Abstract
Objective
Study 90-day cardiac and thromboembolic complications and all-cause mortality following total hip or knee arthroplasty (THA/TKA).
Method
In a population-based cohort of all Olmsted County residents who underwent a THA or TKA between 1994 and 2008, we assessed 90-day occurrence and predictors of cardiac complications (myocardial infarction, cardiac arrhythmia or congestive heart failure), thromboembolic complications (deep venous thrombosis (DVT) or pulmonary embolism (PE)) and mortality.
Results
Among the Olmsted County THA and TKA cohorts, 90-day complication rates were as follows: cardiac, 15.8% and 6.9%; thromboembolic, 4.9% and 4.0%; and mortality, 0.7% and 0.4%, respectively. Unadjusted frequency of cardiac/thromboembolic events differed by history of prior respective event. In multivariable-adjusted logistic regression analyses, ASA class III–IV (OR, 6.1, 95% CI:1.6, 22.8) and higher Deyo-Charlson comorbidity score (OR, 1.2, 95% CI:1.0,1.4) were significantly associated with odds of 90-day cardiac event post-THA in patients with no known prior cardiac event. In those with known prior cardiac disease, ASA class III–IV (OR, 4.4, 95% CI:2.0, 9.9), male gender (OR, 0.5, 95% CI:0.3,0.9) and history of thromboembolic disease (OR, 3.2; 95% CI:1.4,7.0) were significantly associated with odds of cardiac complication 90-day post-THA. No significant predictors of thromboembolism were found in THA patients.
In TKA patients with no prior cardiac history, age >65 years (OR, 4.1, 95% CI:1.2, 14.0), and ASA class III–IV (OR, 2.8, 95% CI:1.1,6.8) and in TKA patients with known cardiac disease, ASA class III–IV (OR, 3.2, 95% CI:1.8,5.7) was significantly associated with odds of 90-day cardiac event. In TKA patients with no prior thromboembolic disease, male gender (OR, 0.5, 95% CI:0.2,0.9) and higher Charlson index (OR, 1.2, 95% CI:1.1,1.3) and in patients with known thromboembolic disease, higher Charlson index score (OR, 1.1, 95% CI:1.1,1.4) was associated with odds of 90-day thromboembolic event.
Conclusion
Older age, higher comorbidity, higher ASA class and prior history of cardiac/thromboembolic disease was associated with an increased risk of 90-day cardiac and thromboembolic complications.
Keywords: Cardiac, Thromboembolic, Total Hip Arthroplasty, Total Knee Arthroplasty, Mortality
Total knee arthroplasty (TKA) and Total Hip Arthroplasty (THA) lead to significant improvement in pain, function and health-related quality of life (HRQoL) [1–2]. Cardiac and thromboembolic complications are not uncommon in the peri- and post-operative period. Since a significant number of elderly patients undergo these common procedures, it is important to study these complications and their predictors. Most estimates for cardiac and thromboembolic complications following THA or TKA have been obtained from single-center studies or national/regional datasets, including Medicare [3–4], National Inpatient Sample (NIS) [5–6] and state-based discharge registries [7–9]. None of these estimates are truly population-based. In an analysis of Medicare data, 90-day cardiac, thromboembolic and mortality rates after primary TKA were 0.8%, 0.8% and 0.6%, respectively [4]. Following THA, 30-day mortality was 2% overall, higher following hip fracture (6%) than other diagnoses (0.9–1.6%) [3].
Very few well-designed studies, with adequate sample size and adjustment for important confounders using multivariable analyses, have examined predictors of cardiac and thromboembolic complications and mortality. Using the NIS sample from 1990–2004, authors found that older age, male gender, larger hospital size, comorbidities, post-operative complications and payer-type were associated with higher inpatient mortality in patients who underwent THA or TKA [5–6]. In an analysis of Medicare data from 1983–85, older age was a significant predictor of 30-day mortality after THA in multivariable-adjusted analyses [3]. Basilico et al. performed a case-control study of 209 cases with post-operative cardiac complications (including pulmonary embolism) compared to 209 controls without any complication following THA or TKA [10]. Prior history of heart disease (arrhythmia, coronary artery disease, myocardial infarction (MI), congestive heart failure (CHF) or valvular heart disease), older age, revision and bilateral surgery were associated with significantly higher odds of 90-day cardiac complication post-arthroplasty [10]. While these studies have provided insight into cardiopulmonary complications following THA and TKA, they have important limitations. These studies are limited in that complications were studied only during inpatient stay (NIS) [5] [6], in certain age groups (≥65 years for Medicare) [3], or they combined cardiac and thromboembolic complications [10]. With the exception of one study [10], data were claims-based.
Our aim was to use a population-based approach using the data from the Rochester Epidemiology Project (REP) to study these complications after THA and TKA. REP is a rich resource to conduct population-based studies [11–13]. The objectives of this study were to: (1) provide population-based estimates of cardiac and thromboembolic events and mortality rates up to 90-days after primary THA and TKA; (2) assess the predictors of these outcomes at 90-days; and, (3) examine the time-trends in these outcomes.
METHODS
Data Sources and Study Population
We used the data from the Mayo Clinic (Rochester) electronic clinical and administrative databases for this study. We merged the data from the Rochester Epidemiology Project (REP) [13] and the prospective Mayo Clinic Total Joint Registry [14]. REP is a population-based records linkage system that captures health care and outcomes of residents of Olmsted County, which is situated in southeastern Minnesota and is comprised of approximately 124,000 people (2000 U.S. census). REP was developed in the 1960s and has been continuously supported by the NIH since then [13]; it has been used to study population-based epidemiology of rheumatic diseases [15–21] and several other conditions [22–26] in the U.S. Previous studies have shown that in any three-year period, over 90% of Olmsted County residents are examined at Mayo Clinic health care system in the Olmsted County [13]. The closest competing medical centers are in Minneapolis, Minnesota (139 kilometers to the north) and LaCrosse, Wisconsin (114 kilometers to the east). The Mayo Clinic provides primary and secondary care to local residents. The total joint registry has been prospectively capturing every hip and knee arthroplasty procedure since the procedures were introduced in 1969 and 1971, respectively. Patients are routinely followed up 1-, 2- and 5-years after hip and knee arthroplasty. For patients who miss their appointments, mailed questionnaires regarding outcomes and complications are requested along with radiographs. Patients not returning for follow-up and not responding to mailed letters/questionnaires are contacted by telephone call from trained, dedicated registry staff, who administers these questionnaires over the phone.
The time period of interest for this study was from 1/1/1994 to 5/31/2008. This longer observation period was chosen to provide us with enough outcome events, have all predictors of interest in our databases and in order to avoid significant change in definitions of cardiac and thromboembolic events that we may have encountered. From the list of all primary Total Hip and Total Knee Arthroplasties (THA/TKA) conducted at the Mayo Clinic, we identified patients residing in the Olmsted County, using the Zip Code + 4 information, to obtain a population-based sample. Since all Olmsted County residents use the Mayo Clinic system for their healthcare, this allowed us to capture all complications following the index arthroplasty. There are 1,744 THA patients and 1,604 TKA patients. After excluding those with hip fracture as the underlying diagnosis to identify only those with elective surgeries, the final sample consisted of 1,195 THA and 1,604 TKA patients.
Study Outcomes
Three study outcomes of interest were cardiac events, thromboembolic events and all-cause mortality within 90-days of the index surgery in the Olmsted County residents during our study period of interest, 1994–2008. We extracted the diagnoses codes for cardiac and thromboembolic events from the Mayo Clinic Medical Indexing system using Mayo Clinic’s Hospital Adaptation of International Code for Diseases (H-ICDA) codes [27] (available from 1935 to current) and International Classification of Diseases ninth version (ICD-9). These codes are valid and are collected for every in- and out-patient at the Mayo Clinic [28]. Cardiac events were defined as new occurrence of myocardial infarction (MI), congestive heart failure (CHF) or cardiac arrhythmia, identified by new codes for these conditions (online supplementary Table S1). We also extracted procedure codes for angioplasty with and without stent and coronary artery bypass grafting. Since addition of these procedure codes led to identification of only 0.9% of additional events in hip and 0.6% additional events in knee patients who underwent the procedures without documentation of diagnostic code for cardiac or thromboembolic event, we restricted our analyses to only those with H-ICDA or ICD-9 codes. Thromboembolic event was defined as occurrence of new codes for deep vein thrombosis or pulmonary embolism (online supplementary Table S1).
Predictors of Outcomes
In univariate analyses, we considered the following variables: (1) Age, categorized as ≤65 and >65 years; (2) Gender; (3) Prior underlying cardiac events (MI, CHF, or arrhythmia), yes/no; (4) Prior underlying thromboembolic event (DVT or PE), yes/no; (5) Body mass index: available from 1988, for each 5-unit increase (values over 80 and under 10 were set to missing); (6) Comorbidity assessed by Deyo-Charlson index, a validated comorbidity assessment consisting of a weighted sum of 19 comorbidities (including cardiac, pulmonary, renal, hepatic disease, diabetes, cancer, HIV, etc.) [29–30] with higher score indicates more comorbidity, per 1-point increase; available from 1994; (7) American Society of Anesthesiologist (ASA) score: a validated measure a validated measure of peri-operative mortality and immediate post-operative morbidity, categorized as class I–II vs. III–IV [31–32] (class I, normal healthy patient; class II, patient with mild systemic disease (with no functional limitation); class III, patient with severe systemic disease (with some functional limitation); class IV, patient with severe systemic disease that is constant threat to life or moribund patient). Since there were only seven bilateral THAs, all 2006 or later, this variable was not analyzed for THAs. Due to an dramatic effect of pre-existing cardiac and thromboembolic disease on occurrence of these events after THA and
TKA, all analyses were done separately for those with and without pre-existing cardiac and pre-existing thromboembolic disease. Specifically, the predictors considered for respective multivariable analyses were as follows: (a) Predictors for 90-day Cardiac event: Age, gender, ASA, BMI, Deyo-Charlson Index and pre-existing thromboembolic disease; (b) Predictors for 90-day thromboembolic event: Age, gender, ASA, BMI, Deyo-Charlson Index and pre-existing cardiac disease. Only predictors statistically significant in univariate analyses (p<0.05) were entered into multivariable regression models. Since 90-day mortality rate was low (<10 each for THA and TKA), we could not perform any analyses for predictors of mortality.
Statistical Analyses
Descriptive statistics are reported as number (percentage) or mean (SD) as appropriate. Olmsted County residents were assessed for the occurrence of a cardiac event, thromboembolic event and all-cause mortality within 90 days of their initial joint arthroplasty. A 90-day cardiac event included a diagnosis of arrhythmia, myocardial infarction, or congestive heart failure within 90 days of the THA/TKA. A 90-day thromboembolic event included a diagnosis of either pulmonary embolism or deep vein thrombosis within 90 days of THA/TKA.
Separate logistic regression models were used for univariate and multivariable-adjusted analyses of 90-day cardiac event and 90-day thromboembolic event. A backward selection method was used to identify the significant variable in the multivariable models. Variables with significant associations with the outcome (p<0.05) in univariate analyses were entered into the multivariable models. Variables assessed for association with each event included: age (categorized as ≤65 vs. >65), male gender, ASA score (categorized as I/II vs. III/IV), body mass index (BMI), Deyo-Charlson Index, thromboembolic event prior to THA/TKA (assessment of 90-day cardiac event), and cardiac event prior to THA/TKA (assessment of 90-day thromboembolic event). Odds ratios (OR) and 95% confidence intervals (CIs), based on the logistic regression model estimates, are reported. Both univariate and multivariable-adjusted estimates are presented in tables, but only multivariable-adjusted estimates are described in results and discussion, to avoid complexity.
Time-trends in cardiac and thromboembolic events was examined for the various study periods using a linear regression with the independent variable being time of the orthopedic surgery. Trends in BMI and comorbidity were analyzed similarly. The alpha-level was set at 0.05 for statistical significance. All analyses were performed using SAS 9.2 by SAS Institute Inc., Cary, NC, USA.
RESULTS
Characteristics of the Study Populations
We identified all Olmsted County residents who underwent primary elective THA (n=1,195) or TKA (n= 1,604) at the Mayo Clinic during 1994–2008, after excluding patients with hip fracture. Cardiac and thromboembolic events and mortality were assessed for this population-based cohort. Olmsted County residents who underwent THA and TKA had mean ages of 67 and 68 years, 43% and 37% were male and the underlying diagnosis was osteoarthritis in 84% and 92%, respectively (Table 1). Only those with primary THA or primary TKA were analyzed, which constituted the majority of all hip and knee arthroplasties, respectively (Table 1).
Table 1.
Clinical Characteristics of the Study Population
| Olmsted County Residents Mean ± SD or n (%) | ||
|---|---|---|
| THA (n=1,195 patients) | TKA (n=1,608 patients) | |
| Age | 66.6 ± 13.1 | 68.1 ± 10.6 |
| Male/Female | 512 (42.9%) | 600 (37.3%) |
| Primary/Revision | 1104 (92.4%) | 1544 (96.0%) |
| % Bilaterala | 46 (3.9%) | 174 (10.8%) |
| % Cemented or hybrid | 1115 (93.3%) | 1547 (90.2%) |
| Operative Diagnosis | ||
| Osteoarthritis | 1005 (84.1%) | 1473 (91.6%) |
| Rheumatoid arthritis | 24 (2.0%) | 30 (1.9%) |
| Otherb | 166 (13.9%) | 105 (6.5%) |
| Body Mass Indexc | 29.0 ± 6.2 | 31.6 ± 6.7 |
| ASA Class | ||
| I–II | 675 (56.5%) | 905 (56.3%) |
| III–IV | 459 (38.4%) | 646 (40.2%) |
| Deyo-Charlson Indexd | 2.0 ± 2.5 | 2.2 ± 2.5 |
Bilateral indicates the number (percent) of patients who underwent bilateral knee or bilateral hip surgeries within 90-days of the index surgery and includes simultaneous bilateral and staged bilateral surgeries;
Other category includes avascular necrosis, failure due to previous surgery etc.;
Body Mass Index data were available starting in 1988;
Deyo-Charlson Index data were available starting in 1994.
THA, Total hip arthroplasty; TKA, Total knee arthroplasty; SD, standard deviation; ASA, American Society of Anesthesiologists
Incidence of complications after THA and TKA
Table 2 shows the population-based incidence of cardiac and thromboembolic events and mortality in THA and TKA in Olmsted County cohort at 7-, 30- and 90-days post-operatively. Overall, among patients from Olmsted County undergoing primary THA (n=1,195), 6.9% had a cardiac event, 4.0% had a thromboembolic event and 0.7% died within 90-days post-THA. Similarly, among Olmsted County patients undergoing primary TKA (n=1,608), 6.7% had a cardiac event, 4.9% had a thromboembolic event and 0.4% died within 90-days post-TKA.
Table 2.
Frequency of Cardiac events, thromboembolic events and mortality in Olmsted County residents
| 7-day | 30-day | 90-day | ||||
|---|---|---|---|---|---|---|
| THA (n=1,195) n (%) |
TKA(n=1,608) n (%) |
THA (n=1,195) n (%) |
TKA(n=1,608) n (%) |
THA (n=1,195) n (%) |
TKA (n=1,608) n (%) |
|
| All Cardiac events | 29 (2.4%) | 33 (2.1%) | 61 (5.1%) | 85 (5.3%) | 82 (6.9%) | 108 (6.7%) |
| Myocardial Infarction | 3 (0.3%) | 2 (0.1%) | 11 (0.9%) | 14 (0.9%) | 14 (1.2%) | 22 (1.4%) |
| Arrhythmia | 28 (2.3%) | 26 (1.6%) | 41 (3.4%) | 65 (4.0%) | 57 (4.8%) | 83 (5.2%) |
| Congestive Heart Failure | 8 (0.7%) | 7 (0.4%) | 23 (1.9%) | 24 (1.5%) | 32 (2.7%) | 32 (2.0%) |
| All Thromboembolic events | 12 (1.0%) | 29 (1.8%) | 34 (2.9%) | 63 (3.9%) | 48 (4.0%) | 79 (4.9%) |
| Deep Vein Thrombosis | 10 (0.8%) | 16 (1.0%) | 26 (2.2%) | 43 (2.7%) | 38 (3.2%) | 54 (3.4%) |
| Pulmonary Embolism | 3 (0.3%) | 14 (0.9%) | 10 (0.8%) | 26 (1.6%) | 14 (1.2%) | 35 (2.2%) |
| All-cause Mortality | 1 (0.1%) | 1 (0.1%) | 4 (0.3%) | 4 (0.3%) | 8 (0.7%) | 7 (0.4%) |
THA, Total Hip Arthroplasty; TKA, Total Knee Arthroplasty
Table 3 shows the distribution of cardiac and thromboembolic events between patients who had prior cardiac or prior thromboembolic events. The rates differed sharply between patients with and without prior cardiac and thromboembolic events. Therefore, all subsequent analyses were done separately for patients with and without prior cardiac and thromboembolic events. Since only 8 THA and 7 TKA patients died within 90-days of the respective procedure, no further analyses of predictors were performed for 90-day mortality.
Table 3.
90-day cardiac and thromboembolic events based on prior similar events in Olmsted County residents
| Total Hip Arthroplasty | Total Knee Arthroplasty | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | No Prior Cardiac event (N=874) | Prior Cardiac event (N=321) | No Prior THE events (N=1,116) | Prior THE events (N=128) | No Prior Cardiac event (N=1,094) | Prior Cardiac event (N=514) | No Prior THE events (N=1,487) | Prior THE events (N=121) |
| Any Cardiac event | 15 (1.7%) | 67 (20.9%) | 66 (5.9%) | 16 (20.3%) | 22 (2.0%) | 86 (16.7%) | 100 (6.7%) | 8 (6.6%) |
| Myocardial Infarction | 4 (0.5%) | 10 (3.1%) | 11 (1.0%) | 3 (3.8%) | 5 (0.5%) | 17 (3.3%) | 20 (1.3%) | 2 (1.7%) |
| Arrhythmia | 5 (0.6%) | 49 (15.3%) | 45 (4.0%) | 12 (15.2%) | 15 (1.4%) | 68 (13.2%) | 76 (5.1%) | 7 (5.8%) |
| Congestive Heart Failure | 6 (0.7%) | 26 (8.1%) | 26 (2.3%) | 6 (7.6%) | 8 (0.7%) | 24 (4.7%) | 27 (1.8%) | 5 (4.1%) |
| Any Thromboembolic event | 30 (3.4%) | 18 (5.6%) | 35 (3.1%) | 13 (16.5%) | 49 (4.5%) | 30 (5.8%) | 54 (3.6%) | 25 (20.7%) |
| Deep Vein Thrombosis | 23 (2.6%) | 15 (4.7%) | 28 (2.5%) | 10 (12.7%) | 37 (3.4%) | 17 (3.3%) | 37 (2.5%) | 17 (14.1%) |
| Pulmonary Embolism | 9 (1.0%) | 5 (1.6%) | 9 (0.8%) | 5 (6.3%) | 17 (1.6%) | 18 (3.5%) | 22 (1.5%) | 13 (10.7%) |
MI, Myocardial Infarction; CHF, Congestive Heart Failure; THE, thromboembolic
Risk Factors for Cardiac and Thromboembolic Events 90-days after Elective Primary THA
In multivariable-adjusted analyses of THA patients with no known prior cardiac disease, higher (worse) ASA class of 3–4 and higher Deyo-Charlson index were associated with significantly higher risk of a cardiac event 90-days post-THA (Table 4). In multivariable-adjusted analyses of THA patients with known cardiac disease, higher (worse) ASA class of 3–4, female gender and prior history of thromboembolic event were significantly associated with higher risk of a cardiac event 90-days post-THA.
Table 4.
Univariate and Multivariable-adjusted 90-day event rates in Olmsted County residents after Total Hip Arthroplasty
| 90-day Cardiac Events Odds Ratio (95% confidence intervals) |
90-day Thromboembolic Events (THE) Odds Ratio (95% confidence intervals) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No history of heart disease | History of heart disease | No history of Thromboembolism | History of Thromboembolism | |||||
| Univariate | Multivariablea | Univariate | Multivariablea | Univariate | Multivariablea | Univariate | Multivariablea | |
| Age >65 (Ref: ≤65 years) | 5.5 (1.2, 25.2) p=0.03 | 2.3 (0.98, 5.3) p=0.055 | 1.6 (0.8, 3.3) p=0.21 | 0.6 (0.2, 2.1) p=0.41 | ||||
| Male gender (Ref: female) | 0.6 (0.2, 2.1) p=0.45 | 0.5 (0.3, 0.8) p=0.01 | 0.5 (0.3, 0.9) p=0.02 | 0.5 (0.3, 1.1) p=0.09 | 1.2 (0.3, 4.0) p=0.81 | |||
| ASA Class 3–4 (Ref: 1–2) | 7.4 (2.0, 27.2) p=0.003 | 6.1 (1.6, 22.8) p=0.007 | 4.7 (2.1, 10.2) p<0.001 | 4.4 (2.0, 9.9) p<0.001 | 0.5 (0.3, 1.001) p=0.0502 | 1.6 (0.5, 5.2) p=0.47 | ||
| Deyo-Charlson (per 1 pt increase) | 1.2 (1.1, 1.5) p=0.006 | 1.2 (1.0, 1.4) p=0.049 | 1.1 (0.97, 1.2) p=0.21 | 1.0 (0.9, 1.2) p=0.61 | 0.7 (0.5, 1.04) p=0.08 | |||
| BMI (per 5-point increase) | 0.9 (0.6, 1.5) p=0.70 | 0.95 (0.8, 1.2) p=0.66 | 1.2 (0.9, 1.5) p=0.25 | 1.1 (0.8, 1.6) p=0.45 | ||||
| Prior history of heart disease | --- | --- | --- | 3.2 (1.4, 7.0) p=0.005 | 1.9 (0.9, 3.8) p=0.08 | 0.8 (0.2, 2.8) p=0.77 | ||
| Prior history of thromboembolism | 1.5 (0.2, 11.7) 0.70 | 4.1 (1.9, 8.8) p<0.001 | <.001 | --- | --- | --- | --- | |
Multivariable backward selection logistic regression models examined all variables that were significant in univariate analyses with p<0.05; for 90-day thromboembolic complications, none of the variables were significant in univariate analyses and therefore, multivariable analyses could not be performed
ASA, American Society of Anesthesiology; BMI, body mass index; NS, not significant;
---, not adjusted since the analyses were done separately for those with and without respective prior events
In THA patients with or without known prior thromboembolic disease, none of the risk factors were significantly associated with 90-day risk of thromboembolic event (Table 4).
Risk Factors for Cardiac and Thromboembolic Events 90-days after Elective Primary TKA
In TKA patients with no known prior cardiac disease, age >65 years and higher (worse) ASA class of 3–4 were associated with higher risk of a cardiac event 90-days post-TKA (Table 5). In TKA patients with known cardiac disease, higher ASA class of 3–4 was significantly associated with higher risk of a cardiac event 90-days post-TKA.
Table 5.
Univariate and Multivariable-adjusted 90-day event rate in Olmsted County residents after Total Knee Arthroplasty
|
90-day Cardiac Events Odds Ratio (95% confidence intervals); p-value |
90-day Thromboembolic Events Odds Ratio (95% confidence intervals); p-value |
|||||||
|---|---|---|---|---|---|---|---|---|
| No history of heart disease | History of heart disease | No history of Thromboembolism | History of Thromboembolism | |||||
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| Age >65 (Ref: <65 years) | 4.4 (1.3, 15.2) p=0.02 | 4.1 (1.2, 14.0) p=0.03 | 2.0 (1.02, 3.8) p=0.04 | 1.7 (0.9, 3.1) p=0.10 | 1.3 (0.5, 3.3) p=0.63 | |||
| Male gender | 1.04 (0.4, 2.6) p=0.94 | 1.4 (0.9, 2.3) p=0.18 | 0.5 (0.3, 0.997) p=0.049 | 0.5 (0.2, 0.9) p=0.02 | 2.5 (1.0, 6.4) p=0.0501 | |||
| ASA Class 3–4 (Ref: 1–2) | 3.0 (1.2, 7.5) p=0.02 | 3.2 (1.8, 5.7) p<0.001 | 3.2 (1.8, 5.7) p<0.001 | 1.4 (0.8, 2.5) p=0.20 | 1.6 (0.6, 4.1) p=0.34 | |||
| Deyo-Charlson Index (per 1 pt increase) | 1.1 (0.9, 1.3) p=0.25 | 1.1 (1.01, 1.2) p=0.03 | 1.2 (1.1, 1.3) p<0.001 | 1.2 (1.1, 1.3) p<0.001 | 1.2 (1.1, 1.4) p=0.004 | 1.2 (1.1, 1.4) p=0.004 | ||
| BMI (per 5-point increase) | 1.0 (0.7, 1.4) p=0.88 | 1.1 (0.9, 1.3) p=0.46 | 0.8 (0.6, 1.2) p=0.25 | |||||
| Prior history of cardiac events | ---- | ---- | ---- | ---- | 1.1 (0.6, 1.9) p=0.79 | 1.6 (0.6, 3.9) p=0.34 | ||
| Prior history of thromboembolic events | 1.0 (0.7, 1.4) p=0.88 | 0.8 (0.3, 1.8) p=0.53 | --- | --- | --- | --- | ||
Multivariable backward selection logistic regression models examined all variables that were significant in univariate analyses with p<0.05
ASA, American Society of Anesthesiology; BMI, body mass index; THE, thromboembolic; NS, not significant;
---, not adjusted since the analyses were done separately for those with and without respective prior events
In TKA patients with no known prior thromboembolic disease, higher Deyo-Charlson index score and female gender were associated with higher risk of 90-day risk of thromboembolic event (Table 5). In TKA patients with known prior thromboembolic disease, higher Deyo-Charlson index score was associated significantly higher risk of thromboembolic event within 90-days of index TKA.
Time Trends in Post-operative Cardiac Events and Thromboembolic Events
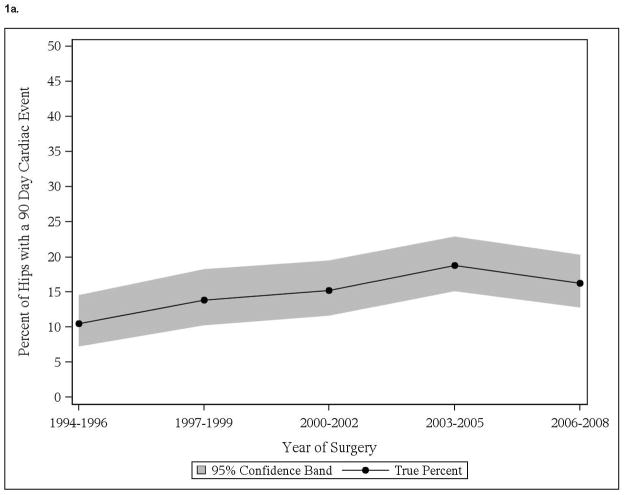
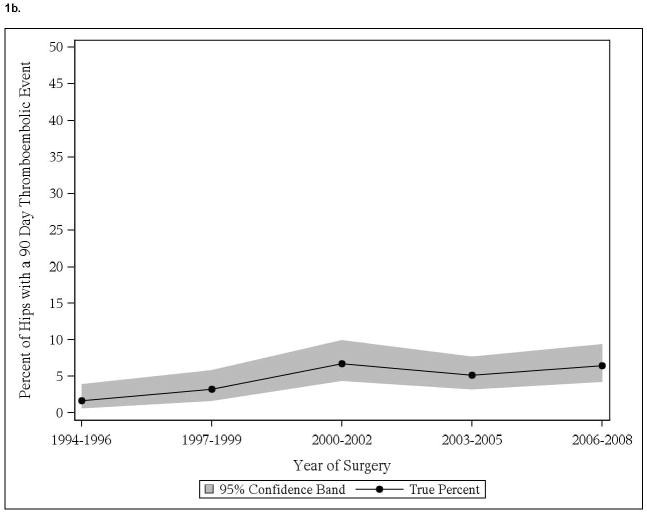
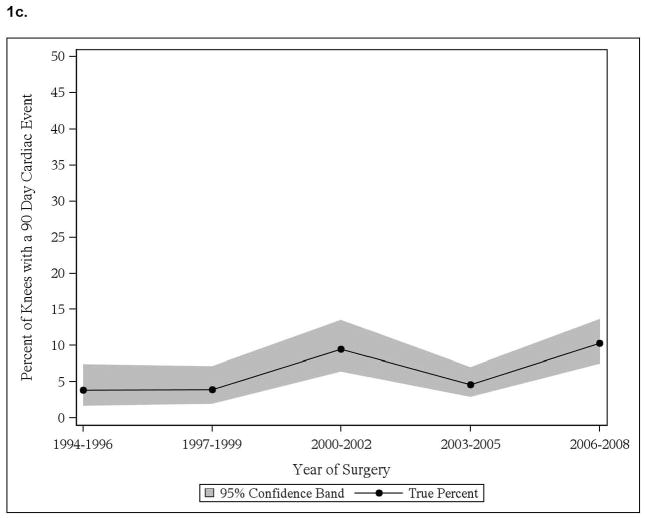
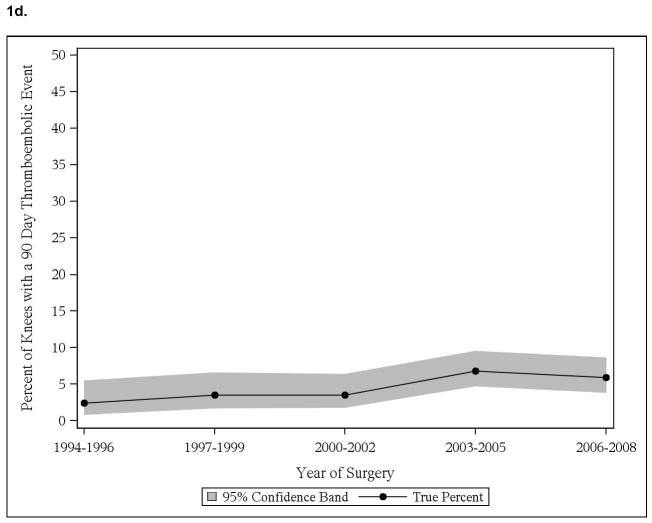
We noted significant time trend with increase in cardiac events in those with pre-existing cardiac disease, both in THA and TKA cohorts (Figure 1a, 1c). No significant time trends in thromboembolic events in patients with thromboembolic events were noted, in both THA and TKA cohorts (Figure 1b, 1d). No significant time-trends in cardiac or thromboembolic events were noted in patients without prior respective events, in both THA and TKA cohorts (data not shown).
Figure 1.
Time trends in cardiac and thromboembolic events in Olmsted county residents
1a. 90-day cardiac events in Olmsted County residents with THA
1b. 90-day thromboembolic events in Olmsted County residents with THA
1c. 90-day cardiac events in Olmsted County residents with TKA
1d. 90-day thromboembolic events in Olmsted County residents with TKA
Interestingly, significant increase was noted in body mass index through the study period (p<0.001), for example BMI increased from 26.5 kg/m2 in 1994–96 to 28.8 kg/m2 in 2006–08 and Deyo-Charlson index during the study period (p=0.02), as an example it increased from 2.0 in 1994–96 to 2.6 in 2006–08 in THA cohort. Similarly in TKA cohort, body mass index increased significantly over study period (p<0.001), for example it increased from 30.3 in 1994–96 to 32.1 in 2006–08.
DISCUSSION
In this population-based study, we provided population-based estimates of cardiac and thromboembolic complications and mortality in a U.S. cohort of primary THA and TKA for the first time. We found that female gender and higher ASA class of III-IV were risk factors for cardiac events after THA. In TKA patients, older age and ASA class of III-IV were risk factors for 90-day cardiac events, and female gender and higher comorbidity index were risk factors for 90-day thromboembolic events. With very few exceptions (cardiac events in those with prior history of cardiac events), we did not note any significant time-trends. Increase in disease diagnosis, closer surveillance for these complications and change in disease definitions may contribute to time-trends. Several findings deserve further discussion.
This study provides population-based estimates for 90-day cardiac and thromboembolic events in a U.S. arthroplasty cohort and adds to the literature. We know of no other population-based U.S. study that has provided incidence rates for post-arthroplasty complications. Overall, in the THA cohort, 6.9% had 90-day cardiac and 4.0% had 90-day thromboembolic events. In the TKA cohort, 6.7% had 90-day cardiac and 4.9% had 90-day thromboembolic events. It is not surprising that the rate of 1.4% for 90-day myocardial infarction in our TKA cohort is very similar to the 0.8% reported for primary TKA Medicare study population from the year 2000 [4]. One difference in these studies was inclusion of all patients in our study versus only those 65 years and older in the previous study that used Medicare data.
Although not directly comparable, the thromboembolic event rates from two previously published non-population-based U.S. studies were 1.9% for THA and 3.0% for TKA patients during the index hospitalization [7] and 2.8% for primary THAs and 2.1% for primary TKAs within 90-days [8]. Differences in study design (population- versus non-population-based), study time-frames and mean age in THA cohort may underlie these differences. Non-population based studies lack the ability to provide true incidence rates, since the denominator is patients seen in a facility and not the population. Therefore, they can over- or under-estimate rates based on patient case-mix. Rates of symptomatic thromboembolic event 90-day postoperatively in a population-based study from Scotland were 2.2% for THA and 1.7% for TKA during 1992–2001 [33]. Differences in outcome event definition (any versus symptomatic thromboembolism), health care delivery systems (U.S. versus Sweden), risk factor profile of patients, patient age (69 versus 71 years for THA) and study time-period differences may explain the differences in the rates between our and the Swedish study.
We identified risk factors for cardiac events following THA and TKA. An important observation was that the factors and the risk associated with them varied by the type of arthroplasty (THA versus TKA) and the history of prior cardiac or thromboembolic disease. In fact, the complication rates were so dramatically different between patients with and without pre-existing disease that all analyses were conducted separately. One notable exception was the lack of difference in rates of thromboembolism by prior such history in the TKA cohort (6.7% versus 6.6%). These observations imply that caution must be taken before combining events in patients with and without prior history of these events and in patients with THA versus TKA.
One of the previous well-designed case-control study combined THA and TKA cohorts and included pulmonary embolism in cardiovascular complications [10]. In this study, prior history of cardiac disease, older age, revision and bilateral surgery were associated with higher odds of 90-day cardiac complication after total joint arthroplasty [10]. We confirm the previously reported old age and pre-existing cardiac disease associations with cardiac complications. In addition, not surprisingly, we identified ASA class as a risk factor for cardiac complications, in both THA and TKA cohorts. ASA has been identified as a risk factor for peri-operative complications, in general [34]. Our study extends this finding to postoperative cardiac complications in arthroplasty cohorts.
We found that there were no additional demonstrable risk factors for 90-day thromboembolic events in THA patients except prior history of thromboembolism (unadjusted rates 5.9% versus 20.3%). Our study confirms a similar finding of association of prior thromboembolic disease with 30-day post-operative symptomatic thromboembolic events in patients undergoing major lower limb surgery, including THA, TKA and hip fracture surgery [35], and extends this to 90-day follow-up. The surgeons may need to be extra vigilant for thromboembolic events in THA patients with prior thromboembolism with regards to the choice and intensity of venous thromboembolism prophylaxis. There is an intense debate regarding the choice of thromboprophylaxis in patients undergoing knee or hip arthroplasty [36] [37]. The American Association of Chest Physicians recommends the use of heparin, coumadin or fondaparinux and recommending not using aspirin or mechanical devices alone [38]. On the other hand, the American Academy of Orthopedic Surgeons recommends aspirin as an option in those at lower risk of thromboembolism and higher risk of bleeding [39].
Women undergoing TKA had significantly higher risk of thromboembolism in our study, confirming similar prior observation [7]. In addition, we found that higher comorbidity was a significant risk factor for thromboembolic events in both patients with and without prior known thromboembolism. A similar observation has been made in THA, but not TKA, cohorts [7]. Our study is limited in that we did not study post-thrombotic syndrome, an important complication after TKA/THA. Deep venous thrombosis occurred more frequently than pulmonary embolism in both THA and TKA cohorts, which is similar to previous studies [40–44]. This is an expected finding due to immobilization of the lower extremity after THA/TKA.
In conclusion, we performed a population-based study to estimate the incidence rates of cardiac and thromboembolic events and mortality up to 90-days after elective primary THA and TKA. Several findings of risk factors from other studies were confirmed and new predictors and risk factors were identified. Findings from this study can guide providers to discuss the risk of these complications depending on whether patients have prior history of cardiac and thromboembolic disease or not. Future studies should explore whether use of certain interventions, including type of thromboprophylaxis and comorbidity management, can reduce these perioperative complications.
Supplementary Material
Acknowledgments
Grant support: NIH CTSA Award 1 KL2 RR024151-01 (Mayo Clinic Center for Clinical and Translational Research) and the Department of Orthopedic Surgery, Mayo Clinic School of Medicine, Rochester, MN.
Footnotes
Financial Conflict: One of the authors (DL) has received royalties/speaker fees from Zimmer, has been a paid consultant to Zimmer and has received institutional research funds from DePuy, Stryker and Zimmer. J.A.S. has received speaker honoraria from Abbott; research and travel grants from Allergan, Takeda, Savient, Wyeth and Amgen; and consultant fees from Savient, Takeda, URL pharmaceuticals and Novartis. Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
“The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.”
References
- 1.Kane RL, Saleh KJ, Wilt TJ, et al. The functional outcomes of total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1719–1724. doi: 10.2106/JBJS.D.02714. [DOI] [PubMed] [Google Scholar]
- 2.Ethgen O, Bruyere O, Richy F, et al. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Whittle J, Steinberg EP, Anderson GF, et al. Mortality after elective total hip arthroplasty in elderly Americans. Age, gender, and indication for surgery predict survival. Clin Orthop Relat Res. 1993:119–126. [PubMed] [Google Scholar]
- 4.Katz JN, Barrett J, Mahomed NN, et al. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–1916. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111:1206–1216. doi: 10.1097/ALN.0b013e3181bfab7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memtsoudis SG, Della Valle AG, Besculides MC, et al. Risk factors for perioperative mortality after lower extremity arthroplasty: a population-based study of 6,901,324 patient discharges. J Arthroplasty. 2010;25:19–26. doi: 10.1016/j.arth.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Lyman S, Jones EC, Bach PB, et al. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005:132–137. doi: 10.1097/01.blo.0000150571.51381.9a. [DOI] [PubMed] [Google Scholar]
- 8.White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525–1531. doi: 10.1001/archinte.158.14.1525. [DOI] [PubMed] [Google Scholar]
- 9.Kreder HJ, Deyo RA, Koepsell T, et al. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79:485–494. doi: 10.2106/00004623-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Basilico FC, Sweeney G, Losina E, et al. Risk factors for cardiovascular complications following total joint replacement surgery. Arthritis Rheum. 2008;58:1915–1920. doi: 10.1002/art.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremers HM, Myasoedova E, Crowson CS, et al. The Rochester Epidemiology Project: exploiting the capabilities for population-based research in rheumatic diseases. Rheumatology (Oxford) 2011;50:6–15. doi: 10.1093/rheumatology/keq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. 1997:61–68. doi: 10.1097/00003086-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Schafer VS, Kermani TA, Crowson CS, et al. Incidence of herpes zoster in patients with giant cell arteritis: a population-based cohort study. Rheumatology (Oxford) 2010;49:2104–2108. doi: 10.1093/rheumatology/keq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kermani TA, Schafer VS, Crowson CS, et al. Malignancy risk in patients with giant cell arteritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2010;62:149–154. doi: 10.1002/acr.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694–1697. [PubMed] [Google Scholar]
- 18.Shbeeb M, Uramoto KM, Gibson LE, et al. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27:1247–1250. [PubMed] [Google Scholar]
- 19.Peterson LS, Nelson AM, Su WP, et al. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24:73–80. [PubMed] [Google Scholar]
- 20.Peterson LS, Mason T, Nelson AM, et al. Juvenile rheumatoid arthritis in Rochester, Minnesota 1960–1993. Is the epidemiology changing? Arthritis Rheum. 1996;39:1385–1390. doi: 10.1002/art.1780390817. [DOI] [PubMed] [Google Scholar]
- 21.Machado EB, Gabriel SE, Beard CM, et al. A population-based case-control study of temporal arteritis: evidence for an association between temporal arteritis and degenerative vascular disease? Int J Epidemiol. 1989;18:836–841. doi: 10.1093/ije/18.4.836. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SE, O’Fallon WM, Beard CM, et al. Trends in the utilization of silicone breast implants, 1964–1991, and methodology for a population-based study of outcomes. J Clin Epidemiol. 1995;48:527–537. doi: 10.1016/0895-4356(94)00209-9. [DOI] [PubMed] [Google Scholar]
- 23.Codd MB, Sugrue DD, Gersh BJ, et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 25.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Kim WR, Benson JT, Therneau TM, et al. Changing epidemiology of hepatitis B in a U.S. community. Hepatology. 2004;39:811–816. doi: 10.1002/hep.20098. [DOI] [PubMed] [Google Scholar]
- 27.Commission on Professional and Hospital Activities, Hospital Adaptation of ICDA. 2. Vol. 1. Ann Arbor, MI: Commission on Professional and Hospital Activities; 1973. [Google Scholar]
- 28.St Sauver JL, Hagen PT, Cha SS, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80:203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Sax FL, MacKenzie CR, et al. Morbidity during hospitalization: can we predict it? J Chronic Dis. 1987;40:705–712. doi: 10.1016/0021-9681(87)90107-x. [DOI] [PubMed] [Google Scholar]
- 31.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–266. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- 32.Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18:693–708. doi: 10.1016/s0883-5403(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 33.Howie C, Hughes H, Watts AC. Venous thromboembolism associated with hip and knee replacement over a ten-year period: a population-based study. J Bone Joint Surg Br. 2005;87:1675–1680. doi: 10.1302/0301-620X.87B12.16298. [DOI] [PubMed] [Google Scholar]
- 34.Perka C, Arnold U, Buttgereit F. Influencing factors on perioperative morbidity in knee arthroplasty. Clin Orthop Relat Res. 2000:183–191. doi: 10.1097/00003086-200009000-00028. [DOI] [PubMed] [Google Scholar]
- 35.Leizorovicz A, Turpie AG, Cohen AT, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman JR, Barnes CL, Lachiewicz PF, et al. Venous thromboembolism debate in joint arthroplasty. J Bone Joint Surg Am. 2009;91 (Suppl 5):29–32. doi: 10.2106/JBJS.I.00364. [DOI] [PubMed] [Google Scholar]
- 37.Eikelboom JW, Karthikeyan G, Fagel N, et al. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest. 2009;135:513–520. doi: 10.1378/chest.08-2655. [DOI] [PubMed] [Google Scholar]
- 38.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. Rosemont, IL: American Academy of Orthopaedic Surgeons (AAOS); 2007. p. 63. [Google Scholar]
- 40.Husted H, Otte KS, Kristensen BB, et al. Low risk of thromboembolic complications after fast-track hip and knee arthroplasty. Acta Orthop. 2010;81:599–605. doi: 10.3109/17453674.2010.525196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotke PA, Steinberg ME, Ecker ML. Significance of deep venous thrombosis in the lower extremity after total joint arthroplasty. Clin Orthop Relat Res. 1994:25–30. [PubMed] [Google Scholar]
- 42.Kume H, Inoue Y, Mitsuoka A, et al. Doppler ultrasonography-aided early diagnosis of venous thromboembolism after total knee arthroplasty. Eur J Vasc Endovasc Surg. 2010;40:664–668. doi: 10.1016/j.ejvs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Dushey CH, Bornstein LJ, Alexiades MM, et al. Short-Term Coagulation Complications Following Total Knee Arthroplasty A Comparison of Patient-Reported and Surgeon-Verified Complication Rates. J Arthroplasty. 2011 doi: 10.1016/j.arth.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Westrich GH, Farrell C, Bono JV, et al. The incidence of venous thromboembolism after total hip arthroplasty: a specific hypotensive epidural anesthesia protocol. J Arthroplasty. 1999;14:456–463. doi: 10.1016/s0883-5403(99)90101-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.