Abstract
Imitation has been considered as one of the precursors for sociocommunicative development. Impairments of imitation in autism spectrum disorder (ASD) could be indicative of dysfunctional underlying neural processes. Neuroimaging studies have found reduced activation in areas associated with imitation, but a functional connectivity MRI network perspective of these regions in autism is unavailable. Functional and effective connectivity was examined in 14 male participants with ASD and 14 matched typically developing (TD) participants. We analyzed intrinsic, low-frequency blood oxygen level dependent (BOLD) fluctuations of three regions in literature found to be associated with imitation (inferior frontal gyrus [IFG], inferior parietal lobule [IPL], superior temporal sulcus [STS]). Direct group comparisons did not show significantly reduced functional connectivity within the imitation network in ASD. Conversely, we observed greater connectivity with frontal regions, particularly superior frontal and anterior cingulate gyri, in the ASD compared to TD group. Structural equation modeling of effective connectivity revealed a significantly reduced effect of IPL on IFG together with an increased influence of a region in dorsal prefrontal cortex (dPFC) on IFG in the ASD group. Our results suggest atypical connectivity of the imitation network with an enhanced role of dPFC, which may relate to behavioral impairments.
Keywords: Asperger's disorder, fcMRI, intrinsic functional connectivity, effective connectivity, structural equation modeling, prefrontal cortex
1. Introduction
Autism spectrum disorder (ASD) encompasses a broad range of pervasive neurodevelopmental disorders with sociocommunicative impairments and repetitive behaviors as core features. One of the many areas affected in children with ASD is the ability to imitate the actions of others, particularly when requested to do so (Hobson & Lee, 1999; Receveur et al., 2005; Rogers, Bennetto, McEvoy, & Pennington, 1996; Rogers, Hepburn, Stackhouse, & Wehner, 2003; Smith & Bryson, 1994; Vivanti, Nadig, Ozonoff, & Rogers, 2008). The degree of this impairment may correlate with the complexity of action and may have paradoxical manifestations, as individuals with autism may display echopraxia, involving automatic imitation without an attentional component (Malvy et al., 1999).
Imitation may be associated with action understanding through simulation and with other precursors for sociocommunicative development (Rizzolatti & Craighero, 2004). In typically developing (TD) children, imitation is thought to contribute to the acquisition of social skills by supporting self-other processing that could help lay the foundation for interpreting the goals and intentions of others in social interactions (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Lepage & Théoret, 2007; Meltzoff & Decety, 2003; Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008). Neuroimaging studies have identified areas of activation for imitation that may overlap with regions of the mirror neuron system (Decety, Chaminade, Grèzes, & Meltzoff, 2002; Iacoboni et al., 1999; Nishitani & Hari, 2002; Rizzolatti & Craighero, 2004) and regions activated when interpreting intentions and predicting actions (Fogassi et al., 2005; Liepelt, Von Cramon, & Brass, 2008; Saxe, Xiao, Kovacs, Perrett, & Kanwisher, 2004). Therefore, various components necessary for social interactions may rely on similar, shared neural processes as those employed for imitation (Hurley, 2008). Early deficits in imitation could in turn affect the development of sociocommunicative and other cognitive systems known to be impaired in ASD.
Despite some controversy regarding the hypothesis of a general imitation impairment (Bird, Leighton, Press, & Heyes, 2007; Hamilton, Brindley, & Frith, 2007; Williams, Whiten, & Singh, 2004), neuroimaging and neurophysiological studies in ASD have found abnormal activation for imitation of various actions (Bernier, Dawson, Webb, & Murias, 2007; Dapretto et al., 2006; Nishitani, Avikainen, & Hari, 2004). Nishitani and colleagues (2004) examined oral-facial imitation in ASD using magnetoencephalography (MEG). The authors observed neural activity that temporally progressed from the primary visual cortex (V1) to superior temporal sulcus (STS) to inferior parietal lobule (IPL) to inferior frontal gyrus (IFG) and finally to primary motor cortex. Comparable sites of activation were seen in TD participants and those with Asperger's disorder, but activity was weaker and delayed in IFG for the Asperger's group. Using fMRI, Villalobos and colleagues (2005) investigated interregional synchronization with visual areas and found decreased functional connectivity between V1 and IFG bilaterally. Thus, current evidence indicates that neural activity and connectivity in regions for imitation may be abnormal in ASD. Reduced activity in comparable locations during performance of other tasks such as multisensory integration (Oberman & Ramachandran, 2008), visuomotor processing (Martineau, Cochin, Magne, & Barthelemy, 2008; Müller, Kleinhans, Kemmotsu, Pierce, & Courchesne, 2003), or face perception (Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007) suggests a more general regional dysfunction (Leighton, Bird, Charman, & Heyes, 2007; Mostofsky et al., 2006).
To date, no functional connectivity MRI (fcMRI) study in ASD has investigated the intrinsic connectivity of brain areas that participate in a neural network for imitation. First observed in the resting state (Biswal, Yetkin, Haughton, & Hyde, 1995), intrinsic fcMRI detects the temporal correlation between spatially discrete low-frequency fluctuations of the blood oxygen level dependent (BOLD) signal, which is considered to reflect network connectivity (Fox & Raichle, 2007). In addition to the empirical support from studies examining low-frequency BOLD fluctuations in putative networks, such as unimodal motor (Biswal et al., 1995; Jiang, He, Zang, & Weng, 2004; Xiong, Parsons, Gao, & Fox, 1999), auditory (Cordes et al., 2001), and visual networks (Nir, Hasson, Levy, Yeshurun, & Malach, 2006), as well as multimodal language (Hampson, Peterson, Skudlarski, Gatenby, & Gore, 2002), executive (Seeley et al., 2007), and default mode networks (Greicius, Supekar, Menon, & Dougherty, 2009), the validity of the fcMRI technique is supported by several lines of evidence. These include the correspondence with anatomical connectivity (Bullmore & Sporns, 2009; Fernández & Galán, 2008; Horwitz et al., 2005), as shown in direct comparisons between DTI tractography and fcMRI findings (Greicius et al., 2009; Honey et al., 2009; Skudlarski et al., 2008; van den Heuvel, Mandl, Kahn, & Hulshoff Pol, 2009) and in studies of callosal agenesis (Quigley et al., 2003) and callosotomy (Johnston et al., 2008), which are associated with loss of typical homotopic interhemispheric fcMRI effects. Electrophysiological studies have further documented corresponding low-frequency (<.1Hz) fluctuations of local field potentials (Leopold, Murayama, & Logothetis, 2003) and strong correlations between slow fluctuations (<.5Hz) of intracranially detected local field potentials and BOLD fcMRI effects in neurosurgery patients (He, Snyder, Zempel, Smyth, & Raichle, 2008).
A few recent fcMRI studies in ASD have implemented resting-state scans to examine the intrinsic connectivity of areas such as in the default mode network (Kennedy & Courchesne, 2008b; Monk et al., 2009; Weng et al., 2009). It is assumed that during rest, the mind automatically goes into a default mode (Greicius, Krasnow, Reiss, & Menon, 2003; Raichle et al., 2001). However, this state is difficult to monitor and may vary across individuals and time, possibly creating uncontrollable confounds (Gilbert, Dumontheil, Simons, Frith, & Burgess, 2007; Hasson, Nusbaum, & Small, 2009; Waites, Stanislavsky, Abbott, & Jackson, 2005). In studies of clinical disorders such as ASD, these confounds may be aggravated by systematic differences in the response to the scanning environment (lying constrained inside a noisy magnet bore), which may prompt participants to engage in unknown and uncontrolled mental activities. As an alternative approach, data acquired during task performance can also be used for fcMRI, since network-specific spontaneous BOLD fluctuations are simultaneously present in time series acquired during task performance and can be separated statistically from task-related responses (Arfanakis et al., 2000; Fair et al., 2007; Fox, Snyder, Zacks, & Raichle, 2006; Gavrilescu et al., 2008). Therefore, isolating low-frequency BOLD fluctuations in data sets acquired during performance of a task unrelated to the network of interest provides a reasonable approach to examining intrinsic functional connectivity. Any potential residual effects from task performance are less likely to affect intrinsic fluctuations in functionally unrelated networks (cf. Jones et al., 2010), and potential systematic differences between groups in mental processes can be partialled out.
In ASD, evidence has recently accumulated suggesting widespread anomalous connectivity, possibly accounting for behavioral deficits. A number of fcMRI studies in ASD have examined task-dependent synchronization without isolating low-frequency fluctuations through the statistical removal of task activity and low-pass filtering. These fcMRI studies have found reduced coordination between areas engaged in sentence comprehension (Just, Cherkassky, Keller, & Minshew, 2004; Kana, Keller, Cherkassky, Minshew, & Just, 2006), face processing (Kleinhans et al., 2008), response inhibition (Kana, Keller, Minshew, & Just, 2007; Lee et al., 2008), verbal working memory (Koshino et al., 2005), problem solving (Just, Cherkassky, Keller, Kana, & Minshew, 2007), and theory-of-mind tasks (Mason, Williams, Kana, Minshew, & Just, 2008). In some studies, partially increased functional connectivity in ASD compared to TD has also been observed (Braeutigam, Swithenby, & Bailey, 2008; Jones et al., 2010; Mizuno, Villalobos, Davies, Dahl, & Müller, 2006; Monk et al., 2009; Murias, Webb, Greenson, & Dawson, 2007; Noonan, Haist, & Müller, 2009; Turner, Frost, Linsenbardt, McIlroy, & Müller, 2006; Welchew et al., 2005). To elucidate the intrinsic connectivity patterns contributing to possible impairments in an imitation network, we examined whole-brain correlations with low-frequency BOLD fluctuations in three regions commonly reported to participate in imitation: IFG, IPL, and STS (Iacoboni et al., 2001; Iacoboni et al., 1999; Nishitani et al., 2004; Pfeifer et al., 2008; Williams et al., 2006). Additionally, we performed structural equation modeling (SEM) of effective connectivity in order to investigate the interregional influence of each node in the network (Büchel & Friston, 2000; McIntosh & Gonzalez-Lima, 1994).
2. Materials and methods
2.1. Participants
The study included 14 male participants with ASD and 14 TD participants matched for gender, age, handedness, and nonverbal IQ (Table 1). Each group included 11 right-handed and 3 left-handed participants. The Institutional Review Boards of San Diego State University, San Diego Children's Hospital Research Center, and the University of California, San Diego, approved the experimental protocol. Informed consent was obtained from all participants. For participants under the age of 18 years, written parental consent was also obtained. Diagnoses in the ASD group were established using DSM-IV (APA; 1994), the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Couteur, 1994), and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). We included a total of eight individuals diagnosed with autism, three with Asperger's disorder, and three with pervasive developmental disorder – not otherwise specified (PDD-NOS). Exclusionary criteria for all participants were any comorbid medical diagnosis that might affect brain development or preterm birth. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). All participants scored above the cutoff for mental retardation (IQ > 70). Hand preference was assessed through self-report.
Table 1.
Characteristics of participants
| TD (n = 14) |
ASD (n = 14) |
p | |
|---|---|---|---|
| Age in years | 24.2 (8.4) | 24.1 (9.5) | .94 |
| 14 – 42 | 15 – 44 | ||
| Verbal IQ (VIQ) | 110 (10.8) | 93 (15.5) | .01 |
| 88 – 129 | 79 – 127 | ||
| Nonverbal IQ (NVIQ) | 114 (11.9) | 107 (13.9) | .25 |
| 95 – 130 | 68 – 115 | ||
| Full scale IQ | 114 (12.1) | 99 (11.7) | .01 |
| 89 – 130 | 80 – 117 |
Values are presented as Mean (Standard Deviation) and Range. Significance value, p, from two-sample independent t-tests for differences between the TD and ASD groups. Groups were matched on age, gender, handedness, and nonverbal IQ.
2.2. MRI data acquisition
Imaging data were acquired on a 1.5T Siemens Symphony MR scanner (Erlangen, Germany). High-resolution structural images were acquired with a T1-weighted sequence (TR: 11.08ms; TE: 4.3ms; flip angle: 45°; field of view [FOV]: 256mm; 256×256 matrix; 180 slices; 1mm3 resolution). Functional T2*-weighted images were obtained using a single-shot gradient-recalled echo-planar imaging sequence. Two runs of functional MRI data were collected while participants performed either a semantic decision or letter detection task that alternated in a block design (see Supplementary Material for additional information). Each run included 114 volumes of 28 contiguous 5mm axial slices (TR: 2.6s; TE: 36ms; flip angle: 90°; FOV: 256mm; 64×64 matrix; 4×4 mm2 in-plane resolution).
2.3. Data preprocessing
Imaging data were preprocessed and analyzed using Analysis of Functional NeuroImages software package (AFNI, Cox & Hyde, 1997). The first five time points of each run were discarded due to image instability. Correction for head motion was performed by registering each functional volume to the middle time point of the run closest to the structural scan. To reduce noise, we spatially smoothed the images (8mm full-width half-maximum), removed linear trends attributable to scanner drift, and included six rigid-body motion parameter regressors.
In resting-state fcMRI, Cordes et al. (2001) showed that the BOLD signal of functionally connected networks has the greatest power in the low-frequency domain (< 0.1Hz), excluding some higher-frequency physiological noise from respiratory and cardiac cycles. Additionally, task-evoked responses may be superpositioned on top of spontaneous BOLD fluctuations, exerting a linear effect on the measured signal (Fox et al., 2006). Therefore, to isolate low-frequency fluctuations, we applied a low-pass filter at 0.1Hz and included orthogonal task regressors in a linear model. Specifically, to separate task-related effects from intrinsic BOLD fluctuations, we modeled box-car gamma waveform regressors for blocks of each trial type (Fair et al., 2007; Gavrilescu et al., 2008; Jones et al., 2010; Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000).
For each participant, the two functional runs were concatenated and a total of 218 time points (9:45min.) were included in the connectivity analyses. The structural volume was transformed into Talairach space (Talairach & Tournoux, 1988) and fully preprocessed functional images were spatially normalized to the standardized structural volume and resampled to 2×2×2 mm3 for statistical analysis.
2.4. Regions-of-interest (ROI) selection
Mean time series were extracted from spherical seeds with a radius of 4mm placed in locations identified from literature. We used seeds in the right hemisphere to further reduce any residual confounds related to left hemispheric activation associated with the language task. Talairach coordinates (x, y, z) for ROIs in IPL (58, −24, 32) and STS (57, −50, 16) were obtained from peak imitation activations reported by Iacoboni et al. (2001; 1999). Williams et al. (2006) in an fMRI study of imitation in ASD reported peaks at corresponding coordinates using the same paradigm. The IFG seed was selected using a cytoarchitectonic probabilistic map and placed in a site with the highest probability for BA 44 (51, 7, 17) (Eickhoff et al., 2005). Although the connectivity maps of these regions may include non-imitation processes that share similar neural mechanisms, for simplicity, we will refer to the network produced by our three seeds as the imitation network.
2.5. Whole-brain functional connectivity analysis
We applied multiple regressions at the individual subject level to investigate the whole-brain covariance of the BOLD signal with the mean time series of each ROI. Group-wise functional connectivity maps for each seed were combined for identification of conjunction sites (i.e., regions of significant fcMRI effects for all three seed ROIs) to 1) confirm connectivity at imitation network nodes, which was specifically expected for the TD group, 2) examine the connectivity of the network in areas outside of the three regions, and 3) directly compare the connectivity for imitation between ASD and TD group. One-sample t-tests were performed to obtain group fcMRI effect maps for each seed. Maps were uniformly corrected for multiple comparisons to a cluster significance level of p < 0.001 using Monte-Carlo simulations (Forman et al., 1995). Three group masks were then created from the surviving regions and combined for within-group conjunction analysis. Additionally, direct group comparisons using two-sample independent t-tests were performed for each ROI, and masks of each difference map were created by thresholding to a voxel-wise significance level of p < 0.005, with cluster correction at p < 0.05. For both group contrasts (TD>ASD and ASD>TD), we examined the combined masks for conjunction sites depicting a convergence of increased connectivity.
2.6. Effective connectivity analysis
2.6.1. Model specification
The superior longitudinal fasciculus connecting the frontal cortex with temporo-parietal areas includes the arcuate fasciculus, forming the direct pathway, and two lateral segments, constituting the indirect pathway. The posterior lateral segment connects the regions IPL and STS, and the anterior portion directly links IFG with IPL (Catani, Jones, & ffytche, 2005; Duffau, 2008; Makris et al., 2005). Although imitation circuitry likely contains bidirectional connections, we modeled only unidirectional paths to satisfy the need for parsimony in SEM. However, a unidirectional model is consistent with the MEG study of oral-facial imitation by Nishitani and colleagues (2002), which reported neural activity progressing from V1 to STS to IPL to IFG to primary motor cortex. Iacoboni et al. (2001) have proposed a similar processing sequence with the addition of STS receiving feedback motor commands from IFG. Combined, the two studies suggest that the flow of information forms a loop starting with the representation of the action in STS, to mirroring and identifying its goals in IPL, to interpreting the context in IFG, and back to STS to confirm the meaning of the action.
In a second model, we included a fourth ROI (12, 29, 45) in the right dorsal prefrontal cortex (dPFC) – a region within the superior frontal gyrus for which significantly greater functional connectivity was found in the ASD (compared to the TD) group, convergent for all three network nodes. We entertained the hypothesis that in ASD this region moderates regions employed for imitation; therefore, we included three unidirectional links from dPFC.
2.6.2. Structural equation modeling
To examine the effective connectivity of the imitation network, we applied SEM as implemented in AFNI 1dSEM (Chen et al., 2007). Although SEM is more commonly performed at the group level, we conducted individual subject level analyses to account for individual variability, which may be pronounced given the known heterogeneity in ASD (Happé, Ronald, & Plomin, 2006). The preprocessed mean time series for each ROI were used to create a covariance matrix for each subject. Residual error estimates for each ROI included .5 of the total variance, which was further adjusted by visually inspecting the fcMRI effect maps to approximate the extent of its connectivity with other regions of the network (Bullmore et al., 2000; McIntosh & Gonzalez-Lima, 1994). The degrees of freedom included for χ2 significance tests accounted for potential autocorrelations within ROIs (refer to http://afni.nimh.nih.gov/sscc/gangc/PathAna.html for details). Each subject was examined for optimal model fit by having a nonsignificant χ2 value (p > 0.1). Mean path coefficients were obtained for both groups and checked for within-group significance using one-sample t-tests. Comparisons between groups for individual paths were performed using two-sample independent t-tests. Furthermore, since within-group variability of our results may be influenced by the total amount of residual error and degrees of freedom included in model estimation, we performed Levene's tests for homogeneity of variance to examine whether group variability affected results.
3. Results
3.1. Functional connectivity
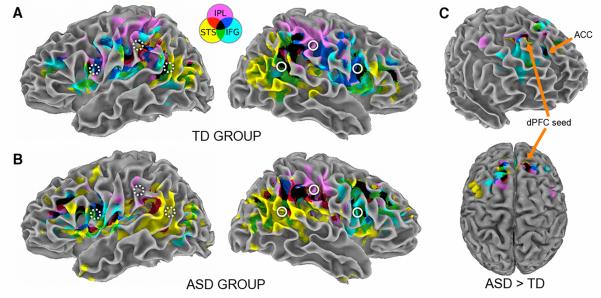
In the TD group, conjunction analyses confirmed concordant low-frequency fcMRI effects for our three seeds in or in close vicinity of our regions selected for their association with imitation (see black conjunction clusters in Fig. 1A and Supplementary Tables). Moreover, we observed conjunction clusters in homotopic sites of the left hemisphere, which reflect bilateral connectivity for imitation processes. In the ASD group, conjunction effects in imitation regions appeared slightly less robust and no sites of convergent connectivity were present in left IPL and STS (Fig. 1B). Unexpectedly, conjunction clusters remote from the typical imitation network were detected in dorsal frontal cortex. In direct group comparisons, we did not find significant differences around our ROIs. No clusters of significantly greater fcMRI effects for the TD (compared to ASD) group were seen anywhere in the brain. However, we found significant inverse effects (ASD>TD), particularly in frontal regions. Among these were several conjunction clusters in bilateral superior frontal and left anterior cingulate gyri, reflecting increased connectivity with these areas by all three network nodes (Fig. 1C).
Figure 1. The imitation network produced by overlapping functional connectivity maps of its three right hemisphere nodes.
(A) In the TD group, concordant connectivity (i.e. conjunction effects for all seeds; black clusters) is seen in the vicinity of each node and in left-hemisphere homologous regions. (B) In the ASD group, large unexpected conjunction clusters are found in dorsal prefrontal cortex (dPFC) bilaterally. (C) On direct group comparison, atypically enhanced connectivity in frontal regions is seen in the ASD group, with conjunction effects in dPFC and left anterior cingulate cortex (ACC). FcMRI effects for each seed are color-coded, as indicated at the top. Seed ROIs are indicated by solid-white circles and their left-hemisphere homologues are indicated by dotted-white circles.
3.2. Effective connectivity
Path coefficients and significance levels are presented for both models in Table 2. Structural equation modeling of an imitation network demonstrated that the two models had acceptable fits in all participants (p > 0.1). In both groups, path coefficients indicated a positive effect on afferent regions, suggesting a positive feedback loop (Fig. 2A). In the ASD group, a reduced effect of IPL on IFG trended toward significance, but no significant differences were detected for other connections. In the second model we added an ROI in right dPFC (superior frontal gyrus; Fig. 1C), for which significantly increased conjunction effects had been observed in ASD compared to TD. We found a significantly reduced effect of IPL on IFG accompanied by a significantly greater effect of dPFC on IFG in the ASD group (Fig. 2B). Levene's test for homogeneity of variance detected no significant between-group differences in variability of path coefficients for IPL to IFG (F(1,26) = 0.06, p = 0.81) and dPFC to IFG (F(1,26) = 0.37, p = 0.55).
Table 2.
Path coefficients for each connection in a right hemisphere imitation network
| Modeled connections | Mean path coefficients |
TD vs. ASDa |
Levene'sb |
|||
|---|---|---|---|---|---|---|
| TD | ASD | t(26) | P | F(1,26) | P | |
| Imitation network model | ||||||
| STS → IPL | .15† (.04) | .13† (.04) | 0.36 | 0.72 | 0.001 | 0.97 |
| IPL → IFG | .46§ (.04) | .32‡ (.06) | 1.88 | 0.07 | 0.01 | 0.91 |
| IFG → STS | .38§ (.04) | .35§ (.06) | 0.36 | 0.72 | 6.74 | 0.02 |
| dPFC moderating imitation network | ||||||
| STS → IPL | .13‡ (.03) | .11* (.04) | 0.32 | 0.76 | 0.69 | 0.41 |
| IPL → IFG | .45§ (.04) | .25‡ (.06) | 3.00 | 0.006 | 0.06 | 0.81 |
| IFG → STS | .36§ (.04) | .29§ (.05) | 1.16 | 0.26 | 1.50 | 0.23 |
| dPFC → STS | .18* (.06) | .33§ (.06) | −1.40 | 0.17 | 0.69 | 0.41 |
| dPFC → IPL | .13* (.06) | .23† (.06) | −1.18 | 0.25 | 0.02 | 0.88 |
| dPFC → IFG | .12 (.08) | .30‡ (.06) | −2.04 | 0.05 | 0.37 | 0.55 |
Path coefficients are given as Mean (Standard Error). Within-group significance level assessed using one-sample t-tests
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001,
p ≤ 0.0001.
Direct group comparisons of each path coefficient performed using two-sample independent t-tests.
Levene's test for homogeneity of variance.
Figure 2. A simple imitation network model.
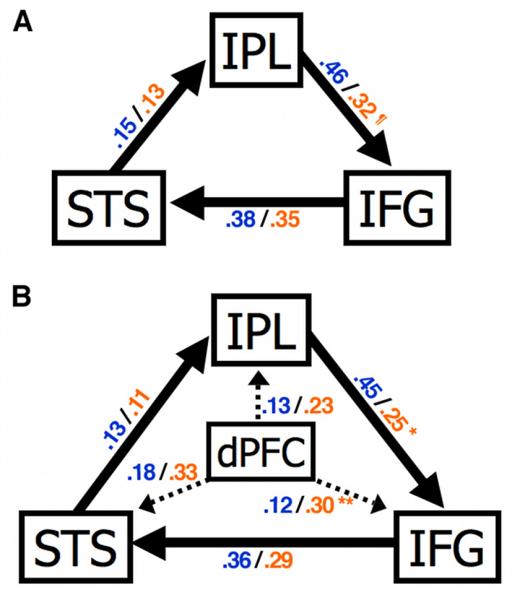
(A) Mean path coefficients for each group are presented as TD/ASD (blue/orange). In a three-node model, there is a trend toward significantly reduced effect of IPL on IFG in the ASD group when compared to the TD group (¶p = 0.07). (B) A second model included dorsal prefrontal cortex (dPFC) as a moderator for the imitation network in the right hemisphere. In direct comparisons, the ASD group showed significantly increased effect of dPFC on IFG (*p = 0.05) and reduced effect of IPL on IFG (**p = 0.006). STS, superior temporal sulcus; IPL, inferior parietal lobule; IFG, inferior frontal gyrus.
4. Discussion
We examined task-independent, low-frequency BOLD fluctuations using three right hemisphere seeds placed in sites considered prominent nodes of an imitation circuit (IFG, IPL, and STS, based on fMRI activation findings from previous imitation studies). In the TD group, we observed conjunction fcMRI effects in or in close vicinity of the three nodes, reflecting tight functional interconnectivity. Moreover, homotopic sites of convergent connectivity were also detected in regions of the contralateral left hemisphere, which is consistent with results indicating the bilaterality of imitation networks (Aziz-Zadeh, Koski, Zaidel, Mazziotta, & Iacoboni, 2006). In the ASD group, the intrinsic synchronicity between regions of the imitation network appeared less robust, but group differences did not reach significance. Moreover, connectivity in homotopic regions of the left hemisphere, as seen in the TD group, was reduced in our ASD group. Consistent with some previous reports of anomalous patterns of functional connectivity in ASD (Braeutigam et al., 2008; Jones et al., 2010; Mizuno et al., 2006; Monk et al., 2009; Murias et al., 2007; Noonan et al., 2009; Turner et al., 2006; Welchew et al., 2005), we observed diffuse and extensive connectivity with bilateral frontal regions. Specifically, the dorsal frontal cortex contained sites of convergent effects, i.e., significantly increased fcMRI effects for all three seeds that were detected only in the ASD group.
Several fcMRI studies in ASD have reported reduced functional connectivity of frontal cortex across various tasks (Just et al., 2007; Just et al., 2004; Kana, Keller, Cherkassky, Minshew, & Just, 2008; Mason et al., 2008). The apparent inconsistency may be explained by methodological differences. In contrast to ours, the aforementioned studies by Just and colleagues did not implement task regressors and low-pass filtering to isolate intrinsic low-frequency BOLD fluctuations. Interestingly, a recent study by the same group (Koshino et al., 2008) applied a low-pass filter – thus reducing the effect of experimental trials presented at a high frequency – and found no evidence of underconnectivity within the fronto-parietal network of their ASD group. Notably, the absence of significant group differences in this network is generally consistent with our own task-regressed and low-pass filtered fcMRI results between IFG and IPL.
Slow, synchronized fluctuations of spontaneous neuronal activity at rest have been found to reflect functional connectivity of putative networks in both animals (Leopold et al., 2003; Shmuel & Leopold, 2008; Vincent et al., 2007) and humans (Nir et al., 2008; Seeley et al., 2007; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008; Xiong et al., 1999). These low-frequency signal fluctuations are also present during task performance and exert a linear effect on measured BOLD responses (Fox et al., 2006). Using low-pass filtering and task regression to isolate intrinsically generated low-frequency BOLD fluctuations may not guarantee complete removal of all task-related activity (Hasson et al., 2009). However, recent studies indicate that the removal of task effects can be effective and yields qualitatively similar results to resting-state fcMRI (Arfanakis et al., 2000; Fair et al., 2007; Gavrilescu et al., 2008; Jones et al., 2010). Notably, this approach provides better protection from uncontrolled spontaneous thought processes at rest, which may naturally differ between ASD and TD participants (Kennedy & Courchesne, 2008a). Thus, the utility of either approach – using resting or task-regressed data – is best weighed with its respective strengths and limitations in mind. Additional information and analyses presented in the Supplementary Material suggest that enhanced connectivity with dPFC detected in the ASD group (relative to the TD group) was unlikely to be driven by task-related effects. It should be noted that our participant groups were matched for nonverbal, but not for verbal IQ. It can therefore not be ruled out that lower VIQ in the ASD group – although expected in a cohort with a sociocommunicative disorder – may have had some impact on our findings.
In a secondary analysis using SEM, we attempted to better characterize the effect of atypically enhanced low-frequency synchronization with the frontal cortex by modeling effective connectivity in the imitation network. Although both models (with and without dPFC; see Fig. 2) had acceptable fits in all participants, dPFC in the ASD group indicated a larger positive influence on regions for imitation, as expected based on our fcMRI results. Notably, once dPFC was included in the model, we observed a significantly reduced influence of IPL on IFG in the ASD group compared to TD controls. This indicates that greater coordination between low-frequency BOLD fluctuations of IPL and dPFC was potentially masking reduced communication between IPL and IFG. Thus, our SEM results suggest that impaired functioning in regions employed by imitation may be accompanied by abnormally greater interactions with regions outside of the typical network. This appears consistent with an earlier study showing atypical prefrontal recruitment during visuomotor coordination tasks in ASD (Müller et al., 2003). However, it remains open whether greater synchronization with dPFC can lead to reduced influence of IPL on IFG, or whether increased coordination between the two regions is a result of already impaired communication between IPL and IFG.
In another fMRI study using SEM, Wicker and colleagues (Wicker et al., 2008) investigated facial expression recognition in adults with ASD. Even though they observed predominantly reduced task-related effective connectivity in the ASD group, an ROI in dorsolateral PFC (DLPFC) was found to have a significantly greater effect on fusiform gyrus in comparison to the TD group. Since there were no behavioral differences, the authors concluded that abnormally increased influence of DLPFC could reflect a compensatory mechanism recruited for emotion-processing of faces. In an analogous interpretation of our results, dPFC may play a compensatory role in support of atypical imitation processing in ASD, which may be reflected in null findings from some behavioral studies (Hamilton et al., 2007).
Our dPFC seed was located in the dorsal portion of BA 8 in the right hemisphere. Rajah, Ames, and D'Esposito (2008) proposed that activity in right BA 8 (and adjacent area 6) involves spatiotemporal working memory manipulation and/or maintenance. Specifically, BA 8 has been implicated in processes such as spatial attention (Simon et al., 2002), episodic memory retrieval (Cabeza, Locantore, & Anderson, 2003), temporal recency judgments (Dudukovic & Wagner, 2007), spatial sensorimotor transformations (Levy, Schluppeck, Heeger, & Glimcher, 2007), and action selection (Cisek & Kalaska, 2005). Using fMRI, Beudel and de Jong (2009) employed a button press paradigm in which the finger or button were either freely selected by the participant or fixed (i.e. determined by a number stimulus). They found that BA 8 activated strongly only for free-selection tasks, which the authors related to involvement in the cognitive decision component of action selection. In another fMRI study, Koch and colleagues (2006), examining working memory retrieval, reported an exponential signal decrease in BA 8/9 across time that significantly correlated with behavioral performance. They concluded that practice-related reductions in BA 8/9 indicated less reliance on frontal region as processing became more efficient. Taken together, increased connectivity with dPFC may suggest that people with ASD rely heavily on cognitive selection and manipulation of actions from memory, further suggesting that greater impairments may be seen for novel, meaningless actions where memories for these movements cannot be easily recalled from past experience (Cattaneo et al., 2007; Hamilton et al., 2007; Rogers et al., 1996). Such reliance on top-down control may result from impaired implicit sensorimotor mechanisms, employed in elicited, intentional imitation, and could reflect compensatory plasticity in ASD.
However, enhanced connectivity with dPFC probably cannot be directly equated with enhanced action control in ASD, given that motor impairments may be a common feature of the disorder (Fournier, Hass, Naik, Lodha, & Cauraugh, 2010; Gidley Larson & Mostofsky, 2008; Mostofsky et al., 2006; Mostofsky et al., 2009; Rogers et al., 1996). Motor control involves the coordination of multiple regions, relying on subcortical, cerebellar, parieto-frontal, and even local circuitry (i.e. between functionally distinct, neighboring regions such as dPFC and supplementary motor area (SMA), or among neurons of discrete functional areas within SMA). Dysfunction at any of the nodes in these circuits can produce deficits. It is still under debate whether imitation impairments in ASD exist independently of motor deficits (Williams et al., 2004; Zachor, Ilanit, & Itzchak, 2009) or can be attributed to perceptual-motor deficits (Vanvuchelen, Roeyers, & De Weerdt, 2007). Even if the latter view is taken, the component processes contributing to imitation impairments in ASD, such as dissociating implicit and explicit motor control ability, remain to be fully understood (Mazzoni & Wexler, 2009). With respect to potential motor components in imitation, Mostofsky and colleagues (2009) found greater reliance on the SMA for simple motor sequencing in ASD relative to TD controls; however, reduced functional connectivity was seen across regions involved in the motor execution network. The authors suggest that greater, but isolated, activation in the SMA with decreased cerebellar activation may indicate impairment in the transfer of learned actions to habitual ones that are under implicit control. However, it is not well understood how such abnormalities in the motor execution network may affect imitation circuits that were examined in the present study.
Alternatively, though not necessarily mutually exclusive with the compensatory interpretation, greater synchronization with cortical areas outside typical networks could also reflect more general patterns of aberrant connectivity in ASD. Patterns of over- and underconnectivity may be the neurodevelopmental ramifications of abnormal brain-growth trajectories (Hazlett et al., 2005; Lewis & Elman, 2008). Courchesne and colleagues (2001) found that the autistic brain undergoes a rapid growth spurt during the first two years of life, with slowed growth thereafter. Among the many regions affected, findings of white matter volume abnormalities in the frontal lobe have been the most consistent (Carper & Courchesne, 2005; Carper, Moses, Tigue, & Courchesne, 2002; Courchesne & Pierce, 2005; Herbert et al., 2004). Given the convergent evidence for premature overgrowth of white matter in autism and the possible link with behavioral impairments (Mostofsky, Burgess, & Gidley Larson, 2007; White, O'Reilly, & Frith, 2009), our results could suggest that early overconnectivity within frontal regions such as dPFC may interfere with normal neural interactions, potentially limiting the development and diversification of functional networks (Ben Bashat et al., 2007; Carper & Courchesne, 2005; Kennedy & Courchesne, 2008b; Sundaram et al., 2008). In an fcMRI study examining response inhibition with a Go/NoGo task, no group differences between 8-12 year old children with ASD and TD children were detected, but reduced connectivity was found to be correlated with increasing age (Lee et al., 2008). Although ROIs were limited to only those involved in the inhibitory network and potential regions of enhanced connectivity were not explored, age-dependent findings may relate to early overgrowth.
Overall, our finding of atypical connectivity with the frontal cortex along with reduced effective connectivity between IPL and IFG suggests atypical organization of the network for imitation in ASD, which may be linked with impaired sociocommunicative development. However, it is unknown how early overconnectivity may affect developing neurofunctional organization in general and the imitation network in particular. Importantly, it still remains open whether regions of increased connectivity could functionally contribute to processing for imitation or add functionally irrelevant noise by interfering with communication between regions. Furthermore, conclusive associations between functional connectivity and white matter have yet to be established in ASD. BOLD synchronization as detected by fcMRI can reflect both monosynaptic and polysynaptic connections, which further complicates the relationship between anatomical and functional connectivity (Greicius et al., 2009; Honey et al., 2009; van den Heuvel et al., 2009). Finally, since we analyzed intrinsic low-frequency BOLD fluctuations (rather than task-driven BOLD changes), observed connectivity may not be exclusively attributed to an imitation network, but could additionally relate to other networks subserved by brain regions implemented as seeds (IFG, IPL, STS). Our focus on the imitation network in the present study is in no way meant to imply a unique or exclusive role of this network in autistic symptomatology. The pattern of findings indicating subtly reduced connectivity within networks and diffusely increased connectivity outside networks (Monk et al., 2009; Noonan et al., 2009; Welchew et al., 2005) possibly reflects general outcomes of early brain growth anomalies potentially affecting many networks.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (R01-DC006155, R01-MH081023), with additional funding from the National Institute on Deafness and Other Communicative Disorders (NIDCD 1T32-DC007361-03; author BK). Special thanks to the children and families who participated and to Gang Chen, Benjamin McKenna, and Wesley Thompson for their help and insight on SEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18(8):921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta JC, Iacoboni M. Lateralization of the human mirror neuron system. J Neurosci. 2006;26(11):2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: A high b value DWI study. Neuroimage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Webb S, Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64(3):228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beudel M, de Jong BM. Overlap and segregation in predorsal premotor cortex activations related to free selection of self-referenced and target-based finger movements. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn254. [DOI] [PubMed] [Google Scholar]
- Bird G, Leighton J, Press C, Heyes C. Intact automatic imitation of human and robot actions in autism spectrum disorders. Proc R Soc B. 2007;274(1628):3027–3031. doi: 10.1098/rspb.2007.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Braeutigam S, Swithenby SJ, Bailey AJ. Contextual integration the unusual way: A magnetoencephalographic study of responses to semantic violation in individuals with autism spectrum disorders. Eur J Neurosci. 2008;27(4):1026–1036. doi: 10.1111/j.1460-9568.2008.06064.x. [DOI] [PubMed] [Google Scholar]
- Büchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Netw. 2000;13(8-9):871–882. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11(4):289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: Evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15(2):249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57(2):126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, et al. Impairment of actions chains in autism and its possible role in intention understanding. Proc Natl Acad Sci U S A. 2007;104(45):17825–17830. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Glen DR, Stein JL, Meyer-Lindenberg AS, Saad ZS, Cox RW. Model validation and automated search in fMRI path analysis: A fast open-source tool for structural equation modeling. Hum Brain Mapp Conference. 2007 [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron. 2005;45(5):801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “Resting-state” Data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23(2-3):153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine. 1997;10 doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chaminade T, Grèzes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15(1):265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Dudukovic NM, Wagner AD. Goal-dependent modulation of declarative memory: Neural correlates of temporal recency decisions and novelty detection. Neuropsychologia. 2007;45(11):2608–2620. doi: 10.1016/j.neuropsychologia.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46(4):927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández GR, Galán RF. On how network architecture determines the dominant patterns of spontaneous neural activity. PLoS ONE. 2008;3(5):e2148. doi: 10.1371/journal.pone.0002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M, Stuart GW, Rossell S, Henshall K, McKay C, Sergejew AA, et al. Functional connectivity estimation in fMRI data: Influence of preprocessing and time course selection. Hum Brain Mapp. 2008;29(9):1040–1052. doi: 10.1002/hbm.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson J, Mostofsky S. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 2008;1(6):341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: The default network and stimulus-independent thought”. Science. 2007;317(5834):43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFC, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45(8):1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106(26):10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archiv Gen Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105(41):16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A. Imitation and identification in autism. J Child Psychol Psychiatry. 1999;40(4):649–659. [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Warner B, Fitzer J, Tagamets M-A, Husain FT, Long TW. Investigating the neural basis for functional and effective connectivity. Application to fMRI. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1093–1108. doi: 10.1098/rstb.2005.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S. The shared circuits model (SCM): How control, mirroring, and simulation can enable imitation, deliberation, and mindreading. Behav and Brain Sci. 2008;31(1):1–22. doi: 10.1017/S0140525X07003123. discussion 22-58. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci U S A. 2001;98(24):13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22(1):63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28(25):6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, et al. Sources of group differences in functional connectivity: An investigation applied to autism spectrum disorder. NeuroImage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fmri study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc Neurosci. 2008:1–18. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008a;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008b;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, von Consbruch K, Nenadic I, Schultz C, Ehle C, et al. Temporal changes in neural activation during practice of information retrieval from short-term memory: An fMRI study. Brain Res. 2006;1107(1):140–150. doi: 10.1016/j.brainres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, et al. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: A fcMRI study of response inhibition. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Bird G, Charman T, Heyes C. Weak imitative performance is not due to a functional ‘mirroring’deficit in adults with autism spectrum disorders. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: Implications for functional brain imaging. Cereb Cortex. 2003;13(4):422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Lepage J-F, Théoret H. The mirror neuron system: Grasping others' actions from birth? Dev Sci. 2007;10(5):513–523. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Levy I, Schluppeck D, Heeger DJ, Glimcher PW. Specificity of human cortical areas for reaches and saccades. J Neurosci. 2007;27(17):4687–4696. doi: 10.1523/JNEUROSCI.0459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Elman JL. Growth-related neural reorganization and the autism phenotype: A test of the hypothesis that altered brain growth leads to altered connectivity. Dev Sci. 2008;11(1):135–155. doi: 10.1111/j.1467-7687.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepelt R, Von Cramon DY, Brass M. How do we infer others' goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage. 2008;43(4):784–792. doi: 10.1016/j.neuroimage.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Malvy J, Roux S, Zakian A, Debuly S, Sauvage D, Barthelemy C. A brief clinical scale for the early evaluation of imitation disorders in autism. Autism. 1999;3(4):357–369. [Google Scholar]
- Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: Is the mirror neuron system involved? Int J Psychophysiol. 2008;68(1):35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew NJ, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Wexler NS. Parallel explicit and implicit control of reaching. PLoS ONE. 2009;4(10):e7557. doi: 10.1371/journal.pone.0007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2(1-2):2–22. [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104(1):160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130(Pt 8):2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc. 2006;12(03):314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: An fmri study of visuomotor learning. Am J Psychiatry. 2003;160(10):1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30(4):1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11(9):1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Avikainen S, Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Ann Neurol. 2004;55(4):558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Viewing lip forms: Cortical dynamics. Neuron. 2002;36(6):1211–1220. doi: 10.1016/s0896-6273(02)01089-9. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller R-A. Aberrant functional connectivity in autism: Evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009 doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. Preliminary evidence for deficits in multisensory integration in autism spectrum disorders: The mirror neuron hypothesis. Soc Neurosci. 2008;3(3-4):348–355. doi: 10.1080/17470910701563681. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39(4):2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Turski P, Moritz C, Haughton V, Seth R, et al. Role of the corpus callosum in functional connectivity. AJNR Am J Neuroradiol. 2003;24(2):208–212. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Ames B, D'Esposito M. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 2008;46(4):1088–1103. doi: 10.1016/j.neuropsychologia.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Receveur C, Lenoir P, Desombre H, Roux S, Barthelemy C, Malvy J. Interaction and imitation deficits from infancy to 4 years of age in children with autism: A pilot study based on videotapes. Autism. 2005;9(1):69–82. doi: 10.1177/1362361305049030. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington B. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Dev. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao D-K, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29(7):751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D. Spatial attention and memory versus motor preparation: Premotor cortex involvement as revealed by fMRI. J Neurophysiol. 2002;88(4):2047–2057. doi: 10.1152/jn.2002.88.4.2047. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: Diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43(3):554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IM, Bryson SE. Imitation and action in autism: A critical review. Psychol Bull. 1994;116(2):259–273. doi: 10.1037/0033-2909.116.2.259. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller R-A. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanvuchelen M, Roeyers H, De Weerdt W. Nature of motor imitation problems in school-aged boys with autism: A motor or a cognitive problem? Autism. 2007;11(3):225–240. doi: 10.1177/1362361307076846. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller R-A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vivanti G, Nadig A, Ozonoff S, Rogers SJ. What do children with autism attend to during imitation tasks? J Exp Child Psychol. 2008;101(3):186–205. doi: 10.1016/j.jecp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (wasi) Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, et al. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57(9):991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, O'Reilly H, Frith U. Big heads, small details and autism. Neuropsychologia. 2009;47(5):1274–1281. doi: 10.1016/j.neuropsychologia.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3(2):135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and 'mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8(2-3):151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachor DA, Ilanit T, Itzchak EB. Autism severity and motor abilities correlates of imitation situations in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2009:1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.