Ankrd 13A, 13B, and 13D constitute a family of ubiquitin-interacting motif (UIM)-bearing cytoplasmic proteins. They are anchored to the plasma membrane, where they recognize the Lys63-linked polyubiquitin chains tagged to ligand-activated EGF receptor and regulate the endocytosis of EGF receptor from the cell surface in mammalian cells.
Abstract
The mechanism of ubiquitin-dependent endocytosis of cell surface proteins is not completely understood. Here we examine the role of the ankyrin repeat domain (Ankrd) 13A, 13B, and 13D proteins, which constitute a functionally unknown family of ubiquitin-interacting motif (UIM)–bearing proteins, in the process. Stimulation of human HeLa cells with epidermal growth factor (EGF) rapidly induced direct binding of Ankrd 13 proteins to ubiquitinated EGF receptor (EGFR) via the UIMs. The binding was inhibited when the Ankrd 13 proteins underwent UIM-dependent monoubiquitination, suggesting that their activity is regulated by ubiquitination of themselves. Ankrd 13 proteins bound specifically to Lys-63–linked ubiquitin chains, which was consistent with a previous report that EGFR mainly undergoes Lys-63–linked polyubiquitination. Ankrd 13 proteins were anchored, via the central region and UIMs, to the plasma membrane, where they colocalized with EGFR. Finally, overexpression of wild-type as well as truncated-mutant Ankrd 13 proteins strongly inhibited rapid endocytosis of ubiquitinated EGFR from the surface in EGF-treated cells. We conclude that by binding to the Lys-63–linked polyubiquitin moiety of EGFR at the plasma membrane, Ankrd 13 proteins regulate the rapid internalization of ligand-activated EGFR.
INTRODUCTION
Ubiquitin (Ub) is a 76–amino acid (aa) protein highly conserved in eukaryotic cells. Via the C-terminal carboxyl group, Ub is conjugated to the ε-amino group of Lys residues in numerous intracellular proteins, a posttranslational modification referred to as monoubiquitination. Ub is also conjugated to one of seven Lys residues or the N-terminal amino group in another Ub molecule, forming eight structurally different polyUb chains. Among them, Lys-48– and Lys-63–linked chains are most abundant in the cell. Target proteins are often conjugated with polyUb chains, and this modification is referred to as polyubiquitination. The fate or function of ubiquitinated proteins is regulated in different ways, depending on the linkage pattern of conjugated Ub (Ikeda and Dikic, 2008; Komander, 2009).
On the plasma membrane, monoUb and/or Lys-63–linked polyUb chains serve as an endocytosis (internalization) signal that is tagged to a variety of cell surface integral membrane proteins in yeast and mammalian cells (Mukhopadhyay and Riezman, 2007; Traub and Lukacs, 2007; Lauwers et al., 2010). The role of Ub in endocytosis, however, is more complicated because not only cargo proteins, but also proteins of the endocytic machinery are regulated by their own ubiquitination (Acconcia et al., 2009). Epidermal growth factor (EGF) receptor (EGFR), belonging to the receptor tyrosine kinase family in mammals, is a cell surface protein whose mechanisms of endocytosis have been studied extensively (Madshus and Stang, 2009; Sorkin and Goh, 2009). Following stimulation with EGF, EGFR undergoes rapid ubiquitination (mainly Lys-63–linked polyubiquitination) by the Cbl family of Ub ligases (Levkowitz et al., 1998; Huang et al., 2006). Inhibition of the Cbl activity results in suppressed internalization of ligand-activated EGFR (Jiang and Sorkin, 2003; Huang et al., 2006; Bertelsen et al., 2011). By contrast, in-frame fusion of Ub to the C-terminal cytoplasmic tail of EGFR induces its internalization in the absence of ligand (Haglund et al., 2003; Mosesson et al., 2003; Bertelsen et al., 2011). These observations suggest that for EGFR, similar to other cell surface proteins, ubiquitination serves as a trigger that drives endocytosis from the cell surface.
The Ub-interacting motif (UIM) is an ∼20-aa sequence that specifically binds to Ub (Hofmann and Falquet, 2001). Although originally identified in the protein S5a, a component of the 19S regulatory subunit of the proteasome (Young et al., 1998), UIMs are found in multiple proteins involved in endocytic trafficking (Hofmann and Falquet, 2001). Among UIM-bearing proteins, epsin and eps15 participate in the clathrin-mediated endocytosis of ubiquitinated cell surface proteins. While binding to the Ub moiety of EGFR via three tandem UIMs, epsin also binds to clathrin, adaptor protein-2, which recruits clathrin to the cytoplasmic face of the plasma membrane, and a plasma membrane lipid phosphatidylinositol-(4,5)-bisphosphate, thereby incorporating ubiquitinated EGFR into the clathrin-coated vesicle (Maldonado-Báez and Wendland, 2006). eps15, which binds to epsin, bears two tandem UIMs and also sorts ubiquitinated EGFR into the clathrin-coated vesicle (Maldonado-Báez and Wendland, 2006). However, depletion of epsin or eps15 using RNA interference only modestly inhibits EGFR internalization (Huang et al., 2004; Kazazic et al., 2009). Binding of the ligand-activated hepatocyte growth factor receptor c-Met, which also undergoes ubiquitination, to eps15 requires the coiled-coil region, but not the UIMs, of eps15 (Parachoniak and Park, 2009). In yeast epsins (Ent1 and Ent2), a role for the UIMs in the interaction between proteins of the endocytic machinery was suggested (Dores et al., 2010). Therefore the overall picture of UIM-mediated endocytosis is unclear. Because there are other UIM-bearing proteins whose cellular functions have not been identified, the possible involvement of these proteins in endocytosis also remains to be examined.
The ankyrin repeat domain (Ankrd) 13 family of proteins consists of four members (13A, 13B, 13C, and 13D), with two characteristic features in primary structure (Figure 1A). In the N-terminal region, they have three ankyrin repeats, a protein–protein interaction domain found in numerous intracellular proteins (Li et al., 2006). In the C-terminal region, Ankrd 13A and 13D harbor four potential UIMs and Ankrd 13B harbors three. Ankrd 13C has no UIM. Recently Ankrd 13C was shown to localize to the endoplasmic reticulum, where it serves as a molecular chaperone that facilitates the trafficking of newly synthesized seven-transmembrane receptors to the plasma membrane (Parent et al., 2010). However, the functions of other Ankrd 13 proteins, which are distinguished from Ankrd 13C by the presence of UIMs, are completely unknown. In this study, we examine the cellular functions of Ankrd 13A, 13B, and 13D and show that these proteins regulate the internalization of ubiquitinated EGFR from the cell surface.
FIGURE 1:
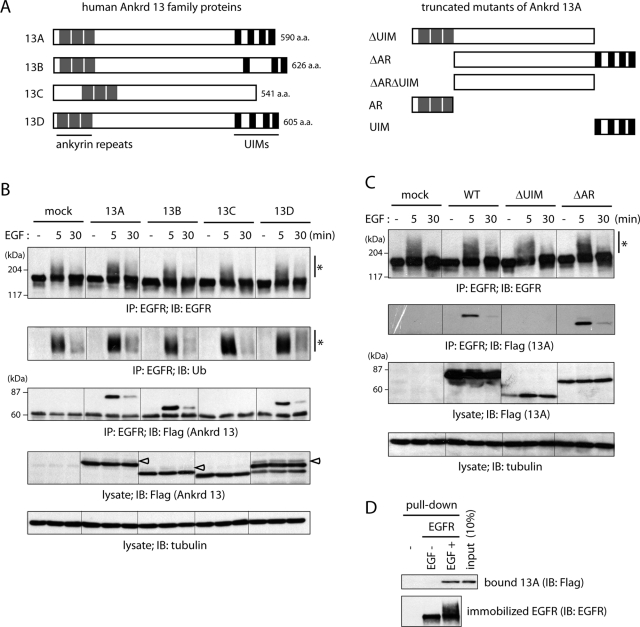
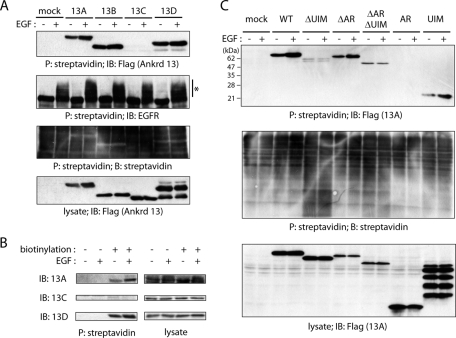
Ankrd 13A, 13B, and 13D bind to EGF-activated EGFR directly. (A) Schematic diagrams of the Ankrd 13 proteins and truncated mutants used in this study. (B, C) HeLa cells were transfected with the indicated FLAG-Ankrd 13 constructs and treated with EGF for 5 or 30 min. Lysates of the cells were immunoprecipitated (IP) with anti-EGFR antibody and immunoblotted (IB) with indicated antibodies. The lysates were also blotted with indicated antibodies. Asterisks and open arrowheads indicate ubiquitinated EGFR and monoubiquitinated Ankrd 13 proteins, respectively. (D) FLAG-Ankrd 13A was precipitated with anti-FLAG antibody from HeLa cells using a hot-lysis method and eluted with the FLAG peptide. EGFR was precipitated from untreated (−) and EGF-treated (+ [5 min]) HeLa cells by the hot-lysis method with anti-EGFR antibody coupled to protein A beads. Eluted Ankrd 13A was incubated with the EGFR-immobilized or unimmobilized beads, and bound proteins were blotted with indicated antibodies. Input (10%) of FLAG-Ankrd 13A is also shown.
RESULTS
Ectopically expressed Ankrd 13A, 13B, and 13D bind to EGF-activated EGFR directly in a UIM-dependent manner
To study the involvement of Ankrd 13 proteins in the endocytosis of ubiquitinated cell surface proteins, we first examined the interaction between Ankrd 13 proteins and EGFR in EGF-stimulated cells. HeLa cells were transfected with Ankrd 13 proteins tagged with a FLAG epitope at the N-terminus. After treatment with EGF for 5 or 30 min, EGFR was immunoprecipitated from the cells and analyzed by immunoblotting. Blotting with anti-EGFR and anti-Ub antibodies detected ubiquitinated EGFR after 5 min of EGF stimulation (Figure 1B, asterisks). Blotting with anti-FLAG antibody showed that Ankrd 13A, 13B, and 13D, but not 13C, bind to EGFR upon EGF treatment (Figure 1B). The binding reached its maximal level within 5 min after the stimulation and decreased in 30 min, correlating with the level of EGFR ubiquitination.
To determine the region required for EGFR binding in Ankrd 13A, we examined the binding of truncated mutants lacking the three ankyrin repeats (ΔAR) or four UIMs (ΔUIM; Figure 1A). Whereas deletion of the ankyrin repeats had no effect on EGFR binding, that of the UIMs completely abolished it (Figure 1C). These results suggested that Ankrd 13A, 13B, and 13D bind via the UIMs either directly to the Ub moiety of ligand-activated EGFR or to another ubiquitinated protein associated with activated EGFR. To discriminate between the possibilities, we performed a pull-down experiment using purified Ankrd 13A and EGFR. FLAG-Ankrd 13A–transfected HeLa cells were lysed at 100°C in the presence of 1% SDS to strip associated ubiquitinated proteins from FLAG-Ankrd 13A (a hot-lysis method; see Figure 3, C and D, later in the paper). FLAG-Ankrd 13A was then precipitated with anti-FLAG antibody from the lysate and eluted with the FLAG-competing peptide. Similarly, untreated and EGF-treated (5 min) HeLa cells were lysed with the hot-lysis method, and endogenous EGFR was precipitated with anti-EGFR antibody coupled to protein A beads. The EGFR-immobilized beads were incubated with eluted FLAG-Ankrd 13A, and, after washing of the beads, bound Ankrd 13A was detected by anti-FLAG immunoblotting. As shown in Figure 1D, binding of Ankrd 13A to EGFR from untreated cells, as well as its nonspecific binding to EGFR-free beads, was undetectable. However, Ankrd 13A was pulled down by EGFR when EGFR was isolated from EGF-treated cells. These results suggested that Ankrd 13 proteins bind to ubiquitinated EGFR directly.
FIGURE 3:
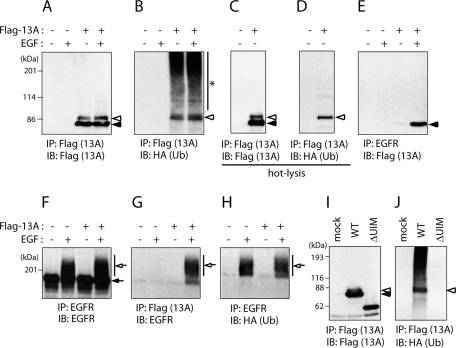
Ankrd 13A undergoes monoubiquitination. (A–H) HeLa cells were cotransfected with FLAG-Ankrd 13A and HA-Ub and treated with (+) or without (−) EGF for 5 min. Lysates of the cells were precipitated and blotted with indicated antibodies. In C and D, cell lysates were prepared with a hot-lysis method. (I, J) HeLa cells were transfected with FLAG-tagged wild-type (WT) Ankrd 13A or ΔUIM together with HA-Ub, and their lysates were precipitated and blotted with indicated antibodies. Closed and open arrowheads indicate unmodified and monoubiquitinated Ankrd 13A, respectively. Closed and open arrows indicate unmodified and ubiquitinated EGFR, respectively. An asterisk in B represents endogenous ubiquitinated proteins bound to FLAG-Ankrd 13A.
Endogenous Ankrd 13A and 13D bind to EGF-activated EGFR
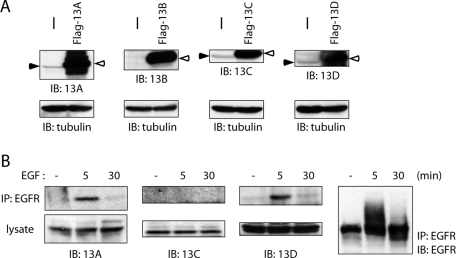
We raised antisera against specific regions of individual Ankrd 13 proteins and next examined the interaction between endogenous Ankrd 13 and EGFR. Expression of endogenous Ankrd 13A, 13C, and 13D, but not 13B, was detected with the antibodies in untransfected HeLa cells, whereas they all detected overexpressed FLAG-tagged versions strongly (Figure 2A). Because expression of Ankrd 13B mRNA was detected by reverse transcriptase–PCR experiments (unpublished data), the antibody was probably not sensitive enough to detect endogenous Ankrd 13B expressed at a low level. To examine the interaction between endogenous Ankrd 13 proteins and EGFR, untransfected HeLa cells were treated with EGF for 5 or 30 min, and EGFR was immunoprecipitated from the cells. Blotting of the precipitates with the anti–Ankrd 13 antibodies showed that endogenous Ankrd 13A and 13D, but not 13C, bind to EGFR upon EGF treatment with the same time course as transfected Ankrd 13 proteins (Figure 2B).
FIGURE 2:
Endogenous Ankrd 13A, 13B, and 13D bind to EGF-activated EGFR. (A) Lysates of untransfected HeLa cells (−) and those transfected with indicated FLAG-tagged Ankrd 13 proteins were blotted with indicated antibodies. Closed and open arrowheads indicate endogenous and ectopically expressed FLAG-Ankrd 13 proteins, respectively. (B) HeLa cells were treated with EGF for 5 or 30 min, and their lysates were precipitated with anti-EGFR antibody. The precipitates as well as the lysates were blotted with indicated antibodies.
Ankrd 13A undergoes monoubiquitination
When FLAG-tagged Ankrd 13A, 13B, and 13D, but not 13C, were expressed in HeLa cells, minor bands that migrate slightly more slowly than the major bands were detected with anti-FLAG antibody (Figure 1B, open arrowheads). Because several other UIM-bearing proteins undergo monoubiquitination (Polo et al., 2002), we examined whether the minor bands represent monoubiquitinated forms of Ankrd 13 proteins. HeLa cells were cotransfected with FLAG-Ankrd 13A and HA-tagged Ub, and their lysates were precipitated with anti-FLAG or anti-EGFR antibody. Again, blotting of the anti-FLAG precipitates with anti-FLAG antibody produced major (Figure 3A, closed arrowhead) and minor (Figure 3A, open arrowhead) bands. Blotting with anti-HA antibody gave the minor band (Figure 3B, open arrowhead), which was still detected when cell lysates were prepared using a hot-lysis method to strip associated proteins from Ankrd 13A (Figure 3C and D), suggesting that it represents monoubiquitinated Ankrd 13A. The Ub-positive, high–molecular weight smear that was coprecipitated with Ankrd 13A (Figure 3B, asterisk) represents endogenous ubiquitinated proteins that bound to the UIMs of overexpressed Ankrd 13A, because it was not precipitated from cell lysates prepared using the hot-lysis method (Figure 3D). Blotting with anti-EGFR antibody showed that FLAG-Ankrd 13A mainly coprecipitates ubiquitinated, but not unmodified, EGFR, indicating that Ankrd 13A specifically binds to ubiquitinated EGFR (Figure 3G). Blotting of the anti-EGFR precipitates with anti-FLAG antibody produced only unmodified Ankrd 13A, indicating that when monoubiquitinated, Ankrd 13A loses the ability to bind to ubiquitinated EGFR (Figure 3E). As with other UIM-bearing proteins (Polo et al., 2002), monoubiquitination of Ankrd 13A did not occur when UIMs were deleted (Figure 3, I and J).
The UIMs of Ankrd 13A, 13B, and 13D bind to Lys-63–linked Ub chains
A previous mass spectrometric analysis demonstrated that EGFR mainly undergoes Lys-63–linked polyubiquitination (Huang et al., 2006). We therefore examined whether Ankrd 13 proteins bind to Lys-63–linked Ub chains. COS-7 cells were transfected with FLAG-Ankrd 13 proteins and lysed with a hot-lysis method to strip Ankrd 13–bound ubiquitinated proteins. FLAG-Ankrd 13 proteins were then precipitated with anti-FLAG antibody coupled to protein A beads, and the Ankrd 13-immobilized beads were incubated with Lys-48– or Lys-63–linked Ub oligomers (dimer [Ub2] to heptamer [Ub7]). After washing the beads, bound Ub chains were detected by anti-Ub immunoblotting. As shown in Figure 4A, Ankrd 13A, 13B, and 13D, but not 13C, pulled down Lys-63–linked, but not Lys-48–linked, Ub chains. The binding affinity was much higher for Ub4-7 than for Ub3, and the binding to Ub2 was undetectable (Figure 4A). Incubation of the Ankrd 13–bound beads with Lys-63–linked Ub chains caused a substantial decrease in the amount of Ankrd 13 proteins bound to the beads, suggesting that for some reason, binding of Ub chains to FLAG-Ankrd 13 proteins destabilizes their binding with anti-FLAG antibody (Figure 4A, bottom). In addition, Ankrd 13A-ΔUIM did not pull down Ub chains, indicating that UIMs are responsible for the binding (Figure 4A).
FIGURE 4:
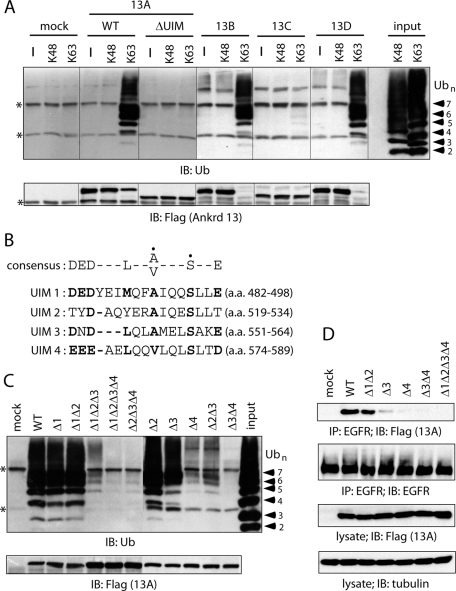
Ankrd 13A, 13B, and 13D bind to Lys-63–linked Ub chains. (A) FLAG-tagged Ankrd 13 proteins were precipitated from transfected COS-7 cells with anti-FLAG antibody–immobilized protein A beads using a hot-lysis method. The beads were incubated with Lys-48– or Lys-63–linked Ub chains, and bound proteins were blotted with indicated antibodies. Asterisks indicate nonspecific bands. (B) Sequence alignment of four UIMs in Ankrd 13A. The Ala/Val and Ser residues indicated with a dot in the consensus sequence were mutated in the mutants used in C and D. (C) FLAG-tagged Ankrd 13A UIM mutants were precipitated from COS-7 cells with anti-FLAG antibody–immobilized beads. The beads were incubated with Lys-63–linked Ub chains, and bound proteins were blotted with indicated antibodies. (D) HeLa cells were transfected with indicated Ankrd 13A UIM mutants and treated with EGF for 5 min. Lysates of the cells were precipitated and blotted with indicated antibodies.
UIMs in the C-terminal side are important for Ub binding of Ankrd 13A
Ankrd 13A has four potential UIMs, designated here as UIMs 1–4 from the N-terminal side (Figure 4B). To examine the contribution of each UIM to the Ub binding of Ankrd 13A, we generated a series of Ankrd 13A mutants with point mutations in the individual UIMs. In these mutants, the invariant Ala/Val and Ser residues (Figure 4B, indicated with a dot in the consensus sequence) were replaced with Gly and Ala, respectively, in each UIM. When UIM 1, 2, or 3 was individually mutated, Ub binding of Ankrd 13A was not significantly reduced compared with that of the wild-type protein (Figure 4C, Δ1, Δ2, Δ3). By contrast, the Ub-binding ability was drastically affected when UIM 4 was mutated (Figure 4C, Δ4). Although a combined mutation in UIMs 1 and 2 did not severely affect the binding (Figure 4C, Δ1Δ2), that in UIMs 2 and 3 considerably reduced it (Figure 4C, Δ2Δ3). A combined mutation in UIMs 3 and 4 resulted in almost a complete loss of Ub binding (Figure 4C, Δ3Δ4). These results suggested that UIM 4 plays a central role in the Ub binding of Ankrd 13A, whereas UIM 3 and, to a lesser extent, UIM 2 are required for maximal binding.
To examine the requirement of individual UIMs for the EGFR binding of Ankrd 13A in living cells, we performed a coimmunoprecipitation experiment in HeLa cells transfected with Ankrd 13A UIM mutants and treated with EGF for 5 min. Immunoprecipitation of the cell lysates with anti-EGFR antibody followed by anti-FLAG immunoblotting showed that UIMs 3 and 4 are important for EGFR binding of Ankrd 13A (Figure 4D), in good correlation with the mutants' affinity for Ub (Figure 4C).
Ankrd 13A, 13B, and 13D colocalize with EGFR on the plasma membrane
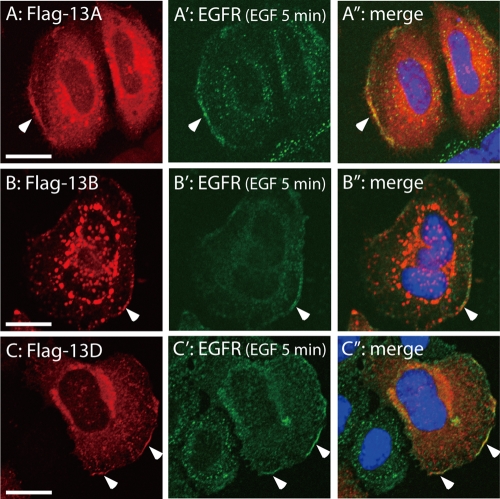
Because Ankrd 13A, 13B, and 13D bound maximally to EGFR when cells were treated with EGF for 5 min (Figure 1), they should colocalize with EGFR in these cells. We tested this possibility by immunofluorescence staining of HeLa cells expressing FLAG-Ankrd 13 proteins. On double staining with anti-FLAG and anti-EGFR antibodies at 5 min after EGF treatment, colocalization of Ankrd 13A, 13B, and 13D with EGFR was observed at the cell periphery in a proportion of the cells (Figure 5, arrowheads). By contrast, the distribution of Ankrd 13 proteins and EGFR never overlapped intracellularly (Figure 5). These results suggested that Ankrd 13 proteins bind to EGFR on the plasma membrane before EGFR undergoes internalization. Because this colocalization was readily observed in unstimulated cells at similar frequency (unpublished data), EGF-enhanced recruitment of Ankrd 13 proteins to the plasma membrane, which will be demonstrated biochemically later (see Figure 6), was undetectable by immunofluorescence staining.
FIGURE 5:
Ankrd 13A, 13B, and 13D colocalize with EGFR on the plasma membrane. HeLa cells were transfected with FLAG-tagged Ankrd 13A (A–A′′), 13B (B–B′′), or 13D (C–C′′), treated with EGF for 5 min, and immunostained with anti-FLAG (A–C) and anti-EGFR (A′–C′) antibodies. A′′–C′′ are merged images in which nuclei were also stained in blue. Arrowheads indicate the plasma membrane region where Ankrd 13 proteins and EGFR colocalize. Bars, 10 μm.
FIGURE 6:
Ankrd 13A, 13B, and 13D are anchored to the plasma membrane. HeLa cells were transfected with indicated FLAG-Ankrd 13 constructs (A, C) or not (B), incubated with (+) or without (−) EGF for 5 min, and labeled with biotin on the cell surface on ice. Lysates of the cells were precipitated with streptavidin beads and blotted with indicated antibodies or streptavidin. The lysates were also blotted with indicated antibodies. An asterisk in A indicates ubiquitinated EGFR.
FLAG-tagged Ankrd 13A, 13B, and 13D were also distributed intracellularly (Figure 5, A–C). Although they exhibited different intracellular localization patterns, 13A and 13B colocalized considerably with a late endosome marker—cation-independent mannose 6-phosphate receptor (CI-M6PR)—in the perinuclear region (Supplemental Figure S1, A–B′′). Ankrd 13D marginally colocalized with CI-M6PR (Supplemental Figure S1, C–C′′). Ankrd 13B, but not 13A or 13D, also colocalized with an early endosome marker—early endosome antigen 1 (EEA1; Supplemental Figure S1, D–F′′). On the other hand, the cytoplasmic reticular distribution pattern of Ankrd 13A and 13D did not overlap with that of CD3δ, a marker for the endoplasmic reticulum, at detectable levels (Supplemental Figure S1, G–I′′). In addition, immunostaining of Ankrd 13–transfected cells with FK2—an anti-Ub antibody that recognizes Ub-protein conjugates but not free Ub (Fujimuro and Yokosawa, 2005)—showed that ubiquitinated proteins are highly accumulated at all the Ankrd 13–positive sites, suggesting that overexpressed Ankrd 13A, 13B, and 13D bind to ubiquitinated proteins also intracellularly (Supplemental Figure S2).
Ankrd 13A, 13B, and 13D are anchored to the plasma membrane
To biochemically demonstrate that Ankrd 13 proteins localize to the plasma membrane, we examined whether they interact with biotinylated cell surface molecules. HeLa cells were transfected with FLAG-Ankrd 13 proteins and treated with or without EGF for 5 min. The cell surface was then labeled with biotin on ice, and biotinylated molecules were precipitated from the cell lysates with streptavidin beads. Immunoblotting of the precipitates with anti-FLAG antibody showed that FLAG-Ankrd 13A, 13B, and 13D, but not 13C, were coprecipitated with biotinylated molecules, suggesting that Ankrd 13A, 13B, and 13D are bound to a cell surface molecule(s) (Figure 6A). Ankrd 13 proteins were readily coprecipitated from unstimulated cells, indicating that they bind to a molecule other than ubiquitinated EGFR. However, since the amount of coprecipitated Ankrd 13 increased upon EGF stimulation, binding to ubiquitinated EGFR probably enhances Ankrd 13 association with the plasma membrane. Similarly, endogenous Ankrd 13A and 13D, but not 13C, were coprecipitated with biotinylated molecules from untransfected cells, and the amounts of plasma membrane-bound endogenous 13A and 13D were elevated after 5 min of EGF stimulation (Figure 6B). It should be noted that the amount of ubiquitinated EGFR on the cell surface was higher in cells overexpressing Ankrd 13A, 13B, or 13D than in mock-transfected cells after 5 min of EGF treatment (Figure 6A, asterisk). The significance of this observation will be further studied later (Figures 7–9).
FIGURE 7:
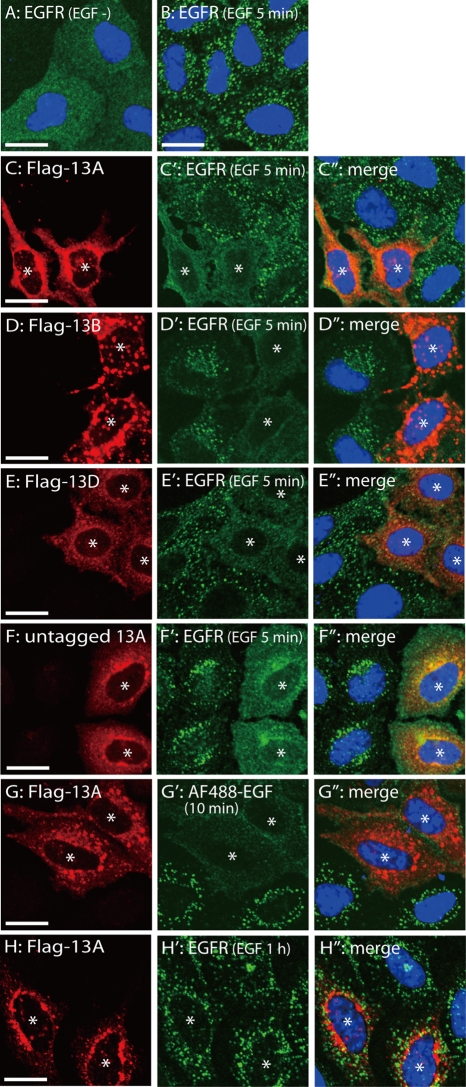
Overexpression of Ankrd 13A, 13B, or 13D inhibits EGFR internalization. (A, B) HeLa cells were untreated (A) or treated with EGF for 5 min (B) and stained with anti-EGFR antibody (green). Nuclei were also stained in blue. (C–E′′) HeLa cells were transfected with FLAG-tagged Ankrd 13A (C–C′′), 13B (D–D′′), or 13D (E–E′′), treated with EGF for 5 min, and stained with anti-FLAG (C–E) and anti-EGFR (C′–E′) antibodies. (F–F′′) HeLa cells were transfected with untagged Ankrd 13A, treated with EGF for 5 min, and stained with anti-Ankrd 13A (F) and anti-EGFR (F′) antibodies. (G–G′′) HeLa cells were transfected with FLAG-Ankrd 13A, incubated with Alexa Fluor 488 (AF488)–conjugated EGF for 10 min, and stained with anti-FLAG antibody (G). G′ shows the fluorescence signal by Alexa Fluor 488. (H–H′′) HeLa cells were transfected with FLAG-Ankrd 13A, treated with EGF for 1 h, and stained with anti-FLAG (H) and anti-EGFR (H′) antibodies. C′′–H′′ are merged images. Asterisks indicate cells overexpressing Ankrd 13 proteins. Bars, 10 μm.
FIGURE 8:
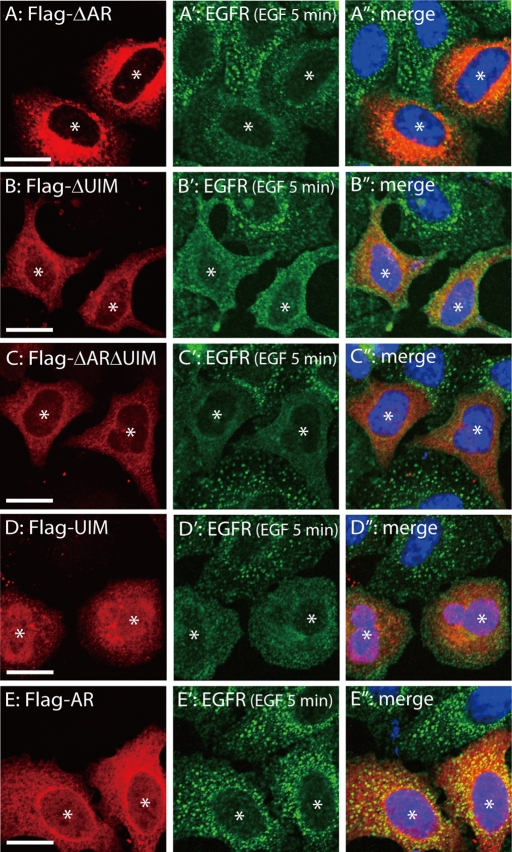
Overexpression of truncated Ankrd 13A mutants inhibits EGFR internalization. HeLa cells were transfected with FLAG-tagged Ankrd 13A-ΔAR (A–A′′), ΔUIM (B–B′′), ΔARΔUIM (C–C′′), UIM (D–D′′), or AR (E–E′′), treated with EGF for 5 min, and stained with anti-FLAG (A–E) and anti-EGFR (A′–E′) antibodies. A′′–E′′ are merged images in which nuclei were also stained in blue. Asterisks indicate cells expressing FLAG-Ankrd 13A constructs. Bars, 10 μm.
FIGURE 9:
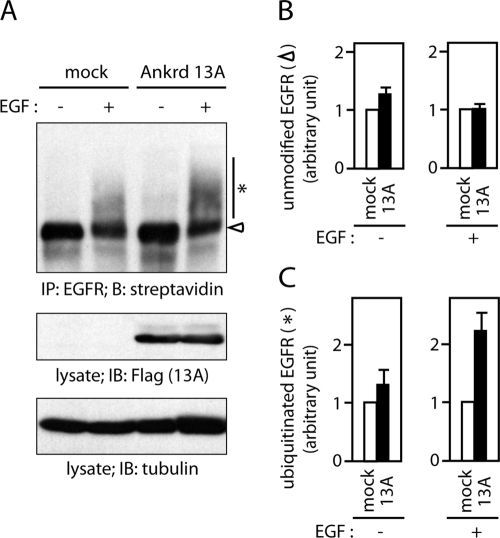
Internalization of ubiquitinated EGFR is inhibited by Ankrd 13A overexpression. (A) HeLa cells were transfected with or without FLAG-Ankrd 13A, incubated with (+) or without (−) EGF for 5 min, and labeled with biotin on the cell surface on ice. Lysates of the cells were precipitated with anti-EGFR antibody and blotted with streptavidin. The lysates were also blotted with indicated antibodies. An open arrowhead and an asterisk indicate unmodified and ubiquitinated EGFR, respectively. (B, C) The intensity of the bands of unmodified and ubiquitinated EGFR in each lane in A was measured. The quantification was performed on three independent experiments, and the amounts of unmodified (B) and ubiquitinated (C) EGFR in FLAG-Ankrd 13A–transfected cells relative to those in mock-transfected cells are shown as mean ± SD.
Whereas FLAG-Ankrd 13A was still precipitated with streptavidin beads when the ankyrin repeats were deleted (Figure 6C, ΔAR), the ankyrin repeats alone were not (Figure 6C, AR). By contrast, the UIMs alone bound to biotinylated molecules (Figure 6C, UIM) and deletion of the UIMs from Ankrd 13A drastically reduced the binding (Figure 6C, ΔUIM). Like full-length Ankrd 13A, the binding of two UIM-bearing mutants—ΔAR and UIM—to biotinylated molecules was enhanced by EGF treatment, again suggesting that the binding to cell surface–ubiquitinated EGFR contributes to the plasma membrane association of Ankrd 13A. Because two mutants bearing the central region but not the UIMs (ΔUIM and ΔARΔUIM) retained the ability to bind to biotinylated molecules and the binding was not enhanced by EGF treatment, the central region of Ankrd 13A participates in the steady-state plasma membrane association through Ub-independent interaction with an unidentified cell surface molecule.
Overexpression of Ankrd 13A, 13B, or 13D inhibits rapid EGFR internalization
To elucidate the biological significance of Ankrd 13 interaction with EGFR, we examined the effect of Ankrd 13 overexpression on the endocytic trafficking of EGFR in HeLa cells. Immunofluorescence staining of FLAG-Ankrd 13–transfected cells with anti-FLAG and anti-EGFR antibodies showed that when unstimulated, transfection of FLAG-Ankrd 13 proteins does not affect the distribution of EGFR at the cell surface (Supplemental Figure S3). Following EGF treatment for 5 min, EGFR was internalized and transported to the early endosome in untransfected cells (Figure 7, A and B). In striking contrast, EGFR was mostly retained at the surface in cells overexpressing FLAG-tagged Ankrd 13A, 13B, or 13D (Figure 7, C–E′′). To exclude the possibility that the effect was due to the tagging of the FLAG epitope, we overexpressed Ankrd 13A bearing no epitope tag and treated the cells with EGF for 5 min. Costaining with anti-Ankrd 13A and anti-EGFR antibodies showed that overexpression of untagged Ankrd 13A similarly inhibits EGFR internalization (Figure 7, F–F′′). We also examined the effect of FLAG-Ankrd 13A overexpression on the uptake of a fluorescence-labeled EGF ligand. Transfected HeLa cells were incubated with Alexa Fluor 488–conjugated EGF for 10 min, fixed, and stained with anti-FLAG antibody. Internalization of the fluorescence-labeled EGF to the early endosome, which was clearly observed in untransfected cells, was drastically inhibited in Ankrd 13A-overexpressing cells (Figure 7, G–G′′). Because the cell surface EGFR level was not reduced by overexpression of Ankrd 13A, 13B, or 13D (Figure 6A and Supplemental Figure S3), these results suggested that Ankrd 13 overexpression inhibits the ligand-induced internalization of EGFR. It should be noted, however, that after 1 h of EGF treatment, the EGFR in cells overexpressing Ankrd 13A, 13B, or 13D was internalized and exhibited the same subcellular distribution as that in untransfected cells (Figure 7, H–H′′, and Supplemental Figure S4), suggesting that Ankrd 13 overexpression does not lead to the complete inhibition of EGFR internalization.
An inhibitory effect on rapid EGFR internalization was similarly observed when Ankrd 13A-ΔAR (Figure 8, A–A′′), ΔUIM (Figure 8, B–B′′), ΔARΔUIM (Figure 8, C–C′′), or just the UIMs (Figure 8, D–D′′) were overexpressed, but the ankyrin repeats alone had no effect (Figure 8, E–E′′). In addition, the uptake of Alexa Fluor 488–conjugated transferrin was also substantially suppressed by Ankrd 13A overexpression (Supplemental Figure S5). These results are considered in the Discussion.
Internalization of ubiquitinated EGFR is inhibited by Ankrd 13A overexpression
To provide further evidence that Ankrd 13 overexpression inhibits EGFR internalization, we detected cell surface EGFR biochemically. HeLa cells were transfected with FLAG-Ankrd 13A and treated with or without EGF for 5 min. The cell surface was labeled with biotin on ice, and EGFR was precipitated from their lysates with anti-EGFR antibody. Biotinylated EGFR was then detected with streptavidin (Figure 9A) and the intensity of the bands corresponding to unmodified (open arrowhead) and ubiquitinated (asterisk) EGFR in the blotting membrane was quantified. The surface level of unmodified EGFR was scarcely affected by Ankrd 13A overexpression in both untreated and EGF-treated cells (Figure 9B). Ankrd 13A overexpression also did not drastically affect the surface level of ubiquitinated EGFR in untreated cells (Figure 9C, left). However, it was elevated by ∼2.2-fold at 5 min after EGF treatment (Figure 9C, right). This must be an underestimate because in our transfection experiments, FLAG-Ankrd 13A was overexpressed in at most 50% of the cells (unpublished data). These results suggested that the internalization of ubiquitinated EGFR is inhibited by Ankrd 13A overexpression. As noted before, the same results were obtained, although somewhat less clearly, in a converse experiment in which biotinylated proteins were first precipitated with streptavidin and the precipitates were blotted with anti-EGFR antibody (Figure 6A, asterisk).
Overexpression of Ankrd 13A, 13B, or 13D does not inhibit EGFR degradation
Degradation of EGFR was not yet observed in HeLa cells at 5 or 30 min after EGF stimulation, and overexpression of wild-type or truncated Ankrd 13 proteins did not affect the amount of EGFR at these time points (Figure 1, B and C, top). To examine whether the inhibition of rapid EGFR internalization by Ankrd 13 overexpression affects the rate of EGFR degradation at later time points, we incubated HeLa cells overexpressing FLAG-Ankrd 13 proteins for 1 or 3 h in the presence of EGF and examined the total cellular level of EGFR by immunoblotting of the lysates. The EGFR level was comparable between untransfected cells and those overexpressing each of the Ankrd 13 proteins throughout the duration of EGF treatment (Supplemental Figure S6). These results were consistent with the observation that EGFR is normally internalized in Ankrd 13–overexpressing cells after 1 h of EGF treatment (Figure 7, H–H′′, and Supplemental Figure S4). The rate of EGFR degradation was also unaffected by overexpression of Ankrd 13A-ΔAR, ΔUIM, or ΔARΔUIM (Supplemental Figure S6).
DISCUSSION
Ankrd 13 proteins participate in EGFR internalization
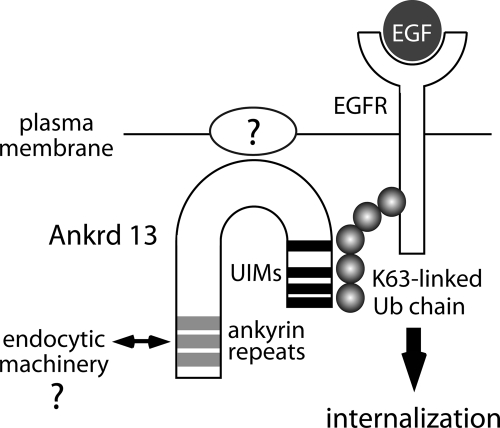
Ubiquitination serves as two distinct sorting signals for cell surface proteins, including EGFR, in the endocytic pathway—an internalization signal from the cell surface and a lysosome-targeting signal from the endosome (Mukhopadhyay and Riezman, 2007; Traub and Lukacs, 2007; Lauwers et al., 2010). The results presented in this study strongly suggest that the Ankrd 13 family of Ub-binding proteins acts at the plasma membrane to regulate the internalization of ubiquitinated cell surface proteins. A schematic model for the function of Ankrd 13A, 13B, and 13D is shown in Figure 10.
FIGURE 10:
Schematic model for the Ankrd 13 function. Ankrd 13A, 13B, and 13D are anchored to the plasma membrane via their central regions by binding to an unidentified cell surface molecule. They recognize the Lys-63–linked Ub moiety of ligand-activated EGFR and facilitate its rapid internalization, possibly through interaction of their ankyrin repeats with the endocytic machinery.
Despite evidence that the ubiquitination of EGFR is necessary for its internalization (Madshus and Stang, 2009), EGFR mutants that lack major ubiquitination sites and therefore do not undergo detectable levels of ubiquitination are still internalized normally upon ligand treatment (Huang et al., 2007). It is unclear whether this observation indicates that ubiquitination of EGFR is dispensable for its internalization or an undetectable level of ubiquitination is sufficient for normal internalization. Our finding that the interaction of Ankrd 13 with ubiquitinated EGFR plays a role in its internalization supports the latter possibility and further implicates EGFR ubiquitination in the internalization process.
Although EGFR internalization was significantly inhibited in Ankrd 13–overexpressing cells at 5 min after EGF treatment, the intracellular distribution of EGFR was unaffected by Ankrd 13 overexpression 1 h after the same treatment (Figure 7 and Supplemental Figure S4). Therefore EGFR internalization was delayed but not completely inhibited in Ankrd 13–overexpressing cells. In addition, the rate at which ligand-activated EGFR is degraded was not affected by Ankrd 13 overexpression either (Figure 1 and Supplemental Figure S6), suggesting that the delay in internalization does not have a profound effect on degradation. On the basis of these results, we propose that Ankrd 13A, 13B, and 13D regulate the rate of rapid internalization but are not essential for internalization. Further study is necessary to elucidate the biological significance of this Ankrd 13 function.
When overexpressed, Ankrd 13 proteins were detected also intracellularly, where they colocalized with endosome markers at least partly (Supplemental Figure S1). Furthermore, the intracellularly overexpressed Ankrd 13 proteins caused the accumulation of ubiquitinated proteins in the same compartments (Supplemental Figure S2). Therefore Ankrd 13A, 13B, and 13D may also play roles intracellularly in the endocytic pathway. To address the possibility, it is important to elucidate whether endogenous Ankrd 13 proteins are also distributed intracellularly. Our anti–Ankrd 13 antibodies, however, failed to provide specific signals in immunostaining of untransfected HeLa cells (Figure 7 and unpublished data).
Mode of Ub binding of Ankrd 13 proteins
The UIMs of Ankrd 13 proteins exhibited binding specificity for Lys-63–linked Ub chains composed of more than three Ub molecules (Figure 4). The low affinity for shorter chains of two or three Ub molecules is a general feature of UIMs (Young et al., 1998; Hawryluk et al., 2006; Barriere et al., 2007; Winborn et al., 2008). Previous studies showed that different UIMs exhibit different binding preferences toward Lys-48– and Lys-63–linked Ub chains. For instance, the UIMs of S5a (Wang et al., 2005) and the deubiquitinating enzymes DUBA (Kayagaki et al., 2007) and ataxin-3 (Winborn et al., 2008) bind to Lys-48– and Lys-63–linked chains with similar affinity. Whereas the UIM of the yeast transcription factor Met4 prefers Lys-48–linked chains (Flick et al., 2006), those of Hrs (Barriere et al., 2007) and epsin (Sato et al., 2009) are specific to Lys-63–linked chains. For Rap80, a component of the DNA repair complex, the distance between two tandem UIMs is shown to confer the specificity for Lys-63–linked Ub chains (Sato et al., 2009). This, however, would not be the case for Ankrd 13 because a series of combined mutations in individual UIMs did not change the specificity of Ankrd 13A toward Lys-63–linked (Figure 4) and Lys-48–linked (unpublished data) Ub chains, although the binding affinity to Lys-63–linked chains was reduced by the mutations. Because several mammalian plasma membrane proteins, including EGFR, undergo Lys-63–linked polyubiquitination (Geetha et al., 2005; Duncan et al., 2006; Huang et al., 2006; Varghese et al., 2008; Goto et al., 2010), the specific binding of Ankrd 13 proteins to Lys-63–linked Ub chains is consistent with their role in the internalization of cell surface proteins in mammalian cells. In addition, the requirement of Ankrd 13A's four individual UIMs for Ub binding differed considerably (Figure 4). Because mutations in the UIMs affected Ub binding more severely when mutated UIMs were located closer to the C-terminal end of the protein, the proximity of UIMs to the C-terminus of Ankrd 13 may be important in determining the contribution to Ub binding.
Ankrd 13A underwent monoubiquitination (Figure 3). Similar to other UIM-bearing endocytic proteins, such as epsin, eps15, and Hrs (Polo et al., 2002), an Ankrd 13A mutant lacking the UIMs was not ubiquitinated, indicating that its own UIMs are required for the modification. In addition, monoubiquitinated Ankrd 13A lost the ability to bind to ubiquitinated EGFR (Figure 3). As previously suggested for other UIM-bearing proteins (Hoeller et al., 2006), this is possibly due to a masking effect of conjugated Ub, which binds to the UIMs of Ankrd 13A and inhibits its interaction with ubiquitinated cargo proteins. Therefore monoubiquitination is likely to serve as a negative-regulatory mechanism of Ankrd 13 function.
How do Ankrd 13 proteins regulate EGFR internalization?
Overexpression of not only wild-type Ankrd 13A, 13B, and 13D, but also of truncated mutants of Ankrd 13A, which were expected to act as dominant-negative versions, inhibited EGFR internalization (Figures 7 and 8), raising the question of whether Ankrd 13 proteins have a stimulatory or inhibitory role in the process. To discriminate between the possibilities, we tried RNA interference experiments that examine the effect of Ankrd 13 depletion on EGFR internalization. We generated multiple small interfering RNAs (siRNAs) that can knock down Ankrd 13A, 13B, and 13D individually in HeLa cells. Because the results in this study strongly suggested a redundant role for Ankrd 13 proteins in endocytosis, we examined cells transfected with a mixture of three siRNAs for Ankrd 13A, 13B, and 13D. When mixed, however, these siRNAs failed to deplete the three Ankrd 13 mRNAs/proteins simultaneously. Therefore, although EGFR internalization was not significantly affected by the siRNAs, this is possibly due to an incomplete inhibition of the Ankrd 13 function (unpublished data). Nonetheless, we suggest a positive-regulatory role for Ankrd 13 for the following reason. Because Ankrd 13A, 13B, and 13D are multidomain proteins, they possibly serve as an adaptor that couples ubiquitinated EGFR to a third protein in the endocytic machinery (Figure 10). If so, when wild-type Ankrd 13 proteins are overexpressed, some bind only to ubiquitinated EGFR, whereas others bind only to the unidentified endocytic protein, thereby preventing the formation of the trimeric complex of EGFR, Ankrd 13, and the endocytic protein. We thus speculate that ectopic overexpression of not only truncated but also wild-type Ankrd 13 inhibits the function of endogenous Ankrd 13. In summary, we propose that Ankrd 13A, 13B, and 13D positively regulate the internalization of ligand-activated EGFR by binding to the Ub moiety of ubiquitinated EGFR at the plasma membrane. The uptake of transferrin was also substantially suppressed by Ankrd 13A overexpression (Supplemental Figure S5). Because the transferrin receptor normally does not undergo ubiquitination, these results suggest that overexpressed Ankrd 13 also inhibits internalization of nonubiquitinated cargo proteins without binding to them. This effect could also be caused by an excessive, unbalanced interaction of overexpressed Ankrd 13 proteins with the endocytic machinery.
Both epsin and esp15 recruit ubiquitinated EGFR to the clathrin-coated vesicle through interaction with the coat components (Maldonado-Báez and Wendland, 2006). To understand the mechanism by which Ankrd 13 proteins facilitate EGFR internalization, we examined the interaction of Ankrd 13A with clathrin heavy and light chains, as well as the α and β2 subunits of adaptor protein-2, in coimmunoprecipitation experiments. However, we did not detect interaction with any of the proteins (unpublished data). We also did not detect any interaction with membrane phospholipids, including various phosphoinositide species, in a lipid–protein overlay experiment using purified Ankrd 13A-ΔUIM as a probe (unpublished data). Nevertheless, the ankyrin repeats are known as protein–protein interaction domains (Li et al., 2006). We also found that the central region of Ankrd 13A contributes to its association with the plasma membrane (Figure 6). The identification of molecules that interact with these regions will help elucidate the precise role of Ankrd 13 in endocytosis.
MATERIALS AND METHODS
cDNA expression constructs
The cDNA for human Ankrd 13A was cloned from a human brain cDNA library (Clontech, Mountain View, CA). The cDNA for human Ankrd 13B was obtained from Open Biosystems (Huntsville, AL). The cDNAs for human Ankrd 13C and 13D were obtained from Invitrogen (Carlsbad, CA). These cDNAs were inserted into the N-terminally FLAG- and hemagglutinin (HA)-tagged mammalian expression vectors pME-FLAG and pME-HA. cDNAs for Ankrd 13A-ΔAR, ΔUIM, ΔARΔUIM, AR, and UIM were amplified from the full-length cDNA by PCR and cloned into pME-FLAG. Ankrd 13A cDNAs with point mutations in the UIMs were generated using the QuikChange Site-Directed Mutagenesis System (Stratagene, La Jolla, CA) and inserted into pME-FLAG. The HA-tagged Ub and FLAG-tagged CD3δ expression vectors were provided by T. Suzuki (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) and N. Nakamura (Tokyo Institute of Technology, Yokohama, Japan), respectively. cDNAs for clathrin light chain and adaptor protein-2 subunits were provided by P. De Camilli and D. Toomre (Yale University, New Haven, CT) and H. Ohno (RIKEN Research Center for Allergy and Immunology, Yokohama, Japan), respectively, and inserted into the expression vector pME-HA.
Cell culture and DNA transfection
HeLa and COS-7 cells were grown in DMEM supplemented with 10% fetal bovine serum, and DNA was transfected into the cells using the FuGENE6 transfection reagents (Roche Diagnostics, Indianapolis, IN) for 48 h. To stimulate cells with EGF, they were grown in the presence of 0.5% fetal bovine serum for 24 h and subsequently incubated with human EGF (100 ng/ml; PeproTech, Rocky Hill, NJ) in the presence of 10 μg/ml cycloheximide or with Alexa Fluor 488–conjugated EGF (200 ng/ml; Invitrogen) at 37°C. To examine transferrin uptake, cells were incubated at 37°C with Alexa Fluor 488–conjugated transferrin (50 μg/ml, Invitrogen). Biotinylation of cell surface molecules was performed on ice, using EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL), according to the manufacturer's instructions.
Anti–Ankrd 13 antibodies
A peptide corresponding to human Ankrd 13A (aa 279–297) was chemically synthesized (Biologica, Nagoya, Japan). cDNAs for unique regions of human Ankrd 13B (aa 535–587), 13C (aa 2–145), and 13D (aa 514–566) were inserted into the plasmid pGEX6P-2 (GE Healthcare, Piscataway, NJ) to generate glutathione S-transferase (GST) fusion proteins in Escherichia coli cells. The synthetic peptide and GST-fusion proteins were used to immunize rabbits.
Cell lysate preparation
Cell lysates were prepared by solubilizing cells in 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 50 mM NaF, 0.5% Nonidet P-40, 1 mM EDTA, 10 mM N-ethylmaleimide, 1 mM phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A and collecting the supernatants after centrifugation. To prepare lysates with the hot-lysis method, cells were lysed in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% SDS, and 1 mM EDTA for 10 min at 100°C. After centrifugation, supernatants were diluted fourfold with 1.33% Triton X-100.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed using standard procedures. Anti-EGFR (0.1 μg; MBL, Nagoya, Japan) and M2 anti-FLAG (1 μg; Sigma-Aldrich, St. Louis, MO) antibodies were used for immunoprecipitation. Biotinylated proteins were precipitated with streptavidin beads (GE Healthcare). Primary antibodies for immunoblotting were as follows: anti-EGFR (0.5 μg/ml; MBL), FK2 anti-Ub (1 μg/ml; MBL), anti-Ub (5 μg/ml; Covance, Princeton, NJ), M2 anti-FLAG (4 μg/ml; Sigma-Aldrich), anti–α-tubulin (0.02 μg/ml; Abcam, Cambridge, United Kingdom), anti-HA (0.1 μg/ml; Roche Diagnostics), anti–Ankrd 13A (1:800), anti–Ankrd 13B (1:800), anti–Ankrd 13C (1:800), anti–Ankrd 13D (1:800), and anti–clathrin heavy chain (0.4 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were peroxidase-conjugated anti–mouse, –rat, and –rabbit immunoglobulin G (IgG) antibodies (GE Healthcare). Peroxidase-conjugated streptavidin (GE Healthcare) was used to detect biotinylated proteins. Blots were detected using the ECL Western Blotting Detection Reagents (GE Healthcare). The intensity of bands in blotted membranes was quantified using ImageJ (National Institutes of Health, Bethesda, MD).
Ub-binding assay
Lys-48– and Lys-63–linked polyUb chains (Ub2-7) were purchased from Affiniti Research Products (Exeter, United Kingdom). M2 anti-FLAG antibody (1 μg; Sigma-Aldrich) was immobilized on protein A–Sepharose beads (10 μl; GE Healthcare) to immunoprecipitate FLAG-Ankrd 13 proteins from lysates of transfected COS-7 cells in a 6-cm dish using the hot-lysis method. The FLAG-Ankrd 13–bound beads were incubated with the Ub chains (1 μg) in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, and 1 mM EDTA for 16 h at 4°C. The beads were washed with the same buffer, and bound Ub chains were detected by immunoblotting.
Immunofluorescence staining
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline, permeabilized in 0.2% Triton X-100 in phosphate-buffered saline, and blocked in 5% fetal bovine serum in phosphate-buffered saline. Cells were then incubated with rabbit anti-FLAG (2 μg/ml; Sigma-Aldrich), M2 mouse anti-FLAG (2 μg/ml; Sigma-Aldrich), anti-HA (1 μg/ml; Roche Diagnostics), anti-EGFR (10 μg/ml; MBL), anti-EEA1 (1 μg/ml, BD Transduction Laboratories, Lexington, KY), anti–CI-M6PR (5 μg/ml; provided by E. Kominami, Juntendo University, Tokyo, Japan), and FK2 anti-Ub (10 μg/ml; MBL) antibodies. Secondary antibodies were Alexa Fluor 488– and Alexa Fluor 594–conjugated anti–mouse, –rat, and –rabbit IgG antibodies (1:1000; Invitrogen). Nuclei were stained with TO-PRO-3 iodide (642/661; 50 μM; Invitrogen) during incubation with the secondary antibodies. Fluorescence images were captured with a laser-scanning confocal microscope (Axiovert 200M; Carl Zeiss, Oberkochen, Germany).
RNA interference
To knock down Ankrd 13A and 13B, plasmid vectors that allow the production of siRNAs for human Ankrd 13A and 13B mRNAs were constructed using the siRNA expression vector pSilencer 1.0-U6 (Ambion, Austin, TX). They target the nucleotide residues 115–133 or 1752–1770 for 13A and 41–59 or 1088–1106 for 13B from the translation initiation codon. Because we failed to obtain effective siRNA expression vectors for 13D, the BLOCK-iT kits (Invitrogen) were used according to the manufacturer's instructions to synthesize double-strand RNA corresponding to nucleotide residues 1851–1967 of human Ankrd 13D mRNA (3′ noncoding region) and process it with the endoribonuclease Dicer in vitro. The siRNA expression vectors and Dicer-processed siRNAs were transfected into cells twice at 48-h intervals.
Phospholipid-protein overlay assay
The Ankrd 13A-ΔUIM cDNA was inserted into pGEX6P-2 (GE Healthcare), and the GST-fusion protein was purified from E. coli cells using glutathione–Sepharose beads (GE Healthcare). The PIP Strips membrane (Echelon Biosciences, Salt Lake City, UT) was incubated with the GST-fusion protein (1 μg/ml), and bound protein was detected with anti-GST antibody (0.2 μg/ml; Santa Cruz Biotechnology) according to the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Akiko Mukai and Kunitoshi Ito for help in the initial phase of this study and Toshiaki Suzuki, Eiki Kominami, Nobuhiro Nakamura, Pietro De Camilli, Derek Toomre, and Hiroshi Ohno for reagents. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (21025011 and 21113505 to M.K.).
Abbreviations used:
- aa
amino acid
- Ankrd
ankyrin repeat domain
- CI-M6PR
cation-independent mannose 6-phosphate receptor
- EEA1
early endosome antigen 1
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- GST
glutathione S-transferase
- siRNA
small interfering RNA
- Ub
ubiquitin
- UIM
ubiquitin-interacting motif
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-09-0817) on February 1, 2012.
REFERENCES
- Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: the network at work. Exp Cell Res. 2009;315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Barriere H, Nemes C, Du K, Lukacs GL. Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. Mol Biol Cell. 2007;18:3952–3965. doi: 10.1091/mbc.E07-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen V, Sak MM, Breen K, Rødland MS, Johannessen LE, Traub LM, Stang E, Madshus IH. A chimeric pre-ubiquitinated EGF receptor is constitutively endocytosed in a clathrin-dependent, but kinase-independent manner. Traffic. 2011;12:507–520. doi: 10.1111/j.1600-0854.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- Dores MR, Schnell JD, Maldonado-Baez L, Wendland B, Hicke L. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11:151–160. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick K, Raasi S, Zhang H, Yen JL, Kaiser P. A ubiquitin-interacting motif protects polyubiquitinated Met4 from degradation by the 26S proteasome. Nat Cell Biol. 2006;8:509–515. doi: 10.1038/ncb1402. [DOI] [PubMed] [Google Scholar]
- Fujimuro M, Yokosawa H. Production of antipolyubiquitin monoclonal antibodies and their use for characterization and isolation of polyubiquitinated proteins. Methods Enzymol. 2005;399:75–86. doi: 10.1016/S0076-6879(05)99006-X. [DOI] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Goto E, Yamanaka Y, Ishikawa A, Aoki-Kawasumi M, Mito-Yoshida M, Ohmura-Hoshino M, Matsuki Y, Kajikawa M, Hirano H, Ishido S. Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization. J Biol Chem. 2010;285:35311–35319. doi: 10.1074/jbc.M110.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Hoeller D, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–350. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–1632. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rødland MS, Traub LM, Stang E, Madshus IH. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, André B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20:196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–3439. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- Maldonado-Báez L, Wendland B. Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 2006;16:505–513. doi: 10.1016/j.tcb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278:21323–21326. doi: 10.1074/jbc.C300096200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- Parachoniak CA, Park M. Distinct recruitment of Eps15 via its coiled-coil domain is required for efficient down-regulation of the met receptor tyrosine kinase. J Biol Chem. 2009;284:8382–8394. doi: 10.1074/jbc.M807607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Roy SJ, Iorio-Morin C, Lépine MC, Labrecque P, Gallant MA, Slipetz D, Parent JL. ANKRD13C acts as a molecular chaperone for G protein-coupled receptors. J Biol Chem. 2010;285:40838–40851. doi: 10.1074/jbc.M110.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yoshikawa A, Mimura H, Yamashita M, Yamagata A, Fukai S. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 2009;28:2461–2468. doi: 10.1038/emboj.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- Varghese B, et al. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28:5275–5287. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Young P, Walters KJ. Structure of S5a bound to monoubiquitin provides a model for polyubiquitin recognition. J Mol Biol. 2005;348:727–739. doi: 10.1016/j.jmb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ, Cohen RE, Peng J, Paulson HL. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J Biol Chem. 2008;283:26436–26443. doi: 10.1074/jbc.M803692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Deveraux Q, Beal RE, Pickart CM, Rechsteiner M. Characterization of two polyubiquitin binding sites in the 26S protease subunit 5a. J Biol Chem. 1998;273:5461–5467. doi: 10.1074/jbc.273.10.5461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.