PtdIns(3,4,5)P3 located at the site of cellular attachment.
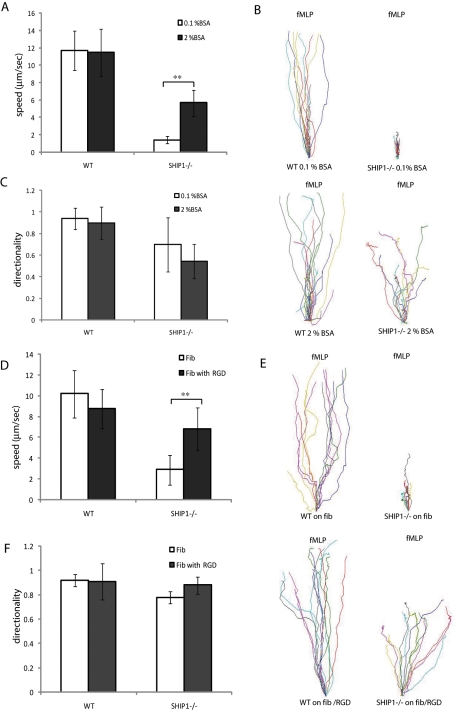
Defects in chemotaxis in SHIP1−/− neutrophils can be rescued by reducing cell adhesion
SHIP1−/− neutrophils display severely impaired migration toward a chemoattractant source (Nishio et al., 2007). We then reasoned that if the impaired chemotaxis is due to excessive cell adhesion, we can improve chemotaxis by reducing surface adhesion. To address this, we studied neutrophil chemotaxis toward fMLP using EZ-TAXIScan assays on glass coverslips either coated with 0.1% bovine serum albumin (BSA), which favors cell adhesion, or 2% BSA, which reduces cell adhesion. We found that wild-type neutrophils could migrate with high speed (11 μm/s) under both conditions. In contrast, SHIP1−/− neutrophils on a 0.1% BSA–coated glass coverslip could sense the chemoattractant gradient and attempted migration, but chemotaxis was extremely inefficient, with a speed of 1.4 μm/s. In contrast, when the surface was coated with 2% BSA to reduce cell adhesion, the impaired migration was partially rescued, with a significant population of cells migrating the entire length of the channel at an average speed of 5.67 μm/s (Figure 5, A–C, and Supplemental Videos S3–S6).
FIGURE 5:
Defects in chemotaxis in SHIP1−/− neutrophils can be rescued by reducing cell adhesion. Neutrophils from wild-type and SHIP1−/− mice were plated on glass coverslips coated with 0.1% or 2% BSA (A–C) or fibronectin in the presence or absence of RGD peptide in the buffer (D–F) in a EZ-TAXIScan device and exposed to a shallow chemoattractant gradient generated by addition of 1 μl fMLP (1 μM). (A, C, D, F) Migrating neutrophils were evaluated (n = 20 cells; **p < 0.05 vs. wild–type neutrophils) for directionality and migration speed as described in Materials and Methods. (B, E) Cell tracks of migrating wild-type (left) and SHIP1−/− (right) neutrophils were traced from captured images, realigned such that all cells started from the same starting point (0, 0), and plotted.
fMLP stimulation by GPCR-mediated inside-out signaling leads to activation of integrins. Activated integrins can then bind to extracellular matrix proteins through interactions with RGD motifs. To examine whether decreased cell adhesion during cell migration can rescue cell migration defects in SHIP1−/− neutrophils, we tested the ability of the neutrophils to migrate on an fMLP gradient on a fibronectin-coated glass surface in a buffer containing 1 μg/ml RGD. The RGD peptide in the buffer reduced cell–substratum adhesion by binding to activated integrins and preventing their association with fibronectin. We found that in the absence of RGD in the buffer, the migration of SHIP1−/− neutrophils was highly impaired. These cells could sense the chemoattractant gradient and initiate chemotaxis, but the adhesive forces restricted cell migration. In contrast, in the presence of 1 μg/ml RGD peptide in the buffer, SHIP1−/− neutrophils could migrate much more efficiently, with a speed of 6.8 versus 2.9 μm/s in the absence of the RGD peptide. The cell migration speed for wild-type neutrophils in the absence or presence of RGD peptide in the buffer was 10.2 and 8.75 μm/s, respectively (Figure 5, D–F, and Supplemental Videos S7–S10). These results indicate that the cell migration defects in SHIP1−/− neutrophils are an effect of enhanced cell adhesion and can be rescued by reducing cell–substratum adhesion.
Altered reactive oxygen species production in SHIP1−/− neutrophils
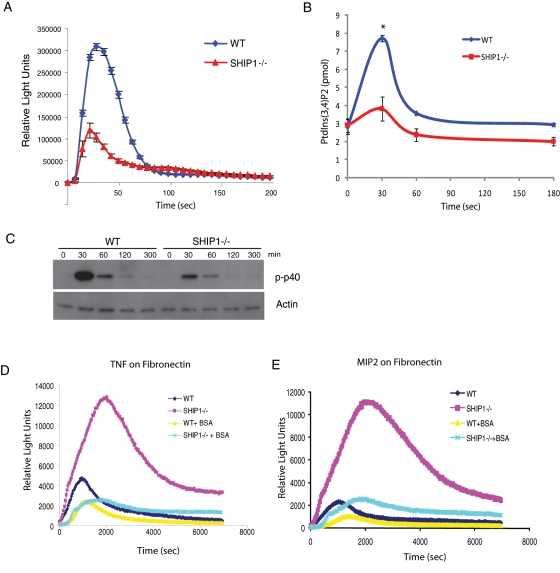
Neutrophil NADPH oxidase is a multicomponent enzyme that includes cytosolic and membrane-bound proteins that remain unassembled in resting cells. Membrane components include heterodimeric flavocytochrome b558, which is composed of two subunits: gp91 (Nox2) and p22phox. Cytosolic components include four soluble factors—p67phox, p47phox, p40phox, and Rac GTPase (Rac1 and Rac2). On cell surface receptor activation, cytosolic factors translocate to the membrane, where NADPH oxidase is assembled and large quantities of superoxide anions, which are precursors for a variety of reactive oxygen species (ROS) that are used to kill pathogens, are released (Groemping and Rittinger, 2005; Sumimoto, 2008). Common agents that activate neutrophils, such as the chemoattractant-activated G protein–coupled receptors, opsonized zymosan, or cell adhesion on extracellular matrix, trigger different signaling pathways that result in NADPH oxidase activation (Hirsch et al., 2000; Sasaki et al., 2000; Cross and Segal, 2004). fMLP stimulation results in a rapid, transient burst in ROS production, peaking within 30 s of stimulation, whereas cell adhesion induces a gradual production of ROS with a peak at 15–30 min. Products of phospholipid signaling PtdIns(3,4,5)P3, PtdIns(3,4)P2, and PtdIns(3)P are important in ROS production, which mediates membrane recruitment of p47phox and p40phox through interaction with their PX domains (Kanai et al., 2001; Ellson et al., 2006).
We observe that when neutrophils are stimulated with fMLP, SHIP1−/− neutrophils produce reduced levels of ROS compared with wild-type neutrophils. Because the loss of SHIP1 does not cause change in PtdIns(3,4,5)P3 levels in suspension, the level of PtdIns(3,4)P2 is possibly lower compared with wild-type neutrophils due to lack of 5-phosphatase activity. Because PtdIns(3,4)P2 can associate with PX-domain protein p47phox and mediate its membrane translocation for the activation of the NADPH oxidase complex, loss of SHIP1 leads to reduced production of ROS in suspension (Figure 6A). We then analyzed the level of PtdIns(3,4)P2 formed in neutrophils upon stimulation with 1 μM fMLP by PtdIns(3,4)P2 mass enzyme-linked immunosorbent assay (ELISA). On stimulation with fMLP, a transient increase in PtdIns(3,4)P2 was observed in wild-type neutrophils, with similar kinetics as for production of ROS, but SHIP1−/− neutrophils showed a significant reduction in PtdIns(3,4)P2 levels upon fMLP stimulation (Figure 6B). The reduced production of PtdIns(3,4)P2 in SHIP1−/− neutrophils explains the reduced production of ROS when stimulated with fMLP in suspension. Phosphorylation of p40phox is known to occur during activation of NADPH oxidase, peaking within 30 s of fMLP stimulation (Bouin et al., 1998). Analysis revealed that phosphorylation of p40phox upon fMLP stimulation was also significantly reduced in SHIP1−/− neutrophils as compared with wild-type neutrophils (Figure 6C).
FIGURE 6:
Altered ROS production in SHIP1−/− neutrophils. (A) ROS production in neutrophils (5 × 105) from wild-type or SHIP1−/− mice after stimulation with 1 μM fMLP was evaluated by monitoring chemiluminescence every 7 s for 200 s. (B) PtdIns(3,4)P2 formation in neutrophils (5 × 106) from wild-type and SHIP1−/− mice after stimulation with 1 mM fMLP measured using PtdIns(3,4)P2 mass ELISA (see Materials and Methods). Data are presented as mean ± SD from three independent experiments. *p < 0.01. (C) Wild-type or SHIP−/− neutrophils were stimulated with fMLP, and the level of phosphorylated p40phox in the cell lysates was analyzed by Western blot. Actin was used as a control. (D, E) Neutrophils (5 × 105) from wild-type or SHIP1−/− mice were primed with TNFα (D) or MIP2 (E), resuspended in a media containing 0.1 or 5% BSA, and allowed to adhere on a fibronectin-coated surface. ROS production was measured by monitoring chemiluminescence every 30 s for 7000 s.
Integrin-mediated neutrophil activation can be attained by plating neutrophils primed with proinflammatory stimuli (such as TNFα or MIP2) and allowed to adhere on a surface coated with integrin ligand (such as fibronectin), which leads to the production of ROS that requires integrin β2 (Nathan et al., 1989; Mocsai et al., 2002). Under these experimental conditions, SHIP1−/− neutrophils could produce very high levels of ROS compared with wild-type neutrophils, but when the surface was coated with 5% BSA to inhibit cell adhesion, the amount of ROS generated was reduced to normal levels (Figure 6, D and E). Enhanced levels of PtdIns(3,4,5)P3 caused by cell adhesion in SHIP1−/− neutrophils would override the deficiency in PtdIns(3,4)P2 (which leads to reduced ROS production in suspension) and lead to activation of the NADPH oxidase complex, causing enhanced ROS production. This suggests that the increased adhesion-induced ROS production is mediated by the increased PtdIns(3,4,5)P3 production upon cell adhesion in SHIP1−/− neutrophils, and this effect can be rescued by reducing cell adhesion. However, fMLP stimulation in suspension causes reduced ROS production due to decreased levels of PtdIns(3,4)P2.
DISCUSSION
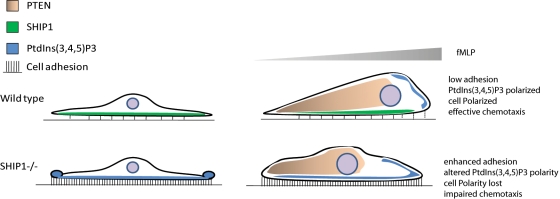
Migration toward a chemoattractant requires proper sensing of the chemoattractant gradient and appropriate cell attachment and detachment during the migratory process. There is a great body of evidence suggesting that PtdIns(3,4,5)P3, a second messenger generated by PI3K, is responsible for maintaining cell polarity in neutrophils by regulating subcellular localization and activation of downstream effectors essential for proper chemotaxis. During chemotaxis, an anterior–posterior PtdIns(3,4,5)P3 gradient is generated inside the cell that acts as a compass to facilitate directional movement along shallow chemoattractant gradients. This compass is largely controlled by the activity of PI3K that is recruited to the front of the cell and by the 3-phosphatase PTEN in Dictyostelium that is recruited to the back of the cell. Of interest, PTEN−/− neutrophils were able to migrate effectively. However, loss of the 5-phosphatase SHIP1 resulted in a dramatic defect in cell migration with enrichment of PtdIns(3,4,5)P3 at the cell cortex, altered F-actin polymerization, and loss of cell polarity. Dictyostelium does not contain the SHIP1 enzyme, so a parallel pathway involving the requirement of SHIP1 cannot be drawn from Dictyostelium models. Neutrophils also have integrins, which are not present in Dictyostelium. In neutrophils, integrins that bind to both the extracellular matrix and actin cytoskeleton have been suspected of functioning as an anchor (Hu et al., 2007). During cell migration, new adhesive contacts are formed at the front of the migrating cell and adhesive contacts are broken at the rear end. Signals from integrin-mediated cell adhesion also lead to the formation of PtdIns(3,4,5)P3 at the cell–substratum interface. We hypothesize that for proper chemotaxis an anterior–posterior PtdIns(3,4,5)P3 gradient is critical in driving F-actin polymerization at the leading edge, and formation of top-down PtdIns(3,4,5)P3 polarity could cause an imbalance in the anterior–posterior PtdIns(3,4,5)P3 gradient. For proper cell migration, formation of a PtdIns(3,4,5)P3 gradient between the top and bottom surfaces of the cell would be extremely limiting, as it would lead to F-actin polymerization at the site of cell adhesion and loss of polarity. This does not occur in normal cells. In this study, we identified the 5-inositol phosphatase SHIP1 as the key regulator necessary for abolishing the formation of a “top–bottom” PtdIns(3,4,5)P3 gradient upon cell adhesion and facilitate formation of new adhesive contacts at the leading edge and loss of adhesive contacts from the rear during cell migration toward a chemoattractant gradient.
We show that SHIP1−/− neutrophils respond to chemoattractant stimuli in suspension similarly to wild-type neutrophils. SHIP1−/− neutrophils polarize F-actin at the leading edge (Figure 1A) upon fMLP stimulation in suspension, producing similar levels of phosphorylated Akt (as a marker for the amount of PtdIns(3,4,5)P3) as wild-type neutrophils (Figure 4A). However, upon cell adhesion to an extracellular matrix protein (such as fibronectin), SHIP1−/− neutrophils lose polarity and F-actin is no longer polarized at the leading edge but is present throughout the cortex (Figure 1, B–D). Extensive Akt phosphorylation was observed in SHIP1−/− neutrophils upon adhesion, which correlated with the enrichment of PtdIns(3,4,5)P3 at the cell–substratum interface. These observations are supported by the finding that SHIP1−/− neutrophils under unstimulated conditions could adhere to a fibronectin-coated surface much more efficiently than wild-type neutrophils, but when stimulated with fMLP, which activates integrins by an inside-out signaling mechanism, both cell types adhere with similar efficiency (Figure 2, A and B).
Although PtdIns(3,4,5)P3 is pivotal in proper cell migration, the 3-inositol phosphatase PTEN and 5-inositol phosphatase SHIP1, which regulate PtdIns(3,4,5)P3 levels in the cell, have different roles in regulating chemotaxis. The 3-inositol phosphatase PTEN is extremely important in regulating PtdIns(3,4,5)P3 levels in the cell through antagonizing the activity of PI3K. The role of PTEN in chemotaxis is well understood from studies in Dictyostelium, where PTEN localized to the sides and rear of a migrating cell and thus amplified the intracellular PtdIns(3,4,5)P3 gradient. As a consequence, Dictyostelium lacking PTEN is defective in chemotaxis and shows an increased frequency of spontaneous protrusions and multiple pseudopods (Iijima and Devreotes, 2002). In some mammalian cell lines, PTEN shows a similar pattern of localization in migrating cells. However, deletion of PTEN in neutrophils demonstrated only minor alterations during chemotaxis toward fMLP but was shown to be involved in prioritizing end-target chemotactic cues (like fMLP and C5a) and preventing distractions from intermediary molecules (like IL-8, MIP2, and LTB4; Heit et al., 2008). When we analyzed the role of PTEN in adhesion, we observed that PTEN−/− neutrophils adhere to fibronectin-coated surfaces with similar efficiency as wild-type neutrophils under both unstimulated and fMLP-stimulated conditions (Figure 2, C and D). We also observed that unlike SHIP1−/− neutrophils, Akt phosphorylation is not increased in PTEN−/− neutrophils upon cell adhesion (Figure 4B). From our studies it is apparent that SHIP1 regulates PtdIns(3,4,5)P3 formation upon cell adhesion and limits PtdIns(3,4,5)P3 accumulation upon stimulation through integrin-mediated cell adhesion. We show that SHIP1 is present in the cytosol in suspended cells and translocates to the cell membrane and is tyrosine phosphorylated upon adhesion. SHIP1 also associates with various signaling molecules present at the sites of adhesion, such as the Src family kinase Lyn, FAK, and β3 integrin (Figure 3). This indicates that SHIP1 activity is indeed present at the site of cell adhesion and is important in preventing the formation of “top–bottom” PtdIns(3,4,5)P3 polarity. We also show that membrane recruitment followed by tyrosine phosphorylation of SHIP1 upon cell adhesion increases the 5-phosphatase activity toward PtdIns(3,4,5)P3 (Figure 3C). Thus this regulation is lost in SHIP1−/− neutrophils, and PtdIns(3,4,5)P3 is enriched at the cell–substratum interface (Figure 4, C and D). The “top–bottom” PtdIns(3,4,5)P3 polarity in the SHIP1−/- neutrophils causes increased cell attachment (Figure 2) and is a driving factor for impaired chemotaxis. Therefore, when we reduce cell attachment either by using excess BSA or blocking integrins with an RGD peptide in the buffer, the chemotaxis defect in SHIP1−/− neutrophils is rescued (Figure 5).
ROS production is regulated by the formation of phospholipids through various receptors. SHIP1 does not play a role in fMLP-mediated PtdIns(3,4,5)P3 formation in suspension, and as a result, we do not see any alteration in the level of phosphorylated Akt. We also show that upon stimulation of fMLP, loss of SHIP1 results in a reduced amount of PtdIns(3,4)P2, another important molecule in the activation of the NADPH oxidase complex, and as a result we observe reduced ROS levels upon fMLP stimulation in suspension. Conversely, upon adhesion, integrin-mediated PtdIns(3,4,5)P3 production in SHIP1−/− neutrophils overrides the deficiency in PtdIns(3,4)P2 levels and results in an increase in ROS production compared with wild-type neutrophils. The resulting increase in ROS production can be reduced by using excess BSA to limit cell adhesion (Figure 6).
Recent studies using knockout mice established a role for both 3-phosphatase PTEN and 5-phosphatase SHIP1 in neutrophil functions, but there is a lack of evidence showing the precise role of PTEN and SHIP1 in specific receptor-regulated PtdIns(3,4,5)P3 synthesis. We propose that the two inositol phosphatases act through different receptor-regulated processes to control spatial accumulation of PtdIns(3,4,5)P3 and establish a proper anterior–posterior PtdIns(3,4,5)P3 compass.
In this study, we show that SHIP1 acts as a negative regulator of integrin-mediated cell adhesion in neutrophils. In wild-type neutrophils, integrin-mediated cell adhesion results in PtdIns(3,4,5)P3 production at the sites of cell adhesion. Concurrently, SHIP1 at the cell–substratum interface is engaged, phosphorylated, and activated. This activity is crucial for dephosphorylating the PtdIns(3,4,5)P3 formed during cell adhesion.
With the combined actions of both SHIP1 and PTEN, PtdIns(3,4,5)P3 polarity is maintained at the leading edge, neutrophils polarize and there is effective cell migration. PTEN is localized to the rear end of a migrating cell to facilitate the accumulation of PtdIns(3,4,5)P3 at the anterior end, and SHIP1 is active at the cell–substratum interface to abolish the PtdIns(3,4,5)P3 gradient being formed by integrin activation. On loss of SHIP1, adhesion-mediated PtdIns(3,4,5)P3 formation is uncontrolled, resulting in the formation of “top-down” PtdIns(3,4,5)P3 polarity. Increased PtdIns(3,4,5)P3 levels enhance cell adhesion. This leads to activation of various PtdIns(3,4,5)P3 effector proteins, including Akt, and various PH domain–containing Rac exchange factors that could potentially activate Rac GTPases (Vedham et al., 2005; Ganesan et al., 2006). Activation of Rac1 would lead to F-actin polymerization throughout the cortex and loss of cell polarity. This impaired cell polarity and enhanced cell adhesion would consequently result in impaired chemotaxis (Figure 7). It is of note that in Dictyostelium, which does not have integrin molecules but can perform chemotaxis using a Gαs-mediated signaling process, lacks SHIP1 but contains a functional PTEN (Eichinger et al., 2005). It is possible that SHIP1 evolved much later during evolution along with integrins for their regulation.
FIGURE 7:
Model for a coordinated role of PTEN and SHIP1 in cell adhesion and chemotaxis. See the text for details.
MATERIALS AND METHODS
Mice
SHIP1+/−, a conditional PTEN-knockout mouse (PTENloxP/loxP), and the myeloid-specific Cre mouse were purchased from Jackson Laboratories (Bar Harbor, ME). SHIP1−/− and SHIP+/+ mice were generated through the mating of SHIP1+/− mice. Myeloid-specific PTEN-knockout mice were generated as previously described (Subramanian et al., 2007). All procedures involving mice were approved and monitored by the Children's Hospital of Boston Animal Care and Use Committee.
Cells, plasmids, and reagents
Mouse bone marrow neutrophils were isolated using a neutrophil enrichment kit (Stemcell Technologies, Vancouver, Canada) according to the manufacturer's protocol. The murine neutrophil isolation protocol routinely yields cell suspension that are >90% neutrophils with >98% viability as determined by Wright–Giemsa staining and trypan blue exclusion, respectively. Mouse bone marrow neutrophils were transfected with Akt-PH-EGFP using an Amaxa Nucleofector Kit (Amaxa Biosystems, Lonza, Cologne, Germany), using program Y001 according to the manufacturer's instructions. HL-60 cells were grown in RPMI + 20% fetal calf serum and differentiated into neutrophil-like cells with 1.3% dimethyl sulfoxide for 6 d.
Antibodies against phospho-Akt (Ser-473), phospho-Akt (Thr-308), SHIP1 (clone P290), Lyn, FAK, total Akt, and actin were obtained from Cell Signaling Technology (Beverly, MA). SHIP1 (clone P1C1) and β3 integrin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the phospho-tyrosine antibody (clone 4G10) was obtained from Millipore (Billerica, MA). Wortmannin and AS252424 were obtained from Cayman Chemical Company (Ann Arbor, MI). RGD peptide was obtained from AnaSpec (Freemont, CA).
Cell adhesion assay
The 96-well plates were coated with 10 μg/ml fibronectin at 37°C for 1 h and were blocked with 1% BSA in phosphate-buffered saline (PBS) for 1 h. Bone marrow neutrophils were isolated, resuspended at a density of 1 × 107/ml, and either left unstimulated or stimulated with fMLP (1 μM) for 2 min. Bone marrow neutrophils were then added (100 μl) to each well and allowed to adhere for 5, 15, or 30 min. Nonadherent cells were removed by washing thrice with PBS. Cells were lysed using 20 μl of 0.5% hexadecyltrimethylammonium bromide (CTAB). Peroxidase activity in the cell lysates was then measured using TMB as a substrate to reflect the amount of cells present. The reaction was stopped between 10 and 15 min, and absorbance at 450 nm was read. Total input (100 μl cells) was used as a control to determine relative cell adhesion for comparison.
Immunoprecipitation
Bone marrow neutrophils were either stimulated with fMLP for 2 min or allowed to adhere on a fibronectin-coated surface for 30 min. Unstimulated neutrophils in suspension were used as a control in either case. Neutrophils were lysed in IP lysis buffer containing 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10% glycerol, 1% Triton X-100, and 2 mM EDTA supplemented with protease inhibitors. Cell lysates were incubated with SHIP1 antibody (clone P1C1; Santa Cruz Biotechnology), and immune complexes were pulled down using Protein A + G agarose beads (Santa Cruz Biotechnology). Pull-down eluates were resolved by SDS–PAGE and probed for SHIP1 and tyrosine-phosphorylated proteins using 4G10 (Millipore).
Chemotaxis assay
The EZ-TAXIScan MIC-1000 (Hirata Corporation of America, Indianapolis, IN) was used to investigate real-time horizontal chemotaxis of mouse neutrophils. The EZ-TAXIScan consists of an etched silicon substrate and a flat glass plate, both of which form two compartments. Glass coverslips (Dow Corning, Midland, MI) coated with 0.1% or 2% BSA or 10 μg/ml fibronectin were placed on the glass plate at the bottom of the compartment. Purified bone marrow–derived wild-type and SHIP1−/− neutrophils in HBSS/Ca2+ + Mg2+ containing 0.1% BSA, 2% BSA, or 0.1% BSA with 1 μg/ml RGD peptide were added to the lower reservoir of each of the six channels and allowed to line up by removing 18 μl of buffer from the upper reservoir. A 1 μl amount of chemoattractant was added to the upper reservoir. Chemotaxis was recorded at 37°C for 20 min with 30-s intervals using a charge-coupled device camera. For analysis of cell tracks, (x, y) coordinates of migrating neutrophils were tracked from sequential images using DIAS imaging software (Soll Technologies, Iowa City, IA). Cell tracks were then realigned such that all of the cells started from the same starting point (0, 0) and were plotted using Matlab (MathWorks, Natick, MA). Directionality was calculated as the straight-line migration distance from the origin divided by total migration length. Migration speed (μm/min) was calculated as an average of cell speeds (migration distance between the current frame and previous frame divided by time between sequential frames, 0.5 min) for each captured frame. Parameters were only calculated for migrating cells during the 5- to 15-min time frame of each movie.
SHIP1 phosphatase assay
A total of 5 × 106 neutrophils either in suspension or adhered to fibronectin coated surface was lysed in IP lysis buffer and SHIP1 pulled down using SHIP1 antibody (clone P1C1) and Protein A + G agarose beads (Santa Cruz Biotechnology). Beads were washed thrice with phosphatase reaction buffer containing 25 mM Tris-HCl, pH 7.4, 140 mM NaCl, 2.7 mM KCl, and 10 mM dithiothreitol. SHIP1 pulled down was incubated with 3000 pmol of water-soluble PtdIns(3,4,5)P3 substrate (diC8 PIP3; Echelon Bioscience, Salt Lake City, UT) for 30–60 min at 37°C. Free phosphate generated from the SHIP1 activity was then quantified by malachite green phosphatase assay kit according to manufacturer's protocol (Echelon Biosciences). Percentage PtdIns(3,4,5)P3 conversion was determined for each time point as [(free phosphate in test reaction, pmol) − (free phosphate in background, pmol)] × 100%/3000 pmol. Free phosphate in background is the value of phosphate in “substrate-only [PtdIns(3,4,5)P3]” controls. Cell lysates were also analyzed for the levels of total SHIP1 and actin.
PtdIns(3,4)P2 ELISA
Bone marrow neutrophils from wild-type and SHIP1−/− mice were resuspended in PBS containing Ca2+/Mg2+ and 0.1% BSA at a density of 2 × 107 cells/ml. Cells (5 × 106) were stimulated with 1 μM fMLP for indicated times and immediately lysed in 0.5 M trichloroacetic acid. PtdIns(3,4)P2 was extracted from cells and subjected to ELISA using a PtdIns(3,4)P2 mass ELISA kit (Echelon Biosciences). Briefly, lipids were extracted with 2.25 ml of MeOH, CHCl3, and 12 M HCl (80:40:1) for 15 min at room temperature and partitioned by centrifugation after the addition of 0.75 ml of CHCl3 and 1.35 ml of 0.1 M HCl. The lower phase was vacuum dried and dissolved in PtdIns(3,4)P2 buffer. Controls, standards, and samples were incubated with PtdIns(3,4)P2 detector, secondary detection reagent, and TMB solution sequentially. The reaction was terminated by adding stop solution (0.5 M H2SO4), and the absorbance was measured at 450 nm. Experiments were repeated twice, and all controls, standards, and samples were run in triplicate per experiment. Data are presented as mean ± SD, and statistical significance was assessed by two-tailed, paired Student's t test.
ROS measurement
To measure ROS upon fMLP stimulation in suspension, murine neutrophils were resuspended in HBSS + 0.1% BSA after isolation (3 × 106 cells/ml) and stimulated with fMLP using a computer-programmed injector built into the luminometer (TriStar LB941; Berthold Technologies, Oak Ridge, TN). To measure the levels of ROS upon cell adhesion, plates were coated with fibronectin, and neutrophils were resuspended in HBSS + 0.1% BSA or HBSS + 5% BSA at 3 × 106 cells/ml. To detect extracellular ROS, 0.5 μM isoluminol and 80 U/μl horseradish peroxidase were added to the cell suspension. The production of ROS is determined by their ability to catalyze the oxidation of isoluminol, which results in light emission that can be detected using a luminometer.
Immunofluorescence microscopy
Differentiated HL-60 cells or mouse bone marrow neutrophils transfected with Akt-PH-EGFP were allowed to adhere on a fibronectin-coated glass surface and fixed. HL-60 cells were stained for SHIP1 (clone P290; Cell Signaling Technology). Images were acquired using a confocal microscope. Images were analyzed using Volocity software (PerkinElmer, Waltham, MA) to generate side-view projection cross-section images. Images were further analyzed using ImageJ (National Institutes of Health, Bethesda, MD) to determine fluorescence intensities across the section.
Statistical analysis
Analysis of statistical significance for indicated data sets was performed using the Student's t test capability on Excel (Microsoft. Redmond, WA).
Supplementary Material
Acknowledgments
The work is supported by National Institute of Health Grants HL085100, AI076471, HL092020, and GM076084 to H.R.L. and by a postdoctoral fellowship from the Deutscheforschungsgemeinshaft (Germany) to S.M. We thank Catlyn Blanchard and Jia Zhong for help with mice lines used in the study.
Abbreviations used:
- EGFP
enhanced green fluorescent protein
- FAK
focal adhesion kinase
- HBSS
Hank's balanced salt solution
- PH
pleckstrin homology domain
- PTEN
phosphatase and tensin homologue deleted in chromosome 10
- RGD
arginine–glycine–aspartic acid peptide
- SHIP1
SH2 domain containing inositol-5-phosphatase
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-10-0889) on February 9, 2012.
REFERENCES
- Axelsson L, Hellberg C, Melander F, Smith D, Zheng L, Andersson T. Clustering of beta(2)-integrins on human neutrophils activates dual signaling pathways to PtdIns 3-kinase. Exp Cell Res. 2000;256:257–263. doi: 10.1006/excr.2000.4816. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. J Biol Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- Condliffe AM, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- Cross AR, Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C, Davidson K, Anderson K, Stephens LR, Hawkins PT. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GJ, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- Ganesan LP, et al. FcgammaR-induced production of superoxide and inflammatory cytokines is differentially regulated by SHIP through its influence on PI3K and/or Ras/Erk pathways. Blood. 2006;108:718–725. doi: 10.1182/blood-2005-09-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P. PTEN functions to “prioritize” chemotactic cues and prevent “distraction” in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- Kriebel PW, Barr VA, Parent CA. Adenylyl cyclase localization regulates streaming during chemotaxis. Cell. 2003;112:549–560. doi: 10.1016/s0092-8674(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Lamkin TD, Walk SF, Liu L, Damen JE, Krystal G, Ravichandran KS. Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J Biol Chem. 1997;272:10396–10401. doi: 10.1074/jbc.272.16.10396. [DOI] [PubMed] [Google Scholar]
- Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung WH, Tarasenko T, Bolland S. Differential roles for the inositol phosphatase SHIP in the regulation of macrophages and lymphocytes. Immunol Res. 2009;43:243–251. doi: 10.1007/s12026-008-8078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell MJ, Yuan Y, Anderson KE, Hibbs ML, Salem HH, Jackson SP. SHIP1 and Lyn kinase negatively regulate integrin alpha IIb beta 3 signaling in platelets. J Biol Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright SD. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- Parry RV, Harris SJ, Ward SG. Fine tuning T lymphocytes: a role for the lipid phosphatase SHIP-1. Biochim Biophys Acta. 2010;1804:592–597. doi: 10.1016/j.bbapap.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem. 2000;275:19090–19097. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Puri KD, et al. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood. 2004;103:3448–3456. doi: 10.1182/blood-2003-05-1667. [DOI] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- Sly LM, Ho V, Antignano F, Ruschmann J, Hamilton M, Lam V, Rauh MJ, Krystal G. The role of SHIP in macrophages. Front Biosci. 2007;12:2836–2848. doi: 10.2741/2276. [DOI] [PubMed] [Google Scholar]
- Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Tu Z, Ninos JM, Ma Z, Wang JW, Lemos MP, Desponts C, Ghansah T, Howson JM, Kerr WG. Embryonic and hematopoietic stem cells express a novel SH2-containing inositol 5'-phosphatase isoform that partners with the Grb2 adapter protein. Blood. 2001;98:2028–2038. doi: 10.1182/blood.v98.7.2028. [DOI] [PubMed] [Google Scholar]
- Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol. 2005;25:4211–4220. doi: 10.1128/MCB.25.10.4211-4220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mantel C, Broxmeyer HE. Flt3 signaling involves tyrosyl-phosphorylation of SHP-2 and SHIP and their association with Grb2 and Shc in Baf3/Flt3 cells. J Leukoc Biol. 1999;65:372–380. doi: 10.1002/jlb.65.3.372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.