Abstract
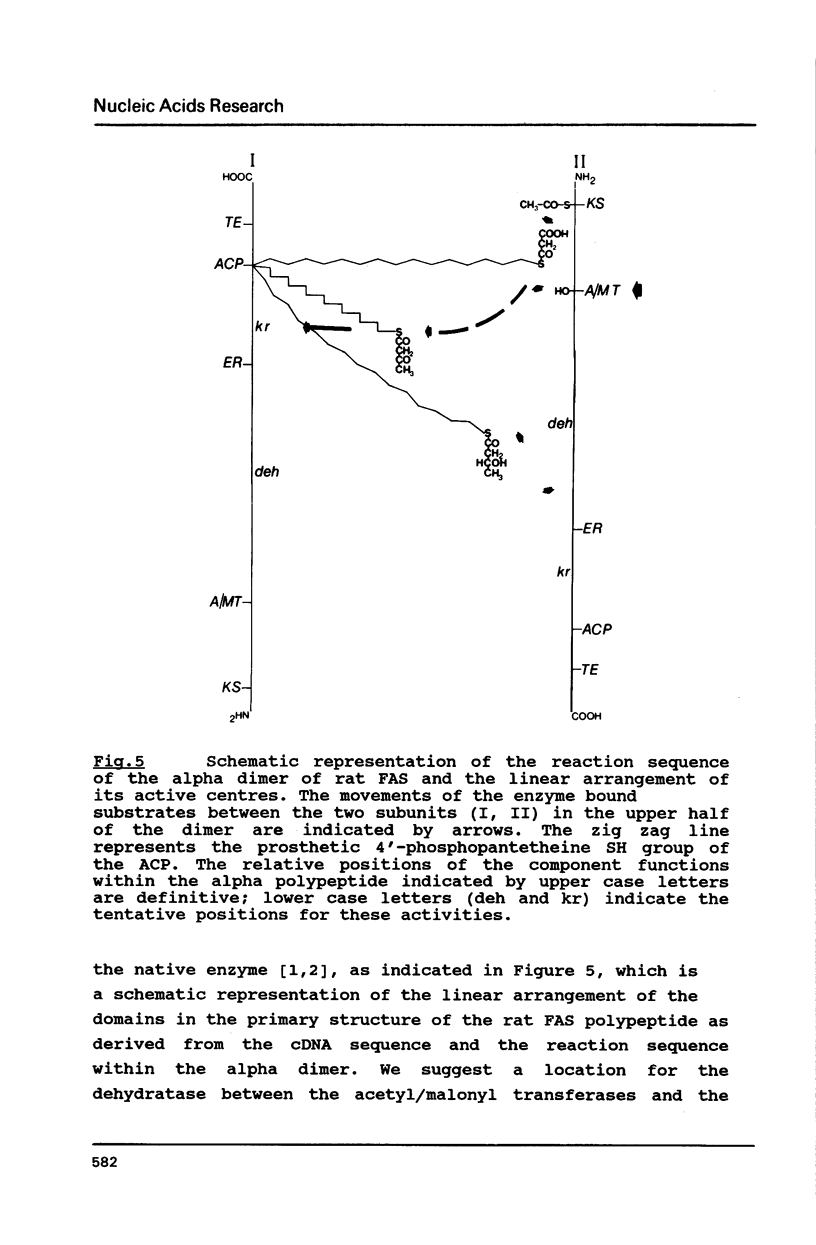
The rat fatty acid synthase (FAS) is active only as a dimer, although the eight component functions are contained in a single polypeptide chain. Using mRNA from lactating rat mammary glands a cDNA expression library was established. With the overlapping immunologically positive clones we have an 8.9kb cDNA sequence for rat FAS. In the 3'-nontranslated region of the rat FAS cDNA we find a prototype polyadenylation/termination signal and 779 nucleotides upstream, a mutated one. Both of these polyadenylation/termination signals are used and give rise to two equally abundant mRNA species which are coordinately regulated. In the derived amino acid sequence we could locate six of the eight component functions; their order is NH2- beta-ketoacyl synthase - acetyl/malonyl transferases -enoyl reductase - acyl carrier protein - thioesterase -COOH. Comparison of FAS from different sources shows that the primary sequence is conserved only for the active residues and the amino acids in their immediate vicinity.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. W., Goldman M. J., Fisch J. E., Ochs R. S., Goodridge A. G. The fatty acid synthase gene in avian liver. Two mRNAs are expressed and regulated in parallel by feeding, primarily at the level of transcription. J Biol Chem. 1986 Mar 25;261(9):4190–4197. [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Boyce S. G., Lueking D. R. Purification and characterization of Rhodobacter sphaeroides acyl carrier protein. Biochemistry. 1987 May 19;26(10):2740–2746. doi: 10.1021/bi00384a013. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dils R. R., Cameron I. R., Skidmore C. J., Weir A. F. Mammary gland fatty acid synthetase messenger RNA. Biochem Soc Trans. 1985 Oct;13(5):830–831. doi: 10.1042/bst0130830. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hardie D. G., McCarthy A. D., Braddock M. Mammalian fatty acid synthase: a chimeric protein which has evolved by gene fusion. Biochem Soc Trans. 1986 Jun;14(3):568–570. doi: 10.1042/bst0140568. [DOI] [PubMed] [Google Scholar]
- Kuo T. M., Ohlrogge J. B. The primary structure of spinach acyl carrier protein. Arch Biochem Biophys. 1984 Oct;234(1):290–296. doi: 10.1016/0003-9861(84)90351-5. [DOI] [PubMed] [Google Scholar]
- Mattick J. S., Tsukamoto Y., Nickless J., Wakil S. J. The architecture of the animal fatty acid synthetase. I. Proteolytic dissection and peptide mapping. J Biol Chem. 1983 Dec 25;258(24):15291–15299. [PubMed] [Google Scholar]
- Mattick J. S., Zehner Z. E., Calabro M. A., Wakil S. J. The isolation and characterization of fatty-acid synthetase mRNA from rat mammary gland. Eur J Biochem. 1981 Mar;114(3):643–651. doi: 10.1111/j.1432-1033.1981.tb05192.x. [DOI] [PubMed] [Google Scholar]
- McCarthy A. D., Aitken A., Hardie D. G. The multifunctional polypeptide chain of rabbit mammary fatty acid synthase contains a domain homologous with the acyl carrier protein of Escherichia coli. Eur J Biochem. 1983 Nov 15;136(3):501–508. doi: 10.1111/j.1432-1033.1983.tb07769.x. [DOI] [PubMed] [Google Scholar]
- McCarthy A. D., Hardie D. G. The multifunctional polypeptide chains of rabbit-mammary fatty-acid synthase. Stoichiometry of active sites and active-site mapping using limited proteolysis. Eur J Biochem. 1983 Jan 17;130(1):185–193. doi: 10.1111/j.1432-1033.1983.tb07135.x. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. B., Schweizer M., Dykstra C. C., Kushner S. R., Giles N. H. Genetic organization and transcriptional regulation in the qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5783–5787. doi: 10.1073/pnas.78.9.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulauskis J. D., Sul H. S. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J Biol Chem. 1988 May 25;263(15):7049–7054. [PubMed] [Google Scholar]
- Poulose A. J., Bonsall R. F., Kolattukudy P. E. Specific modification of the condensation domain of fatty acid synthase and the determination of the primary structure of the modified active site peptides. Arch Biochem Biophys. 1984 Apr;230(1):117–128. doi: 10.1016/0003-9861(84)90092-4. [DOI] [PubMed] [Google Scholar]
- Poulose A. J., Kolattukudy P. E. Sequence of a tryptic peptide from the NADPH binding site of the enoyl reductase domain of fatty acid synthase. Arch Biochem Biophys. 1983 Feb 1;220(2):652–656. doi: 10.1016/0003-9861(83)90459-9. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Bradshaw R. A., Vagelos P. R. Acyl carrier protein. XIX. Amino acid sequence of liver fatty acid synthetase peptides containing 4'-phosphopantetheine. J Biol Chem. 1972 Oct 10;247(19):6234–6242. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckenbach T., Wobser H., Lynen F. The palmityl binding sites of fatty acid synthetase from yeast. Eur J Biochem. 1977 Oct 17;80(1):13–23. doi: 10.1111/j.1432-1033.1977.tb11850.x. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Case M. E., Dykstra C. C., Giles N. H., Kushner S. R. Cloning the quinic acid (aq) gene cluster from Neurospora crassa: identification of recombinant plasmids containing both qa-2+ and qa-3+. Gene. 1981 Jun-Jul;14(1-2):23–32. doi: 10.1016/0378-1119(81)90145-1. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Case M. E., Dykstra C. C., Giles N. H., Kushner S. R. Identification and characterization of recombinant plasmids carrying the complete qa gene cluster from Neurospora crassa including the qa-1+ regulatory gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5086–5090. doi: 10.1073/pnas.78.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Lebert C., Höltke J., Roberts L. M., Schweizer E. Molecular cloning of the yeast fatty acid synthetase genes, FAS1 and FAS2: illustrating the structure of the FAS1 cluster gene by transcript mapping and transformation studies. Mol Gen Genet. 1984;194(3):457–465. doi: 10.1007/BF00425558. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Roberts L. M., Höltke H. J., Takabayashi K., Höllerer E., Hoffmann B., Müller G., Köttig H., Schweizer E. The pentafunctional FAS1 gene of yeast: its nucleotide sequence and order of the catalytic domains. Mol Gen Genet. 1986 Jun;203(3):479–486. doi: 10.1007/BF00422073. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Roberts L. M., Höltke J., Takabayashi K., Müller G., Hoja U., Höllerer E., Schuh B., Scheizer E. Molecular structure and expression of fatty acid synthetase genes in yeast. Biochem Soc Trans. 1986 Jun;14(3):572–574. doi: 10.1042/bst0140572. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Takabayashi K., Geiger T., Laux T., Biermann G., Buhler J. M., Gauthier F., Roberts L. M., Heinrich P. C. Identification and sequencing of cDNA clones for the rodent negative acute-phase protein alpha 1-inhibitor 3. Eur J Biochem. 1987 Apr 15;164(2):375–381. doi: 10.1111/j.1432-1033.1987.tb11068.x. [DOI] [PubMed] [Google Scholar]
- Smith S., Stern A., Randhawa Z. I., Knudsen J. Mammalian fatty acid synthetase is a structurally and functionally symmetrical dimer. Eur J Biochem. 1985 Nov 4;152(3):547–555. doi: 10.1111/j.1432-1033.1985.tb09230.x. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Arslanian M. J., Oh Y. H., Aune K. C., Vanaman T. C., Wakil S. J. Presence of two polypeptide chains comprising fatty acid synthetase. Proc Natl Acad Sci U S A. 1975 May;72(5):1940–1944. doi: 10.1073/pnas.72.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops J. K., Wakil S. J. Animal fatty acid synthetase. Identification of the residues comprising the novel arrangement of the beta-ketoacyl synthetase site and their role in its cold inactivation. J Biol Chem. 1982 Mar 25;257(6):3230–3235. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Wong H., Mattick J. S., Wakil S. J. The architecture of the animal fatty acid synthetase complex. IV. Mapping of active centers and model for the mechanism of action. J Biol Chem. 1983 Dec 25;258(24):15312–15322. [PubMed] [Google Scholar]
- Vanaman T. C., Wakil S. J., Hill R. L. The complete amino acid sequence of the acyl carrier protein of Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6420–6431. [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Witkowski A., Naggert J., Mikkelsen J., Smith S. Molecular cloning and sequencing of a cDNA encoding the acyl carrier protein and its flanking domains in the mammalian fatty acid synthetase. Eur J Biochem. 1987 Jun 15;165(3):601–606. doi: 10.1111/j.1432-1033.1987.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yuan Z. Y., Hammes G. G. Fluorescence studies of chicken liver fatty acid synthase. Segmental flexibility and distance measurements. J Biol Chem. 1986 Oct 15;261(29):13643–13651. [PubMed] [Google Scholar]




