Abstract
Oral spironolactone has been used for over two decades in the dermatological setting. Although it is not generally considered a primary option in the management of female patients with acne vulgaris, the increase in office visits by post-teenage women with acne vulgaris has recently placed a spotlight on the use of this agent in this subgroup of patients. This article reviews the literature focusing on the use of oral spironolactone in this subset of women with acne vulgaris, including discussions of the recommended starting dose, expected response time, adjustments in therapy, potential adverse effects, and patient monitoring.
Acne vulgaris (AV) is usually perceived as a disorder that affects primarily teenagers; however, preteens and postadolescents are commonly affected. Outpatient visits by patients 25 years of age or older has increased over the past 10 years.1 AV can have a significant impact on the emotional and psychological wellbeing of affected individuals and has been compared to other major disease states in terms of adverse impact on quality of life (QoL).2 While many individuals experience AV that regresses soon after they complete their teenage years, there is a subset of patients that notes persistence throughout later adulthood, with some noting the onset of AV in their adult life, the latter especially in women. Adults may be more conscious of their acne because it is considered a “disease of teenagers,” experiencing more social anxiety as they are wondering why they are still having acne breakouts or are first developing acne lesions when their teenage years have passed. Women seem to make up the majority of patients with adult AV.1 It has also been shown that postadolescent patients fail conventional treatment for AV in 79 to 82 percent of cases.1 Oral isotretinoin treatment failure has also been reported to occur in 16 to 32 percent of this subgroup of patients.1 The recognition that postadolescent women with AV respond to antiandrogenic hormonal therapies has prompted a sustained interest in oral spironolactone.
Background on Spironolactone
Oral spironolactone (hereafter referred to as spironolactone) is used for a variety of indications in the medical arena. First developed in 1957, spironolactone is an aldosterone antagonist that was used initially as a potassium-sparing diuretic in the treatment of hypertension and congestive heart failure. Structurally, its backbone is a basic steroidal nucleus with four rings. The primary metabolite of spironolactone is canrenone, which is an active metabolite that is also an antagonist of aldosterone, and thus promotes diuresis.
The antiandrogenic effects of spironolactone were first discovered when it was being used to treat hypertension in women with concurrent polycystic ovary syndrome (PCOS) and hirsutism.3 It has been used frequently in the dermatology clinic for women with hormonal-pattern AV, defined clinically as primarily inflammatory papules, many deep-seated and tender, that are located predominantly on the lower half of the face and anterior-lateral neck region. Currently, dermatologists prescribe oral spironolactone for off-label use, with AV being a non-United States Food and Drug Administration (FDA)-approved indication. The rationale for using spironolactone in the treatment of AV is that is has been shown to inhibit sebaceous gland activity. As increased size of sebaceous glands and increased sebum secretion are essential components in the development of AV lesions, inhibition of sebaceous gland functions leads to reduced formation of acne lesions.4 Studies have shown that spironolactone decreases androgen-stimulated sebocyte proliferation in vitro and inhibits sebaceous activity in Syrian hamsters in a dose-dependent fashion.4,5 Clinically, women with high androgenic states will also have increased sebum production due to an increase in circulating androgens, with development or worsening of AV a common sequelae of androgen excess. However, the majority of women who present with late-onset or post-teen persistent AV, even with classic hormonal-pattern AV, do not exhibit an increase in serum androgen levels. Regardless, this latter subset still benefits substantially from oral spironolactone in most cases, with the onset of therapeutic effect often noted within 4 to 8 weeks.
Studies have shown that women with hormonal pattern AV who have normal circulating androgen levels exhibit increased levels of the tissue-derived androgens, 3-alpha-androstanediol glucuronide and androsterone glucuronide, both of which appear to act locally on target tissues to promote development of AV in female patients.6,7 Additionally, it has also been noted that acne-prone skin has greater activity of type-1 5-alpha-reductase activity, and non-acne-prone skin has greater activity of 17-beta-hydroxysteroid dehydrogenase activity.8 So where does spironolactone fit into this puzzle of acne lesion formation, especially in the post-teen female patient? Spironolactone decreases 5-alpha reductase activity via increased clearance of testosterone secondary to augmented liver hydroxylase activity. In addition, it increases the level of steroid hormone binding globulin (SHBG), thus providing a sink that reduces circulating free testosterone as more is bound by the increased quantity of SHBG. The resultant effect of reducing free testosterone in circulation is an increased estrogenic state, which can lead to gynecomastia or decreased libido, especially when higher doses of oral spironolactone are used. Spironolactone also acts locally by competing with dihydrotestosterone (DHT) for cutaneous androgen receptors, thereby inhibiting testosterone and DHT binding. The ability of spironolactone to inhibit androgens at different physiological levels has led to its use in women with androgenic alopecia, hirsutism, and excess sebum production, with successful outcomes noted in some patients.9
What is the Current Perspective of the Epidemiology of Acne Vulgaris?
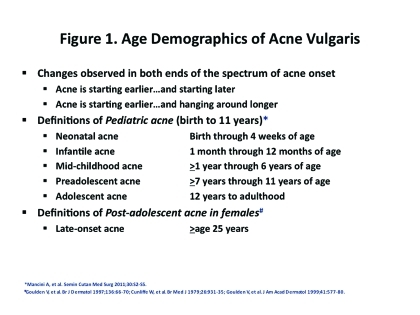
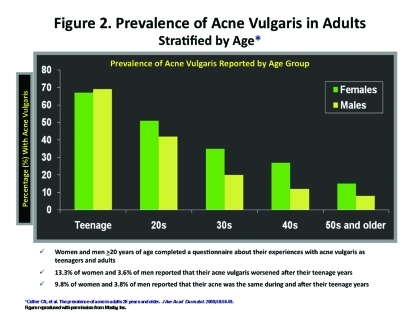
The spectrum of onset of AV is changing with cases emerging both earlier and later than what has been noted in the past (Figure 1). There are a significant number of people of both genders who report experiencing episodes of AV past the teenage years (Figure 2). In some cases, this is persistent AV, meaning that they had AV during their teenage years that did not spontaneously resolve once they progressed into the third decade of life (“the twenties”). They continue to develop lesions of AV either continuously or intermittently. Another subset is late-onset AV, which starts in the postadolescent years (after age 25) with no previous history of acne during the teenage years. By arbitrary designation in the literature, late-onset AV is defined as AV that begins at 25 years of age or older. Late-onset AV has been reported to occur in 18.4 percent of women and 8.3 percent in men.10 In addition, it has been noted that male patients tend to have a higher prevalence of AV before the age of 16 years; however, female patients tend to have a higher prevalence of AV after the age of 23 years.11 Patients with post-teenage AV present at a mean age of 24 years, and approximately 10 percent of office visits for AV are by patients between the ages of 35 and 44 years of age.10 Exogenous factors, such as cosmetics or skin care products, medications, and/or occupation have not been found to be precipitating or exacerbating factors.1 Some researchers have suggested that AV in adulthood may be associated with a strong familial tendency. In one study (N=200), 50 percent of patients reported having first-degree relatives with postadolescent AV.11 The risk of adult AV occurring in a relative of a patient with adult AV is significantly greater than those of unaffected individuals (p<0.001).11 Other studies suggest that genetic factors may determine the failure of acne-prone follicles to evolve into treatment-resistant follicles in early adult life.12 However, the cutaneous microflora of the adolescent with persistent AV as compared to adults with late-onset AV do not differ.13 This study suggests that microflora in adults may not be the predominating driving force for AV in this late-onset age group. To add, this group of patients seems to exhibit higher treatment failure rates with traditional acne therapies. In a study of women over the age of 25 with persistent AV, approximately 82 percent failed therapy with multiple courses of antibiotics and 32 percent had relapsed after treatment with one or more courses of oral isotretinoin.14
Figure 1.
Age demographics of acne vulgaris
Figure 2.
Prevalence of acne vulgaris in adults, stratified by age
What are the Clinical Features of Adult Acne Vulgaris and are there any other Associated Features?
Women with late-onset AV may present similar in appearance to AV that is seen in teenagers, and with the same range of severity. However, as discussed above, late-onset AV commonly presents with a distinctive clinical pattern, often referred to as a hormonal pattern of AV, characterized by a predominance of inflammatory papules concentrated along the lower half of the cheeks, jawline, chin, and lateral neck. In this subset of adult females, it is common for many of the inflammatory papules to be deep in palpable quality and sometimes with nodules present. Tenderness of some of the lesions can also be a common complaint. Another feature of late-onset AV in women is the relative low number or absence of comedonal lesions in many cases, although this is a variable observation. Nevertheless, this onset pattern is the reverse of what is usually noted in preteens and early adolescents, the latter often presenting with a predominance of comedonal lesions. Adult women often exhibit a cyclic exacerbation of AV lesions with perimenstrual flares, with one study reporting that women over 33 years of age have a higher rate of perimenstrual flares as compared to women 20 to 33 years of age (p=0.03).15 In one study, 63 percent of women with AV experienced a 25-percent increase in the number of inflammatory lesions associated with perimenstrual flaring of AV.16 AV in adult women can also be associated with menstrual irregularities, hirsutism, or androgenic alopecia, all clinical features that correlate with a high androgenic state. However, most adult women who present with AV have normal circulating androgen levels.
Persistence of AV into adulthood or late onset of AV in women who also exhibit clinical features of hyper-androgenism, may warrant further investigation for underlying hormonal abnormalities, such as an endocrinopathy or a hormone-secreting tumor. In adult women with AV, especially those with a hormonal pattern clinically, who do not exhibit androgen excess on laboratory evaluation, or in many with hyperandrogenism associated with endocrine disorders, such as polycystic ovary syndrome (PCOS), oral spironolactone may be an ideal therapy because of its ability to block systemic androgen production and diminish the effects of peripheral androgens on target end organs (Table 1).
TABLE 1.
Potential indications for oral spironolactone in post-teenage female patients with acne vulgaris* 20, 22–27
| POTENTIAL INDICATIONS WHERE ORAL SPIRONOLACTONE THERAPY CAN BE CONSIDERED IN WOMEN WITH POST-TEENAGE ACNE VULGARIS |
|---|
| Women with acne flares that cycle with menstruation |
| Women on oral contraceptives or wanting to be on oral contraceptives who exhibit moderate-to-severe acne vulgaris (AV), especially with a “hormonal pattern” clinically (see text) |
| Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin |
| Women with late-onset AV, or persistent-recurrent AV past the teenage years, even in the absence of clinical signs of hyperandrogenism and with normal results of hormonal level testing |
| Women with late-onset AV (acne tarda) or sudden onset of AV |
| Clinical signs of hyperandrogenism, such as hirsutism, androgenic alopecia, and/or increased sebum production with AV |
These are suggested indications where use of oral spironolactone may be considered as a therapeutic option. However, it is important that each patient be evaluated fully to determine the applicability of this option and to exclude any reasons why spironolactone would not be a good choice (i.e., past medical history, possible drug interactions), at least without proper monitoring or further evaluation.
What are some Underlying Disorders that a Clinician must Exclude and What are the Laboratory Tests that should be Ordered?
Although androgens are a major causative factor in AV, most adult women with AV do not have elevated circulating hormone levels or clinical evidence of hyperandrogenism.7 Thus, end-organ sensitivity of the pilosebaceous unit to androgens may be a factor. Persistent and/or treatment resistant AV, or AV of sudden onset, are clinical clues suggestive of abnormal androgen excess. The possibility of an ovarian or adrenal tumor must be considered in this scenario, especially if other signs of hyperandrogenism have emerged, even if subtle. Signs of hyperandrogenism in women include androgenic alopecia, seborrhea (i.e., new onset “oily skin”), AV, hirsutism, Cushingoid features, increased libido, clitoromegaly, deepening of the voice, hyperhidrosis, and acanthosis nigricans. Women experiencing these abnormalities should be screened for underlying endocrine disorders, and preferably referred to an endocrinologist.
The two most common causes of excess circulating androgen levels in women are PCOS and congenital adrenal hyperplasia (CAH).1 The overall prevalence of PCOS is estimated to be 3 to 6 percent, with approximately 23 to 35 percent of women with PCOS exhibiting AV clinically.17 In turn, it has been suggested that severe AV in women is highly suggestive of underlying PCOS, with approximately 83 percent of women with severe AV found to have PCOS in one report.17 These patients present initially with infertility or menstrual irregularities caused by anovulation. It is prudent for clinicians to evaluate the patient for a family history of PCOS, hirsutism, diabetes, and infertility. Adrenal enzyme deficiencies, such as 21-hydroxylase deficiency (late-onset congenital adrenal hyperplasia), although rare, are important to consider in refractory cases. These adrenal deficiency syndromes are seen with a higher prevalence in those of Eastern European Jewish descent when evaluating patients with adult AV.18 Anti-androgen therapy may be helpful in female patients with severe seborrhea, acne, hirsutism, and alopecia, or the so-called seborrhea/acne/hirsutism/alopecia syndrome (SAHA syndrome); late-onset AV (acne tarda); and hyperandrogenism of ovarian or adrenal origin.
If an endocrine abnormality is suspected, screening tests to help diagnose excess circulating androgens are suggested. These tests include dehydroepiandrosterone sulfate (DHEAS), total testosterone, free testosterone, and the luteinizing hormone/follicle-stimulating hormone (LH/FHS) ratio. If DHEAS levels are markedly increased (level >8000ng/dL), an adrenal tumor is an important diagnostic consideration, and an endocrinologist should be consulted for further workup. Levels in the 4000 to 8000ng/dL range can indicate CAH. If 17-hydroxyprogesterone is elevated >200ng/dL, late-onset CAH may be the cause. The most common cause of CAH that is noted in 95 percent of cases is deficiency of the enzyme 21-hydroxylase.19 Other enzyme deficiencies include 17-alpha hydroxylase, 11-beta hydroxylase, and 3-beta hydroxysteroid dehydrogenase. If the testosterone level is elevated >200ng/dL, the source may be an androgen-secreting ovarian tumor. Elevation of the testosterone level >150ng/dL coupled with an LH:FSH ratio of >2:3 is suggestive of PCOS (Table 2).
Table 2.
Endocrine evaluation results and diagnostic considerations19
| LABORATORY TEST | PLASMA HORMONE LEVEL (ng/dL) | SUSPECTED DIAGNOSIS |
|---|---|---|
| DHEAS* | •Elevated >8000 | •Adrenal tumor |
| •Elevated 4000—8000 | •Congenital adrenal hyperplasia (CAH) | |
| •Elevated >700 | •Androgen-secreting tumor | |
| •Normal/slightly elevated | •Polycystic ovarian syndrome (PCOS); •Cushing syndrome |
|
| TOTAL TESTOSTERONE | •>200 | • Ovarian androgensecreting tumor |
| •100—200 | • PCOS •CAH •Cushing syndrome |
|
| FREE TESTOSTERONE | • Elevated from normal range | • Hyperandrogenism |
| LH:FHS RATIO# | •>2:3 | • PCOS |
| • Normal | • CAH •Adrenal tumor • Cushing syndrome • Androgen-secreting tumor |
|
| •17-HYDROXYPROSTERONE | • Elevated >200 | • Late-onset congenital adrenal hyperplasia |
| • Normal/increased | • PCOS • Adrenal tumor •Cushing syndrome • Androgen-secreting tumor |
DHEAS: dehydroepiandrosterone sulfate
LH:FSH ratio: leutinizing hormone:follicle-stimulating hormone ratio
The optimal time for checking these laboratory values is during the luteal phase of the menstrual cycle, that is right before the onset of menses. Patients should be instructed to stop any oral contraceptives at least one month before testing these laboratory values to optimize the accuracy of the results. It is important to consider that a hormone-releasing intrauterine device (IUD) may also affect testing results. It is prudent to repeat testing of hormonal laboratory abnormalities before starting therapy to establish an accurate baseline for comparison if needed at a later date. Lastly, the clinician should keep in mind that oral spironolactone has a role in the treatment of adult women with AV, even when test results that evaluate androgenic status are normal.
What is the starting dosage for oral spironolactone and what is the expected response time?
Spironolactone is used in doses of 25 to 200mg/day for treatment of AV in women; however, it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on the clinical situation. Although it is not often used initially before trying other options, spironolactone can be initiated as monotherapy in adult women with cyclic or late-onset AV, or it can be used in combination with other topical and oral agents, depending on severity and clinical presentation. It has been shown to be efficacious and safe in treating AV, including in those with seborrhea. At a lower dosage range of 50 to 100mg/day, spironolactone has been shown to reduce sebum excretion rate by 30 to 50 percent and improve adverse events.20,21 The efficacy of spironolactone has been established by several studies showing improvement, with lesion reductions ranging from 50 to 100 percent in women treated for AV.22 In most of these studies, the dosage range used was 100 to 200mg daily, with response noted over approximately three months.22 However, the authors find that many adult females with AV are well controlled on 50mg daily of spironolactone.
There is also data to suggest that spironolactone not only improves facial AV, but is efficacious in improving truncal AV as well. One study of adult women on 75 to 150mg of spironolactone daily, over a mean treatment duration of 17 months, reported at least a 50-percent improvement of facial AV and truncal AV in 37.5 percent of the cases.23
Additional studies have shown spironolactone to be useful for AV in adult women. In a 12-week, randomized, placebo-controlled trial of male and female patients (N=36) with severe AV treated with 50 to 200mg of spironolactone daily, the greatest therapeutic outcomes were noted in 11 of 15 patients treated with 100 to 200mg, both objectively and subjectively, although no statistical analysis was reported.23 This is likely due to the small number of subjects included in the trial, especially after breaking down specific dosage groups. Another randomized, placebo-controlled trial of 21 women on 200mg daily of spironolactone for 12 weeks revealed significant improvement of AV evaluated subjectively (p<0.001), by objective evaluation using inflammatory lesion counts (p<0.001) and by photographic documentation (p<0.02).24 However, as noted earlier, lower doses of 50 to 100mg daily often produce good clinical results and have the advantage of fewer side effects.25 In a 12-week, randomized, placebo-controlled study of spironolactone 50mg daily, 24 of 34 patients were clear of acne lesions as compared to improvement in 2 of 31 patients in the placebo group (p<0.001).26
After a desired endpoint of control of acne lesion flaring has been reached, maintenance doses of spironolactone typically range from 25 to 50mg daily. This low dose of spironolactone is sufficient for some women with sporadic outbreaks of inflammatory or isolated cystic lesions. In a thoroughly structured, evidence-based medicine review, due to small sample sizes and the limited number of trials, spironolactone has been labeled as level B evidence for the treatment of AV; however, it is a well-recognized option that is part of the standard of care for treatment of AV in female patients.27
Is there a topical formulation for spironolactone?
Spironolactone has also been studied as a topical formulation, although a proprietary formulation is not available. Topical application of spironolactone has shown limited antiandrogenic activity or effects. In Europe, topical 5% spironolactone lotion and cream have been used to treat grade II acne, which has shown similar efficacy to that of topical antibiotic therapy, although the data are very limited.28 It has also been shown to decrease sebum secretion in young adults.29 Topical spironolactone is not currently available commercially in the United States.
Is spironolactone safe for long-term use and what is the risk of developing breast cancer with prolonged intake?
There are sufficient data to suggest that long-term use of spironolactone appears to be safe overall. This was revealed in one long-term study with patients who received spirono-lactone for up to eight years for the treatment of AV.30 In this eight-year study, questionnaires were sent to 210 patients, 91 of whom replied with a mean length-of-treatment time being 28.5 months (range 0.5-122 months).30 Although 60 percent of patients experienced some side effects (lightheadedness, polyuria, gastrointestinal upset), only 15 percent discontinued medications with no serious adverse events reported.30 The most common side effects were diuretic effects (29%), menstrual irregularities (22%), and breast tenderness (17%).30 The authors concluded that long-term use of spironolactone in the treatment of AV appears to be safe. Although some side effects were relatively common, they were usually not troublesome or severe enough to result in cessation of the drug.30 There were no cases of breast carcinoma in this long-term study; however, four patients underwent breast biopsies with benign outcomes.30 It was unknown if these benign breast lesions were linked to spironolactone.
Early concerns regarding the potential link between breast cancer and spironolactone use was first raised in 1975, with no data definitively demonstrating a correlation since the question has been posed. It is recommended that spironolactone be avoided in women with an increased risk for breast cancer or estrogen-related tumors (either through personal or family history).31 The concerns stemmed from a report of breast tumors in rodents, with no proven association noted in humans. In the rodent study, two years of ingesting spironolactone (at 25–250 times the exposure dose in humans) resulted in benign adenomas of the thyroid and testes, malignant mammary tumors, and proliferative changes in the liver.31 The potential for spironolactone-related breast cancer was also raised in 1975 after a case report of breast carcinoma that occurred in five women who were concurrently using several medications, including spironolactone.32,33 This led the manufacturer to recommend avoiding unnecessary long-term use in a black box warning.31
The potential for spironolactone to induce estrogen-dependent malignancies still remains controversial. However, many believe there is not sufficient evidence to suggest spironolactone has a direct causal link to the development of breast cancer. Another study of 1,475 individuals prescribed spironolactone and followed for 3 to 7 years reported nine cases of breast cancer compared with an age-specific rate of 8.3 cases.34 Another study showing similar results based on data from 461 person-years during three years of follow-up after spironolactone exposure demonstrated no relationship between spironolactone use and breast cancer.31 Five case-controlled studies have also shown no overall increase in the relative risk for breast carcinoma.35 The data suggests that there is no definitive documented association between breast carcinoma and spironolactone ingestion after more than 30 years of spironolactone availability in the marketplace.36
Which Oral Contraceptives can be used with Spironolactone? Is there a Concern for Hyperkalemia in Oral Contraceptives with the Progestin Drospirenone when used in Combination with Spironolactone?
Spironolactone can be used as monotherapy or in combination with other drugs, such as oral contraceptives (OCs). It can be used as monotherapy, particularly in women who are intolerant of OCs exhibit concerns or are at increased risk for possible complications of OCs (such as thromboembolism or stroke) or do not want the side effect of OC-associated melasma. When spironolactone is used in combination with an OC, there may be added benefits in improving AV, along with alleviating concerns about unwanted pregnancy while taking spironolactone. To add, women of childbearing potential must be on a reliable form of birth control with spironolactone to avoid exposure during pregnancy and feminization of the male fetus. Use of an OC in combination with spironolactone also decreases menstrual-related side effects, such as dysmenorrheal, irregular menses, and breast tenderness, all of which can occur with spironolactone monotherapy, especially at higher doses. Also, the combination of an OC with spironolactone may in some cases allow women with AV to be on a lower daily dose of spironolactone without a sacrifice in efficacy.38
OCs can play a significant role in treatment of AV in women because they reduce free serum androgen levels by increasing sex hormone-binding globulin (SHBG) regardless of the type of progestin or concentration of estrogen. The OC brands approved by the FDA for the treatment of AV are ethinylestradiol (EE) 20/30/35µg and norethindrone (NET) 1mg (Estrostrep®), EE 35µg and norgestimate 0.18/0.215/0.25 (Ortho Tri-Cyclen®), and EE 20 and drospirenone (DROSP) 3mg (Yaz®). Data on treatment of AV is also available with other OCs that are not FDA approved for this indication (Table 3). OCs can help to further improve response to therapy for AV in adult women, especially for those with resistant cases and not wanting to be on oral isotretinoin.
TABLE 3.
Selected oral contraceptives for the treatment of acne vulgaris19
| FOOD AND DRUG ADMINISTRATION-APPROVED | CLINICAL DATA AVAILABLE TO SUPPORT USE |
|---|---|
| EE 20/30/35μg and NET 1mg (Estrostrep) | Levonorgestrel 100μg and 20μg EE (Alesse) |
| EE 35μg and norgestimate 0.18/0.215/0.25 (Ortho Tri-Cyclen) | EE 35μg and cyproterone acetate 2mg (Diane-35)* |
| EE 20μg and DROSP 3mg (Yaz) | EE 30μg and DROSP 3mg (Yasmin) |
Not available in the United States
It is recommended that OC therapy be started on the first day of the next menses, or it can be started on the first Sunday during or directly after menses. OCs can also be started immediately after obtaining a negative pregnancy test, referred to as the “quick start program.” Any estrogen given in sufficient doses of at least 100ug can decrease sebum production and suppress ovulation. However, caution should be taken when patients combine spironolactone use with EE 20µg and DROSP 3mg (Yaz®) or EE 30µg and DROSP 3mg (Yasmin®). Both OCs are also approved for premenstrual dysphoric disorder and have natiuretic properties that counteract estrogen-induced weight gain. The progestin component in both of these OCs is drospirenone (DROSP), which is a 17-alpha spironolactone derivative with anti-mineralocorticoid activity, and a potassium-sparing diuretic effect. A 3mg dose of DROSP has been reported to be equivalent to 25mg of spironolactone.
Since spironolactone is associated with risk of hyperkalemia, serum potassium monitoring may be considered with use of a DROSP-containing OC, especially when used in combination with spironolactone. However, although hyperkalemia is a potential concern, there is some data to suggest that using a DROSP-containing OC in combination with spironolactone is safe and effective. In one study of 27 women with severe-nodulocystic facial AV on EE 30µg and DROSP 3mg (Yasmin®) plus spironolactone 100mg daily, reported side effects were not significant enough to cause cessation of treatment.38 Baseline serum potassium levels were measured and a second level was obtained 4 to 6 weeks later during therapy. None of the patients demonstrated elevated serum potassium levels. No patients discontinued therapy from adverse events and 85 percent of women experienced excellent improvement or were entirely clear after six months of the combination treatment.38
EE 20µg and DROSP 3mg (Yaz®) has also been shown to improve AV in two multicenter, double-blind, randomized, placebo-controlled trials and was approved by the FDA in 2007 for the treatment of AV. These trials followed 889 subjects over six cycles with both studies showing a 42- to 46-percent reduction in total acne lesion counts, with the onset of visibly apparent improvement sometimes delayed for approximately three cycles.23,39,40 However, before starting a patient on an OC, the physician should be aware of the contraindications, such as personal or family history of thromboembolic disorders, smoking (over the age of 35 years), estrogen-dependent neoplasms (or undiagnosed uterine bleeding), migraine cephalgia, and pregnancy.
Can Oral Spironolactone be used in Combination with Oral Antibiotic Therapy for Acne Vulgaris in Adult Women?
Spironolactone can also be used in combination with oral antibiotic therapy for AV. One report included patients that failed on previous systemic acne treatment with antibiotics, oral isotretinoin, or OCs, and who presented clinically with hormonal pattern AV (N=85). Patients in this analysis were treated with 50 to 100mg of spironolactone daily as monotherapy in combination with an oral antibiotic or in combination with an OC, specifically EE 35ug and NET 1mg (Estrostrep®).25 The patients were monitored monthly or bimonthly and were treated over a duration of 2 to 24 months. Ultimately, 73 patients had data complete enough for evaluation in this retrospective evaluation. Complete clearing or marked improvement of AV were observed in 48 women (66%) who received a low daily dose of spironolactone (50 to 100mg).25 Also, statistical analysis of the data on length of treatment demonstrated a significant trend toward increased benefit with a longer duration of therapy (2-24 months, mean=10 months) (p=0.032).25 There were marked improvements noted with spironolactone alone (20.8%), spironolactone plus an oral antibiotic (58.3%), and spironolactone plus the OC (8.3%).25 Oral antibiotics used in this patient collection were minocycline, erythromycin, or tetracycline. The majority of patients (57.5%) experienced no adverse effects.25 Menstrual irregularities were present in 14 patients (18%), 10 of whom were not receiving the OC (which would have decreased these menstrual-related side effects).25 Clinically relevant hyperkalemia was not noted, and was not anticipated with use of spironolactone in this study population, as patients were relatively young and were not on any concomitant medications that predisposed them to an increase in serum potassium levels. The response rates were similar to previous studies with 150 to 200mg daily of spironolactone for women with AV, showing improve-ments ranging from 56- to 80-percent reduction of acne lesions after 3 to 6 months of treatment.23,42
It is important to recognize that the use of oral antibiotic therapy for AV, especially long-term use, including in combination with spironolactone, is not recommended without concomitant topical therapy. More specifically, use of a benzoyl peroxide-containing formulation is suggested to reduce the risk of emergence of antibiotic-resistant strains of Propionibacterium acnes.
Are there any Oral Antibiotics that should not be used with Spironolactone or that Require Additional Caution?
Spironolactone has been used safely and effectively in combination with oral antibiotics including tetracyclines, erythromycin, and amoxicillin.25,43 Trimethoprim-sulfa-methoxazole (TMP-SMX) has been found to be just as effective as oxytetracycline in the treatment of AV, and is sometimes used to effectively treat cases of AV that are poorly responsive to other oral antibiotics.44 In evaluating a group of patients with AV treated with spironolactone in combination with other agents, among three patients who used a combination of spironolactone and TMP-SMX, one patient experienced marked improvement and the other two achieved complete response.43 Another patient experienced hypotension, which disappeared after one week of cessation of spironolactone.
Although some dermatologists may be intimidated by the potential for severe side effects associated with TMP-SMX, major side effects, such as severe cutaneous reactions or hematological abnormalities are rare, and in some reports not statistically significantly increased when compared to placebo.44–46 However, this data may be misleading as severe reactions associated with grave morbidity and risk of mortality, such as toxic epidermal necrolysis and erythema multiforme major, are well-known risks of TMP-SMX use, warranting careful consideration and discussion of benefit versus risk when treating AV with this agent. These reactions may be rare, but in those patients that are affected, the implications are significant, and the cause is usually very evident.
More recently, another potential risk possibly associated with use of TMP-SMX has been noted.47 Hyperkalemia has been reported to occur with high doses of TMP-SMX in human immunodeficiency virus (HIV)-infected patients, the elderly, and patients with renal insufficiency.47 Another study also indicates that older patients treated with angiotensin-converting enzyme (ACE) inhibitors or angiotensin-II receptor blockers (ARBs), who are also on TMP-SMX, can experience hyperkalemia.48 Additionally, TMP-SMX can be a major cause of hyperkalemia-associated hospitalization relative to other antibiotics.48 During a 14-year study period with 4,148 admissions, the use of TMP-SMZ was associated with a nearly 7-fold increased risk of hyperkalemia-associated hospitalization.47 Other antibiotics should be considered if patients have many comorbidities including use in combination with spironolactone.48 It may be prudent for the clinician to avoid TMP-SMX in combination with spironolactone for treatment of AV until further information becomes available regarding the true risk of hyperkalemia associated with TMP-SMX and the associated predisposing risk factors.
Are there Concerns about an Increase in the Contraceptive Failure Rate when Using an Oral Antibiotic and an Oral Contraceptive in Combination, Especially with Concurrent Use of Spironolactone?
Some women with AV that are refractory to treatment may need to be on a topical regimen along with spironolactone and an oral antibiotic in order to achieve adequate control. The clinician and/or patient may be concerned about the reported decrease in efficacy of an OC when an oral antibiotic is prescribed, since most female patients on spironolactone will often be using some form of birth control, including an OC. There has been some questions about combining an OC with an oral antibiotic, believed to be related to reduction in gut bacterial flora leading to reduced enterohepatic circulation of estrogen.20 However, there is no definitive evidence to support that oral antibiotics (other than rifamycins such as rifampin) reduce the blood levels of the hormones in OCs or their efficacy. To add, the only antibiotics that have been shown to decrease estrogen levels and reduce contraceptive efficacy are rifamycins, most specifically rifampin, through a different mechanism of induced hepatic metabolism of estrogen.49 Studies have shown that, overall, there is no difference in the OC failure rate of patients who took OCs with antibiotics compared with those who did not.20,50–52
On the other hand, current data do not exclude the possibility that a small subset of women who are using an OC may be at a greater risk of pregnancy when an oral antibiotic is administered. Based on current information, the risk appears unlikely and minimal, if it exists at all. However, the caution is still included in the FDA-approved package inserts for many antibiotics, including those commonly used to treat AV. If a patient is on an OC and spironolactone, an antibiotic can be added to treat AV if needed. However, if the patient is a sexually active female of childbearing potential, it is prudent to educate her and obtain her acknowledgment and consent to this combination approach with the understanding that she needs to be fully adherent with her contraceptive therapy and use an additional birth control method in order to reduce the risk of unintended pregnancy.
What Clinical Evaluation and Laboratory Testing should be Done Before Initiation of Therapy with Oral Spironolactone and What Follow-Up Recommendations are Suggested?
A thorough general examination, including past medical history, medication list, past surgical history, family history, social history, and physical examination are important components of the overall management of the patient. Also a gynecological and an obstetric history are both important in female patients and may be particularly relevant before prescribing a hormonal therapy, such as spironolactone or an OC. It is important to include in the dermatological physical examination of the adult woman with facial AV an assessment of chest, back, shoulders, and upper arms, as AV often involves these locations in addition to the face. It is recommended that each clinician incorporate his or her own consistent system to grade acne severity in different body locations so accurate comparisons can be made from visit to visit. If present, the type, location, and severity of acne scarring should also be documented. Other factors, such as alopecia, hirsutism, and skin oiliness (seborrhea), may also be assessed and documented.
It is important to establish prior to initiating therapy with spironolactone that there is no history of renal disease, that the patient does not utilize salt substitutes (many contain potassium in place of sodium), and is not utilizing potassium supplements, other potassium-sparing diuretics (i.e., amiloride, triamterene), ACE inhibitors, or ARBs. Many clinicians do not obtain baseline laboratory testing, including serum electrolytes, in young healthy female patients with AV with no medical problems who are not on any medication that may put them at risk for hyperkalemia when combined with spironolactone. Nevertheless, obtaining a complete blood cell count and chemical profile (including serum electrolytes) is a prudent approach to establish a baseline and to exclude some potential unexpected concerns in the given patient, such as pre-existing hyperkalemia or impaired renal function. After starting spironolactone for AV, clinical monitoring every 4 to 6 weeks, with adjustment of dose based on clinical response and assessment of side effects, is recommended until stabilization occurs.35 Laboratory monitoring in general is not essential overall, but some clinicians choose to test for hyperkalemia during the initial three months of treatment and periodically thereafter (Table 4).35 Serum potassium level monitoring and other testing, if applicable, can always be incorporated based on clinical judgment for individual patients who exhibit certain risk factors, such as older individuals and those on OCs containing DROSP.34 It is uncommon to experience hyperkalemia from spironolactone in healthy individuals; however, new-onset muscle cramps or weakness can be important clinical clues.
TABLE 4.
Monitoring oral spironolactone therapy in post-teenage women with acne vulgaris and hyperandrogenism: Guidelines to be considered before starting therapy and after three months of treatment38
| TIMEPOINT | DHEAS* | FREE TESTOSTERONE | BLOOD PRESSURE | SERUM POTASSIUM | COMPLETE BLOOD CELL COUNT |
|---|---|---|---|---|---|
| Pretreatment | Yes | Yes | Yes | Yes | Optional |
| 1 month of treatment | Optional if abnormal | Optional if abnormal | Yes | Yes | Optional |
| Every 3 months | Yes if abnormal | Yes if abnormal | Optional | Optional | Optional |
| Last month of treatment | Yes if abnormal | Yes if abnormal | Yes | Yes | Optional |
DHEAS: dehydroepiandrosterone sulfate
As a general suggestion, laboratory monitoring of abnormal circulating androgens (free testosterone or DHEAS) is recommended every 3 to 4 months to ensure successful androgen suppression in those with endocrine abnormalities. One study revealed that women (N=24) had decreased mean DHEAS levels with significant clinical improvement of AV (p<0.05) after three months of treatment with spironolactone 100mg/day.52 Complete therapeutic suppression usually occurs within 4 to 12 months of continued spironolactone therapy. Androgen follow-up testing is not necessary if the values are normal at baseline. In some cases, the therapeutic benefits of spironolactone for AV may plateau after approximately a year and the clinician may need to add another therapy to sustain remission. However, the authors have found, at least in adult women with AV including those with a hormonal clinical pattern and without other signs of hyperandrogenism, that once an effective maintenance dose is established, that control is sustained in most cases as long as they continue using spironolactone at that same dose each day.
What are the Most Common Side Effects of Spironolactone? What is the Risk of Hyperkalemia? Is there a Difference in Long-Term Side Effects as Opposed to Short-Term Side Effects?
The majority of adverse effects associated with spironolactone are dose-dependent. Low-dose therapy using 25 to 50mg daily is generally very well tolerated, and even 100 mg daily is not problematic in most cases.53 Higher dose therapy using >100mg/day is more likely to cause hyperkalemia, particularly when there is cardiac or renal compromise.53 Other dose-dependent side effects include menstrual irregularities (metrorrhagia, amenorrhea, breakthrough bleeding), breast tenderness and enlargement, orthostatic hypotension, hyperkalemia, and reduced libido.53 Menstrual irregularities have been reported to be as high as 18 percent on low-dose spironolactone (50-100mg daily) as opposed to 70 percent in patients on 200mg daily.54 Breast tenderness has been reported in five percent of patients on low-dose spironolactone compared to 30 percent on 200mg daily.54 Most patients experience at least one side effect related to spironolactone, but they are usually not severe enough to discontinue therapy, and some can be tempered by concomitant use of OC therapy (i.e., menstrual irregularities, breast tenderness).55 One long-term study found that the most common side effects were menstrual irregularities (22%), tiredness (16.5%), and breast tenderness (17%), all of which were mild and rarely caused cessation of the medication.30 Most side effects are dose-dependent, except central nervous system (CNS) symptoms (lethargy, headache, lightheadedness, dizziness), which do not appear to always be dose-related.24 If menstrual abnormalities do not improve within three months of monotherapy, the clinician may consider the following options: 1) decrease spironolactone to a dosage range of 50 to 75mg daily if applicable, 2) add an OC to reduce menstrual dysfunction, or 3) “cycling” of the spironolactone with 21 consecutive days of therapy followed by seven days off.
Hyperkalemia is a commonly feared potential consequence of spironolactone therapy, although it is generally not a relevant concern in a healthy population of young women. Spironolactone is best avoided in patients with renal insufficiency, as this increases the risk of hyperkalemia. In patients with heart failure, the incidence of hyperkalemia may be increased as was shown in one study of patients with left ventricular systolic dysfunction who were on spironolactone therapy (N=134).56 Some patients had to discontinue therapy with spironolactone because of hyperkalemia (17.1%), renal function deterioration (14.5%), gynecomastia (5.3%), and other side effects (1.3%).56 This study suggests that serum potassium levels should be closely monitored along with serum creatinine and blood urea nitrogen (BUN) levels in this subset of patients. Studies from the Randomized Aldactone Evaluation Study (RALES) showed that daily doses of 12.5 to 25mg of spironolactone coadministered with ACE inhibitors, loop diuretics, and digoxin were safe provided that serum potassium levels were monitored carefully.57
There are some differences regarding long-term and short-term side effects that have been observed with spironolactone. According to one long-term study, one or more side effects were reported in 59 percent (54/91) of survey respondents on spironolactone 50 to 100mg daily.24 Although the adverse effects were not serious, 15 percent of women stopped therapy because of side effects. This is in comparison to a recent short-term study with spironolactone showing that 42.5 percent of patients treated for two years experienced side effects.54 There was an increase in diuretic effects reported in 29 percent (26/91) of patients in a long-term study compared to five percent (4/80) in short-term studies.24,54
What Drugs should be Avoided with Oral Spironolactone and What are some Contraindications with its Usage?
Certain drugs when used in combination with spironolactone can potentiate specific adverse side effects. The most concerning is hyperkalemia, which has been reported to occur at a rate of 2.9 to 9.9 percent in the outpatient setting in patients without cardiac disease.58 Concurrent ingestion of spironolactone and potassium supplements, potassium-rich foods, and/or salt substitutes should be avoided. Hyperkalemia can occur in any patient with excess potassium intake, especially those with renal insufficiency. One study reported that the most common cause of death associated with hyperkalemia was due to use of spironolactone along with potassium supplementation.59 Hyperkalemia may cause fatal cardiac irregularities, paresthesia, muscle weakness, fatigue, flaccid paralysis of the extremities, bradycardia, and shock. If hyperkalemia is suspected, patients should be advised to go to the emergency room.
There are some contraindications the clinician should also be aware of before starting a patient on spironolactone. The use of diuretics and potassium-sparing drugs (e.g., amiloride, triamterene) with spironolactone is contraindicated. To add, spironolactone should not be used in combination with an ACE inhibitor as this is considered a relative contraindication because of the potential for severe hyperkalemia and inhibition of aldosterone formation. However, clinical studies in patients with moderate to severe congestive heart failure have shown that the addition of low dose spironolactone (25–50 mg daily) to the standard therapy of an angiotensin-converting-enzyme (ACE) inhibitor and a loop diuretic has decreased mortality and hospitalization.60,61 Nevertheless, it is best to avoid prescribing spironolactone for a patient with AV who is taking an ACE inhibitor.
Cautious use with other medications along with spironolactone has been noted. Alcohol, barbiturates, and/or narcotics are best avoided as they can potentiate orthostatic hypotension. Use with corticosteroids or adrenocorticotropin hormone (ACTH) may intensify electrolyte depletion, particularly hypokalemia. Spironolactone reduces the clearance of lithium and concomitant use is not recommended for AV treatment. Increase in digoxin serum concentrations and digoxin toxicity has been suggested as a potential complication with concurrent use of spironolactone. Non-depolarizing neuromuscular blocking agents can also be potentiated if used with spironolactone.62
What is the Risk Associated with Spironolactone use in Pregnancy and Lactation?
Spironolactone should be avoided in pregnancy due to the theoretical risk of teratogenicity, especially in a male fetus. It is also best avoided in men due to the risk of impotence, gynecomastia, and loss of libido. It is rated as pregnancy category C and exhibits this potential based on exposure and reproduction data in animals with concerns regarding hypospadias, feminization of a male fetus, and other possible adverse effects.20 However, the risk of masculinization of the male fetus occurs approximately six weeks post-conception, and if inadvertent administration is discontinued at an early stage, the potential risk to the male fetus is negligible.63
Currently, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposures in pregnant women.64 However, in a surveillance study conducted between 1985 and 1992 involving 229,101 completed pregnancies, 31 newborns had been exposed to spironolactone during the first trimester of pregnancy. Two major birth defects were noted with one expected for other reasons and the other an oral cleft defect; no other anomalies in five major categories were noted among these 31 newborns, including hypospadias, cardiovascular defects, spina bifida, polydactyly, and limb reduction defects.64 Importantly, there is at least one case report of two appropriately developed, healthy males after exposure to high-dose treatment with spironolactone (200-400 daily) and potassium supplementation for maternal Bartter syndrome.65
Potential teratogenicity risks with spironolactone are based on findings demonstrated in male rodents and rabbits and have been reviewed elsewhere.64 Studies in mice using exposures far below the maximum recommended human dose (MRHD) with correction for body surface area revealed no embryotoxicity or adverse effects on the fetus. In rabbits dosed at approximately the MRHD, an increase in fetal resorption and a decrease in the number of viable fetuses were noted. In male rats exposed to spironolactone during late embryogenesis and fetal development, using a daily dosage 10-fold higher than with the mice or rabbits, feminization of male rat fetuses was documented, and another study showed permanent alterations in the reproductive tracts of both male and female rats exposed in utero to 50–100mg/kg/day of spironolactone during late gestation.64
It is unknown whether the parent compound, spironolactone, is excreted in human breast milk; however, the active and predominant metabolite, canrenone, does achieve levels in human breast milk.64,66 It has been estimated that the maximum exposure of the breast-fed infant to canrenone would be 0.2 percent of the maternal daily dose, which is felt to be insignificant.67 Although it is believed that maternal ingestion of oral spironolactone is “probably compatible” with breastfeeding, and the American Academy of Pediatrics classifies spironolactone as compatible with breastfeeding, consideration should be given to the tumorigenic potential of spironolactone in rats, at least from a medicolegal perspective, based on the comfort level of the clinician and parents.66,68
Conclusion
Although there have been considerable advances in acne therapy, treatment failure can occur especially in adult female patients. Overall, spironolactone as a monotherapy or in combination with other agents is well tolerated if properly dosed and adjusted and has been shown to be beneficial for women with AV, especially in those exhibiting the hormonal pattern clinically. Although not first line, some women may benefit, including in cases where an endocrine disorder is suspected and oral isotretinoin therapy is not desired. Use of spironolactone for women with AV is not limited to those who exhibit hyperandrogenism clinically, as spironolactone can be used in women with normal circulating androgen levels. Spironolactone is usually reserved for recalcitrant cases of AV in adult women that are resistant to conventional treatment. Importantly, spironolactone may be used in conjunction with an OC to enhance therapeutic benefit for AV, to achieve birth control in women of childbearing potential, and to decrease menstrual-related side effects. There are also sufficient data to suggest that short- and long-term use with spironolactone is deemed safe overall, provided certain contraindications and risk-factor exclusions are identified before starting treatment. More studies are needed to confirm these results. Concerns regarding breast cancer and hyperkalemia should not intimidate the clinician from using spironolactone for AV when it is clearly needed in young, healthy individuals, provided specific exclusions are identified up front. In conclusion, data from medical literature, clinical experience over many years support that, overall, spironolactone is a safe and efficacious therapy for adult women with AV in many clinical circumstances. Table 5 provides a practical checklist for the clinician when considering the use of spironolactone, with emphasis on treatment of AV in women. Spironolactone should be considered as a major agent in the armamentarium for treatment of adult women with AV. Its use, either as monotherapy or in rational combination with other agents, can provide many patients legitimate hope of achieving reasonable improvement that is usually sustained with continued use, with complete or near-complete control of their acne flares observed in many adult women with AV.
TABLE 5.
Pretreatment checklist before prescribing oral spironolactone including in post-teenage female patients with acne vulgaris1,11,14,17,20,22–27,30–39,43,47,48,52–62,65,68
| QUESTION | CLINICAL CONSIDERATION |
|---|---|
| Is the patient a candidate for spironolactone based on past history and evaluation of acne vulgaris (AV) on face and trunk? Are there other signs, symptoms, or details in patient history or examination that suggest hyperandrogenism? | Obtain thorough history including time courses of AV and treatments used; evaluate for clues suggesting hyperandrogenism, such as irregular menses, infertility, abnormal hair growth (if not evident, ask if she uses hair-removal techniques especially on the face); seborrhea (very oily skin); androgenic alopecia; history of refractory AV, rapid onset of AV (sometimes explosive over days or weeks), and/or repeated recurrence of AV after oral isotretinoin use with short periods of remission (often less than 6–9 months after stopping oral isotretinoin); if hyperandrogenism suspected or confimed, then a plan for further evaluation and follow up coupled with communication with patient's primary physician, and consultation with appropriate specialist(s) is warranted |
| Is the patient pregnant? Is the patient considering or actively trying to become pregnant? | Avoid oral spironolactone based primarily on animal data; data limited in humans |
| Is the patient breastfeeding? | Probably compatible but avoidance may still be prudent as active metabolite (canrenone) achieves low levels in human breast milk |
| Is the patient taking an oral contraceptive (OC), and if so, does the OC contain drosperinone? | Concurrent use of an OC and spironolactone in adult women with AV reduces menstrual irregularities and breast tenderness that may occur with spironolactone, especially at higher doses (>100mg/day); use of spironolactone in combination with an OC may provide augmented benefit for AV as compared to each agent alone; drosperinone is reported to be equivalent to 25mg of spironolactone, which the clinician may wish to consider with regard to dosing and the potential for increased risk of adverse effects (i.e., hyperkalemia) |
| Is there a personal or family history of breast cancer? | Avoid spironolactone if personal history of breast cancer; probably also best to avoid if family history of breast cancer, especially if in first-degree relative(s); available data and experience suggest that risk of development of breast cancer from use appears to be negligible; more data are needed before a definitive statement can be made, including in specific patient subsets and in those with breast cancer history or positive family history; a reasonable, cautious, and informed approach is suggested |
| Does the patient have renal disease or cardiac disease, especially congestive heart failure (CHF)? | Risk of hyperkalemia higher in these patient subsets; although women with AV tend to be a younger healthy population overall, renal insufficiency due to diabetes or other causes seen in younger patients may be encountered on occasion; although CHF not common in adult female population with AV, some cases may occur especially in those with congenital or acquired cardiac valve or septal disorders or primary cardiomyopathies |
| Does the patient have hypertension? Is she currently using any angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), potassium-sparing diuretics, or salt substitutes (many replace sodium with potassium) | Hypertension can cause renal insufficiency, which is a risk factor for hyperkalemia; risk of hyperkalemia increased if spironolactone is used concurrently with potassium supplements, high potassium intake, potassium-sparing diuretics (i.e., amiloride, triamterene), ACE inhibitors, and/or ARBs; coadministration for AV best avoided, especially without consulting first with the patient's physician(s) |
| Is patient on treatment with trimethoprim-sulfamethoxazole (TMP-SMX)? | Possible increased risk of hyperkalemia reported; probably best to avoid coadministration for AV |
| Is the patient on treatment with lithium carbonate? | Avoid coadministration; spironolactone may increase serum lithium levels resulting in toxicity; consider role of lithium as a causative or exacerbating factor related to AV; may wish to consult with physician responsible for prescribing lithium therapy for the patient |
Footnotes
DISCLOSURE:Dr. Kim reports no relevant conflicts of interest. Dr. Del Rosso is a consultant, speaker, and/or researcher for Coria/Valeant, Allergan, Galderma, Graceway, Intendis, Medicis, Onset Dermatologics, Obagi Medical Products, Ortho Dermatologics, PharmaDerm/Nycomed, Promius, Ranbaxy, Stiefel/GSK, TriaBeauty, Triax, Unilever, and Warner-Chilcott.
REFERENCES
- 1.Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J of Dermatol. 1997;136:66–70. [PubMed] [Google Scholar]
- 2.Mallon E, Netwon JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672–6. doi: 10.1046/j.1365-2133.1999.02768.x. [DOI] [PubMed] [Google Scholar]
- 3.Ober KP, Hennessy JF. Spironolactone therapy for hirsutism in a hyperandrogenetic women. Ann Intern Med. 1978;89:643–644. doi: 10.7326/0003-4819-89-5-643. [DOI] [PubMed] [Google Scholar]
- 4.Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253–255. doi: 10.1111/1523-1747.ep12506612. [DOI] [PubMed] [Google Scholar]
- 5.Zouboulis CC, Xia L, Akamatsu H, et al. The human sebocyte culture model provides new insights into development and management of seborrhea and acne. Deramotolgy. 1998;196:21–31. doi: 10.1159/000017861. [DOI] [PubMed] [Google Scholar]
- 6.Lookingbill DP, Horton R, Demers LM, et al. Tissue production of androgens in women with acne. J Am Acad Dermatol. 1985;12:481–487. doi: 10.1016/s0190-9622(85)70067-9. [DOI] [PubMed] [Google Scholar]
- 7.Carmina E, Lobo RA. Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J Clin Endocrinol Metab. 1993;76:1111–1114. doi: 10.1210/jcem.76.5.8496299. [DOI] [PubMed] [Google Scholar]
- 8.Thiboutot D, Harris G, Iles V, et al. Activity of the type 1 5-alpha-reductase exhibits regional differences in isolated sebaceous glands and whole skin. J Invest Dermatol. 1995;105:209–214. doi: 10.1111/1523-1747.ep12317162. [DOI] [PubMed] [Google Scholar]
- 9.Burke BM, Cunliffe WJ. Oral spironolactone therapy for female patients with acne, hirsutism or androgenic alopecia. Br J Dermatol. 1985;112:124–125. doi: 10.1111/j.1365-2133.1985.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 10.Collier CN, Harper JC, Cantrell WC. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Cunliffe W, Gould D. Prevalence of facial acne vulgaris in late adolescence and in adults. Br Med J. 1979;26:931–935. doi: 10.1136/bmj.1.6171.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouden V, McGeown CH, Cunliffe WJ. The familial risk of adult acne: comparison between first-degree relatives of affected and unaffected individuals. Br J Dermatol. 1999;141(2):297–300. doi: 10.1046/j.1365-2133.1999.02979.x. [DOI] [PubMed] [Google Scholar]
- 13.Till AE, Goulden V, Cunliffe WJ, et al. The cutaneous microflora of adolescent, persistent and late-onset acne patients does not differ. Br J Dermatol. 2000;142(5):885–892. doi: 10.1046/j.1365-2133.2000.03467.x. [DOI] [PubMed] [Google Scholar]
- 14.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580. [PubMed] [Google Scholar]
- 15.Stoll S, Shalita AR, Webster GF. The effect of menstrual cycle on acne. J Am Acad Dermatol. 2001;45:957–960. doi: 10.1067/mjd.2001.117382. [DOI] [PubMed] [Google Scholar]
- 16.Lucky AW. Quantitative documentation of a premenstrual flare of facial acne in adult women. Arch Dermatol. 2004;140:423–424. doi: 10.1001/archderm.140.4.423. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther. 2006;19(4):210–223. doi: 10.1111/j.1529-8019.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien RF and Emans SJ. Polycystic ovary syndrome in adolescents. J Ped Adolesct Gyn. 2008;21(3):119–128. doi: 10.1016/j.jpag.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Lolis MS, Bowe WP, Shalita AR. Acne and systemic disease. Med Clin North Am. 2009;93(6):1161–1181. doi: 10.1016/j.mcna.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22:419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human faciall sebocytes and acts antagonistically to testosterone and 5-alpha-dihidrotestosterone in vitro. J Invest Dermatol. 1993;100:660–662. doi: 10.1111/1523-1747.ep12472325. [DOI] [PubMed] [Google Scholar]
- 22.Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57–67. doi: 10.1159/000067823. [DOI] [PubMed] [Google Scholar]
- 23.Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improve acne vulgaris and reduced sebum excretion. Br J Dermatol. 1984;111:124–125. doi: 10.1111/j.1365-2133.1984.tb04045.x. [DOI] [PubMed] [Google Scholar]
- 24.Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227–321. doi: 10.1111/j.1365-2133.1986.tb05722.x. [DOI] [PubMed] [Google Scholar]
- 25.Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498–502. doi: 10.1067/mjd.2000.105557. [DOI] [PubMed] [Google Scholar]
- 26.Mansurul A, Maidul Islam AZM. Effect of spironolactone on acne vulgaris—a double blind study. Bangladesh J Dermatol Venereol Leprol. 2000;171:1–4. [Google Scholar]
- 27.Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009(2):CD000194. doi: 10.1002/14651858.CD000194.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Califano L, Cannavo, Siragusa M, et al. Experience in the therapy of acne with topical administration of spironolactone as an antiandrogen. Clin Ther. 1990;135:193–199. [PubMed] [Google Scholar]
- 29.Yamamoto A, Ito M. Topical sprionolactone reduces sebum secretion rates in young adults. J Dermatol. 1996;23:243–246. doi: 10.1111/j.1346-8138.1996.tb04006.x. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JC, White LE. Long-term safety of spironolacone in acne: results of an 8 year follow-up study. J Cut Med and Surg. 2002;12:541–545. doi: 10.1007/s10227-001-0152-4. [DOI] [PubMed] [Google Scholar]
- 31.Danielson DAN, Jick H, Hunter JR, et al. Nonestrogenic drugs and breast cancer. Am J Epidemiol. 1982;116:329–332. doi: 10.1093/oxfordjournals.aje.a113416. [DOI] [PubMed] [Google Scholar]
- 32.Brest AN. Spironolactone in the treatment of hypertension: a review. Clin Ther. 1986;8:585–586. [PubMed] [Google Scholar]
- 33.Loube SD, Quirk RA. Breast cancer associated with administration of sprionolactone [Letter] Lancet. 1975;1:1428–1429. doi: 10.1016/s0140-6736(75)92645-8. [DOI] [PubMed] [Google Scholar]
- 34.Friedman GD, Ury HK. Initial screening for carcinogenicity of commonly used drugs. J Natl Cancer Inst. 1980;65:723–733. doi: 10.1093/jnci/65.4.723. [DOI] [PubMed] [Google Scholar]
- 35.Barker DJP. The epidemiological evidence relating to spironolactone and malignant disease in man. J Drug Dev. 1978;1(Suppl 1. 2):22–25. [Google Scholar]
- 36.Shaw JC. Hormonal therapies in acne. Exper Opin Pharmacother. 2003;3(7):865–874. doi: 10.1517/14656566.3.7.865. [DOI] [PubMed] [Google Scholar]
- 37.Harper JC. Antiandrogen therapy for the skin and hair disease. Dermatol Clin. 2006;24:137–143. doi: 10.1016/j.det.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Shaw JC. Spironolactone in dermatologic therapy. J Am Acad Dermatol. 1991;24:236–243. doi: 10.1016/0190-9622(91)70034-y. [DOI] [PubMed] [Google Scholar]
- 39. Maloney JM, Lee-Sugh S, Kunz M, et al. Drosperinone 3mg/ethinyl estradiol 20µg in the treatment of acne vulgaris: investigator and subject self-assessment. Poster presented at the 55th Annual Clinical Meeting of the American College of Obstetricians and Gynecologist; San Diego, CA: May 5-9, 2007. [Google Scholar]
- 40.Koltun W, Lucky AW, Thiboutot, et al. Efficacy and safety of 3mg drospirenone/20mcg ethinylestradiol oral contraceptive adminstered in 24/4 regimen in the treatment of acne vulgaris: A randomized, double-blind, placebo-controlled trial. Contraception. 2008;77:249. doi: 10.1016/j.contraception.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Pasekova V, Chroust K. Occurrence of bleeding in women using combined hormonal contraceptives (ethinylstradiol 35 micrograms/norgestimate 250micrograms) in relation to regularity of administration and cycle start day. Ceska Gynekol. 2003;68:84. [PubMed] [Google Scholar]
- 42.Muhlemann MF, Carter GD, Cream JJ, et al. Oral sprionolactone an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227–232. doi: 10.1111/j.1365-2133.1986.tb05722.x. [DOI] [PubMed] [Google Scholar]
- 43.Turowski CB, James WD. The efficacy and safety of amoxicillin, trimethoprim-sulfamethoxazole, and spironolactone for treatment-resistant acne vulgaris. Advances in Dermatol. 2007;23:155–163. doi: 10.1016/j.yadr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Cotterill JA, Cunliffe WJ, Forster RA, et al. A comparison of trimethoprim-sulphamethoxazole with oxytetracycline in acne vulgaris: a double-blind study. Dermatologica. 1972;145:187–191. [Google Scholar]
- 45.Hersle K. Trimethoprim-sulphamethoxazole in acne vulgaris: a double-blind study. Dermatologica. 1972;145:187–191. doi: 10.1159/000252042. [DOI] [PubMed] [Google Scholar]
- 46.Macdonald RH, Macconnell LE, Dunsmore IR. Trimethoprim-sulphamethoxazole versus placebo in acne vulgaris. Br J Clin Pract. 1972;26:97–98. [PubMed] [Google Scholar]
- 47.Velaquez H, Perazella MA, Wright FS, et al. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:269–301. doi: 10.7326/0003-4819-119-4-199308150-00008. [DOI] [PubMed] [Google Scholar]
- 48.Antoniou T, Gomes T, Jurrlink DN. Trimethoprim-sulfamethoxazole-induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system. Arch Intern Med. 2010;170(12):1045–1049. doi: 10.1001/archinternmed.2010.142. [DOI] [PubMed] [Google Scholar]
- 49.Gollnick H, Albring M, Brill K. Efficacite de l’acetate de cyproterone oral associe a l’ethinylestradiol dans le traitement de l’acne tardive de type facial. Ann Endocrinol. 1999;60:157–166. [PubMed] [Google Scholar]
- 50.London BM, Lookingbill DP. Frequency of pregnancy in acne patients taking oral contraceptives. Arch Dermaol. 1994;130:392–393. [PubMed] [Google Scholar]
- 51.Helms SE, Bredle DL, Zajic J, et al. Oral contraceptive failure rates and oral antibiotics. J Am Acad Dermatol. 1997;36:705–710. doi: 10.1016/s0190-9622(97)80322-2. [DOI] [PubMed] [Google Scholar]
- 52.Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. JEADV. 2005;19:163–166. doi: 10.1111/j.1468-3083.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 53.Katsambas A, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? Facts and controversie. Clinics in Derm. 2010;28(1):17–23. doi: 10.1016/j.clindermatol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Hughs BR, Cunliffe WJ. Tolerance of spironolactone. Br J Dermatol. 1988;118:687–691. doi: 10.1111/j.1365-2133.1988.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 55.Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60–62. doi: 10.1016/j.jaad.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 56.Lopes RJ, Lourenco AP, Mascarenhas J, et al. Safety of spironolactone use in ambulatory heart failure patients. Clin Cardiol. 2008;31(11):509–513. doi: 10.1002/clc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Rales investigators. Effectiveness of spironolactone added to an angiotensin-cnoverting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 58.Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;18:340. doi: 10.1136/bmj.c1768. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro S, Slone D, Lewis GP, et al. Fatal drug reactions among medical inpatients. JAMA. 1971;19(3):467–472. [PubMed] [Google Scholar]
- 60.Weber KT. Aldosterone and spironolactone in heart failure. N Engl J Med. 1999;341:753–754. doi: 10.1056/NEJM199909023411009. [DOI] [PubMed] [Google Scholar]
- 61.Struthers AD. Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in congestive heart failure. J Card Fail. 1996;2:47–54. doi: 10.1016/s1071-9164(96)80009-1. [DOI] [PubMed] [Google Scholar]
- 62.Chicago, IL: Searle; 2003. Aldactazide® (spironolactone with hydrochloro-thiazide) tablets [package insert] [Google Scholar]
- 63.Rathnayake D, Sinclair R. Use of spironolactone in dermatology. Skinmed. 2010;8:328–332. [PubMed] [Google Scholar]
- 64.Briggs GG, Freeman RK, Yaffe S. In: Drugs in Pregnancy and Lactation, 7th ed. Briggs GG, Freeman RK, Yaffe S, editors. Philadelphia: Lippincott-Williams & Wilkins; pp. 1480–1481. [Google Scholar]
- 65.Groves TD, Corenblum B. Spironolactone therapy during human pregnancy. Am J Obstet Gynecol. 1995;172(5):1655–1656. doi: 10.1016/0002-9378(95)90549-9. [DOI] [PubMed] [Google Scholar]
- 66.Beermann B, Groschinsky-Grind M. Clinical pharmacolkinetics of diuretics. Clin Pharmacokinet. 1980;5:221–245. doi: 10.2165/00003088-198005030-00003. [DOI] [PubMed] [Google Scholar]
- 67.Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi: 10.1002/jps.2600660841. [DOI] [PubMed] [Google Scholar]
- 68.Committee on Drugs. American Academy of Pediatrics. The transfer of drugs and other chemicals in human breast milk. Pediatrics. 2001;108:776–789. [Google Scholar]