Abstract
The X-linked inhibitor of apoptosis (XIAP) and other members of the inhibitor of apoptosis (IAP) family can suppress apoptosis induced by a diverse variety of triggers. Functional studies done to date have focused on tissue culture models and adenovirus overexpression of XIAP and other IAP proteins. Here we report the phenotype of an engineered transgenic mouse overexpressing a human IAP, as well as assessing the long-term consequence of IAP overexpression. We document the relative protein expression levels of the endogenous mouse homologue to XIAP, mouse inhibitor of apoptosis (MIAP 3), within thymocyte and T cell subpopulations. The consequence of lymphoid-targeted overexpression of XIAP in transgenic mice suggests a physiological role for the endogenous protein, MIAP3. Xiap-transgenic mice accumulated thymocytes and/or T cells in primary and secondary lymphoid tissue, T cell maturation was perturbed, and transgenic thymocytes resisted a variety of apoptotic triggers both in vitro and in vivo. These observations imply a possible key function for the intrinsic cellular inhibitor XIAP in maintaining the homeostasis of the immune system.
Apoptosis, or programmed cell death (PCD), is a key component of normal development and tissue homeostasis, especially of the immune system (1). Dysregulation of PCD is acknowledged as an essential component for the development of cancer and autoimmunity (2). A family of cysteine proteases called caspases are the central components orchestrating the orderly breakdown of cells during the apoptotic process (3). Both the Bcl-2 and IAP gene families encode antiapoptotic proteins that regulate PCD pathways. The mechanism of X-linked inhibitor of apoptosis (XIAP) inhibition is direct interaction and inhibition with critical initiator (caspase-9) and effector caspases caspase-3 and -7 (4, 5). An evolutionarily more ancient subgroup of inhibitors of apoptosis (IAPs), including mammalian Survivin and its yeast (Saccharomyces cerevisiae) and nematode (Caenorhabditis elegans) homologues, have been identified that play a role in chromosome segregation and cytokinesis (6, 7).
Apoptosis shapes thymocyte development through the elimination of aberrantly developed T cells and potentially harmful self-reactive T lymphocytes. Apoptosis also plays a role in eliminating activated effector T cells in the periphery when an immune response has started to wane. Thymocyte subpopulations represent different stages of T cell differentiation and development. Progression of T cell maturation within the thymus primarily commences with a multipro-T stage proliferation, initiated by IL-7, to a double negative (DN) T cell stage (CD4−CD8−) and then proceeds to a double positive (DP) CD4+CD8+ stage. The final phase of maturation is completed with the down-regulation of either CD4+ or CD8+ on a DP thymocyte, leading to the formation of a mature single positive (SP) (CD4−CD8+ and CD4+CD8−) T cell, which is then exported to the periphery. Differentiating thymocytes undergo selection processes that are mediated by apoptosis during several stages of maturation. First, at the DN stage, thymocytes that fail to express an early functional pre-T cell receptor (TCR) Vβ, or later the full TCRαβ, are incapable of receiving a positive signal via the TCR and therefore die via apoptosis, a process termed death by neglect (8). Second, thymocytes with correctly rearranged TCRαβ that cannot bind to major histocompatibility (MHC) complex molecules (MHC I/II) also die of neglect. The process of classical positive selection is the rescue of cells bearing the full TCRαβ with low-to-moderate avidity for self MHC from death by neglect. Last, high-affinity interaction of TCRαβ with MHC I/II proteins associated with self-peptide triggers PCD in a process known as negative selection or clonal deletion (9). Negative selection within the thymus occurs during the immature thymocyte developmental stage, DP, and the early mature SP phases of growth.
Although contested, the current model of thymic development of TCRαβ T cells states that Bcl-2 inhibits some, but not all, apoptotic physiological pathways. Bcl-2 was demonstrated to inhibit thymic apoptosis caused by a lack of an IL-7R signal in the early stages of pro-T cell proliferation (10) and of DP thymocytes destined to die during the process of classical positive selection (11). However, Bcl-2 has no inhibitory effect on the directed deletion of thymocytes that fail to express an early fully functional pre-T cell receptor (10) or autoreactive thymocytes (12).
Overexpression of XIAP inhibits apoptosis in tissue culture cells challenged with a wide variety of apoptotic triggers, such as glucocorticoids, tumor necrosis factor (TNF)α, or serum withdrawal (13). T cell and thymocyte-triggered PCD as well as T cell function/activation have been demonstrated to be partially dependent on activation of caspases through their proteolytic processing (14). Interestingly, high levels of human xiap mRNA expression have been shown within developing T cells of the thymus and peripheral lymph nodes (13). Because XIAP functions primarily through the direct inhibition of caspase protease function, we believe that endogenous XIAP may play a role in T cell development and function.
Here we investigate the effect of XIAP overexpression in T cell development and function by engineering a transgenic mouse overexpressing a human xiap transgene under the control of a T cell-specific promoter, lck. We demonstrate that transgenic mice thymocytes and/or T cells accumulate in primary (thymus) and secondary (spleen) lymphoid tissues relative to wild-type littermates. Elevated DN thymocytes and SP CD4-CD8+ levels suggest that T cell maturation was perturbed because of enhanced apoptotic resistance as demonstrated in both in vitro and in vivo experiments.
Materials and Methods
Construction of lckpr-xiap Vector and Production of Transgenic Mice.
A 1.5-kb cDNA fragment containing the coding region of human xiap was inserted into the lck-human GH vector (15). Transgenic mice were generated at the Biomedical Research Center at the University of British Columbia. Mouse strain C57BL/6 was used to isolate oocytes for transgene microinjection and for crossing of the transgenic founder animals.
Southern Blot Analysis.
Genomic DNA was isolated by standard methods and digested with BamHI/XhoI, separated on agarose gels, and transferred to Biodyne Nylon Paper (Life Technologies, Rockville, MD). 32P-labeled full length xiap cDNA probes were prepared by using Rediprime (Amersham Pharmacia) and 32P-dCTP (Amersham Pharmacia) according to the manufacturer's directions. Final washes were carried out in 0.1 × SSC/0.1% SDS at 65°C.
Western Blot Analysis.
Mouse tissue was lysed in five volumes of homogenization buffer (10 mM Tris⋅HCl, pH 6.8/150 mM NaCl/2 mM MgCl2/1 mM phenylmethylsulphonly fluoride) and then crushed, followed by centrifugation at 250 × g for 2 min. The supernatant (S/N) was then adjusted to 2% SDS and boiled for 20 min, followed by centrifugation at 14 K for 10 min. The S/N was transferred to a new microfuge tube and centrifuged again at 14 K for 15 min. Protein content was assayed by bicinchoninic acid kit (Pierce), and equal amounts of protein samples of the S/N lysate were loaded per lane, separated by SDS/PAGE, and analyzed by Western blotting by using rabbit polyclonal antibodies to XIAP or mouse inhibitor of apoptosis (MIAP) 3 (1:3,000 dilution) and developed by using an enhanced chemiluminescence kit (Pierce).
Flow Cytometry.
T cells and thymocytes were isolated from mouse lymphoid tissue by first mincing and then pressing the tissue through a 10-μm metal mesh and were then counted by using trypan blue exclusion. Cells (1 × 106) were incubated with the following conjugated monoclonal antibodies: anti-Thy1.2-FITC, CD4-phycoerythrin, CD8a-Cy-Chrome, and CD45R-FITC (PharMingen). Flow cytometric analyses were performed on a Coulter XL cytometer (Coulter Canada).
Primary Tissue Culture and Death Assays.
Primary cultures were maintained in RPMI 1640 supplemented with 10% FCS, 50 μM β-mercaptoethanol, 125 mM l-glutamine, penicillin, and streptomycin. Thymocytes (5 × 106/ml) were cultured in 24-well plates and exposed to different apoptotic triggers at the time periods indicated. Some wells were coated with anti-CD3 antibody (clone 145–2C11, PharMingen) (10 μg/ml) overnight and then washed with PBS. Cell death/apoptosis was measured by trypan blue exclusion and annexin V-FITC staining (Immunotech, Luminy, France) and then analyzed on a Coulter XL cytometer.
In Vivo Apoptotic Death.
For in vivo experiments, mice were injected i.p. with PBS (anti-Fas antibody control), saline (dexamethasone control), mouse-specific anti-Fas antibody (clone Jo2, 100 μg) (PharMingen), or dexamethasone (Sigma). Thymocytes from dexamethasone and anti-Fas antibody-injected mice were harvested at 48 and 2 h, respectively. All thymocytes were isolated as described and viability assessed by annexin V-FITC. Thymocytes from dexamethasone-treated mice were further stained to assess affected T cell subpopulations.
T Lymphocyte Isolation.
Lymphocytes were isolated as described and separated into CD4+ and CD8+ population by using antibody coupled beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's directions.
Results
Establishment of p56lck-xiap Transgenic Mice.
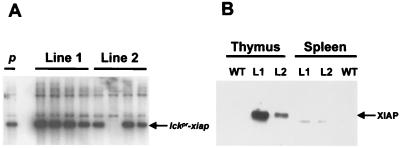
To assess the effect of XIAP on T cell development, a transgenic mouse model was generated. The transgene construct contained the human cDNA encoding xiap downstream of the proximal promoter of the p56 lck tyrosine kinase gene (lckpr). The proximal promoter limits transgene expression primarily to thymocytes (16). Two C57BL/6 founder lines were identified (Fig. 1A), and expression of human XIAP in the thymus was demonstrated by Western blot analysis (Fig. 1B). Line 1 offspring expressed significantly more XIAP at roughly twice that of line 2.
Figure 1.
Characterization of lckpr-xiap transgenic mice. (A) Southern analysis of offspring from founder lines 1 and 2. Lane one (p) is the control lane that contains BamHI/XhoI-digested plasmid DNA, which contains the human cDNA fragment of lckpr-xiap. (B) Western analysis of XIAP expression in the thymus and spleen of founder lines 1 (L1), 2 (L2), and wild-type (WT) mice.
Overexpression Inhibits Apoptosis of Thymocytes in Vitro.
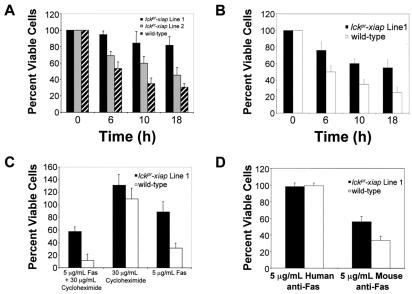
Pre-T cells and thymocytes are highly susceptible to apoptosis, relative to SP and naïve T cells. The vast majority of these developing T cells are destined to die during the maturation process in the thymus. Adenovirus overexpression of XIAP protects a variety of tissue cell lines, including the human Jurkat T lymphoid cell line, from a broad range of in vitro apoptotic triggers (13). We therefore evaluated the ability of XIAP to rescue apoptotic-sensitive thymocytes from general apoptotic triggers, such as C2 ceramide, UV radiation, and anti-Fas antibody. Ceramide triggers apoptosis by directly activating caspase 3 and should therefore be suppressed by XIAP. We compared viability of wild-type and lckpr-xiap thymocytes after C2 ceramide exposure (10 μM) in vitro. Trypan blue exclusion counts were verified by quantifying apoptotic cells by using an Annexin V-FITC kit (Immunotech). As expected, lckpr-xiap thymocytes demonstrated reduced in vitro apoptosis, with only 20% cell death (n = 16, P < 0.01) relative to untreated lckpr-xiap thymocytes over an 18-h time course. In contrast, viable thymocytes from wild-type littermates were reduced by 70% over the same time span (Fig. 2A). Apoptosis proceeds via two major intracellular pathways: (i) initiation via engagement of death receptors, such as Fas and tumor necrosis factor receptors, or (ii) initiation via release of apoptotic factors, such as cytochrome c, as triggered by agents like glucocorticoids or DNA damage by UV radiation. We then assessed the ability of XIAP to inhibit both major apoptotic pathways by comparing wild-type and lckpr-xiap thymocyte viability after exposure to UV radiation (1,000 μJ/cm2) and after engagement of the Fas death receptor by using a mouse-specific anti-Fas antibody (Jo2, 5 μg/ml). Lckpr-xiap thymocytes proved resistant to apoptosis triggered by both pathways. UV radiation-induced apoptosis was reduced compared with wild-type thymocytes (viability: 60% transgenic vs. 30% wild type) (Fig. 2B) (n = 8, P < 0.01) 18 h after irradiation. Fas receptor ligation-induced apoptosis was also lower in transgenic-derived thymocytes both in combination with cycloheximide [60% viability vs. 10% for wild-type littermates at 12 h after treatment (n = 24, P < 0.01)] and without cycloheximide [90% viability vs. 30% for wild-type littermates (n = 24, P < 0.01)] (Fig. 2C). Human specific anti-Fas antibody had no effect on either the transgenic or wild-type thymocytes (Fig. 2D).
Figure 2.
XIAP inhibits general apoptotic triggers, in vitro. (A) Thymocytes from lckpr-xiap lines 1 (n = 16, P < 0.01) and 2 (n = 12, P < 0.01), and wild-type thymocytes (5 × 106 cells/ml) were exposed to C2 ceramide (10 μM) for the time periods indicated. (B) Lckpr-xiap line 1 and wild-type thymocytes (5 × 106 cells/ml) were exposed to UV radiation (1,000 μJ/cm2) (n = 8, P < 0.01) for the time periods indicated. (C) Lckpr-xiap line 1 and wild-type thymocytes (5 × 106 cells/ml) were treated with either anti-Fas antibody (Jo2, 5 μg/ml) in combination with cycloheximide (30 μg/ml) (n = 24, P < 0.01), with cycloheximide (30 μg/ml) alone, or with anti-Fas (n = 24, P < 0.01) alone for 12 h. (D) Lckpr-xiap line 1 and wild-type thymocytes (5 × 106 cells/ml) were treated with either mouse Fas antibody (5 μg/ml) (n = 20, P < 0.01) or human specific anti-Fas antibody (5 μg/ml) (n = 20, P < 0.01) for 18 h. All mice used were between 4 and 5 weeks old.
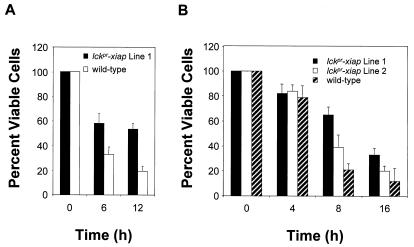
We next investigated the ability of XIAP to interfere with T cell-specific apoptotic triggers by using anti-CD3 antibody and dexamethasone. Elimination of pernicious self-reactive T cells, believed to be the primary mediators of autoimmunity, occurs via a thymus-specific negative-selective apoptotic process known as clonal deletion. In vitro treatment of thymocytes with anti-CD3 antibody is widely accepted as a model for in vivo negative selection, whereas the elimination of thymic endogenous glucocorticoids has been shown to accelerate the thymocyte maturation process and disrupt thymocyte positive selection (17). Both anti-CD3 antibody and dexamethasone trigger in vivo and in vitro apoptosis of thymocytes (12). Lckpr-xiap thymocytes treated with dexamethasone (100 nM) or anti-CD3 antibody in vitro, at a concentration optimal for inducing death in wild-type cells (10 μg/ml), demonstrated enhanced resistance to apoptosis. The number of viable lckpr-xiap thymocytes was approximately double at each time point for both dexamethasone (n = 30, P < 0.01) and anti-CD3 (n = 16, P < 0.01) relative to thymocytes from wild-type littermates (Fig. 3 A and B, respectively).
Figure 3.
XIAP inhibits T cell-specific apoptotic triggers in vitro. (A) Lckpr-xiap lines 1 (n = 30, P < 0.01) and 2 (n = 18, P < 0.01) and wild-type thymocytes (5 × 106 cells/ml) were exposed to dexamethasone (100 nM) for the time periods indicated. (B) Lckpr-xiap line 1 and wild-type thymocytes (5 × 106 cells/ml) were exposed to anti-CD3 antibody (10 μg/ml) (n = 16, P < 0.01) for the time periods indicated. All mice used were between 4 and 5 weeks old.
The broad resistance to apoptosis demonstrated by lckpr-xiap-derived thymocytes was attributable to the overexpression of XIAP and correlated with expression levels of the transgene. Both expression levels (Fig. 1A) and suppression of apoptosis of line 2 (Figs. 2A and 3B) lckpr-xiap mice were approximately half that of line 1.
Endogenous MIAP 3 (Murine XIAP) Expression.
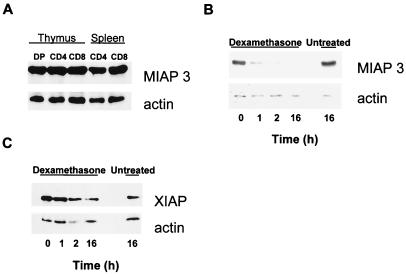
Apoptosis susceptibility is known to vary during the lifespan of thymocytes/T cells, specifically during certain stages of maturation (up to and including the T cell effector stage), suggesting tight regulation of antiapoptotic proteins. Here we investigated the possibility that regulated expression of MIAP 3 may determine apoptotic susceptibility of distinct T lymphocyte populations. MIAP 3 protein expression levels within thymocyte and T cell populations has not been previously reported. As seen in Fig. 4A, MIAP 3 is expressed ubiquitously throughout the total thymocyte and T cell subpopulations (>94% purity). This result was not unexpected, because it was previously demonstrated that relatively high levels of XIAP mRNA were found in all human tissues examined (18). Given that XIAP and MIAP 3 are key antiapoptotic proteins, then why are pre-T, DP, and effector T cells so highly sensitive to apoptosis? Previously it was shown that exposure of thymocytes to dexamethasone triggers selective elimination of XIAP before overt death (19). To assess the potential fate of endogenous and transgene encoded protein in apoptotic-sensitive DP thymocytes, we monitored protein levels by Western blot during thymocyte exposure to dexamethasone (100 nM) in vitro. As seen in Fig. 4B, in wild-type thymocytes treated with dexamethasone, endogenous MIAP 3 protein was eliminated by the 2-h interval, whereas apoptosis was not observed until 4 h after exposure (data not shown). However, transgene-encoded protein levels were reduced but still detectable at 2 h in lckpr-xiap thymocytes treated with dexamethasone. Transgene-encoded XIAP protein was not eliminated, possibly because of an excess of both endogenous MIAP 3 and the transgene XIAP proteins present in the lckpr-xiap thymocytes. It should be noted that anti-XIAP antibody used here is specific to XIAP protein and does not react with MIAP 3, whereas anti-MIAP 3 antibody crossreacts with XIAP protein. These results suggest that the sensitivity of DP thymocytes to apoptosis may be the result of the rapid elimination of endogenous MIAP 3 before apoptotic death. More importantly, these results also suggest that the apoptotic resistance of lckpr-xiap thymocytes compared with wild-type littermates is directly attributable to the continued presence of the transgene protein XIAP.
Figure 4.
MIAP 3 protein is eliminated on treatment of wild-type thymocytes with dexamethasone in vitro. (A) Western analysis of normal endogenous MIAP 3 expression in thymus/T cell subpopulations. (B) MIAP 3 protein levels of isolated wild-type thymocytes that had been treated with dexamethasone (100 nM) in vitro. (C) XIAP transgene protein levels of isolated lckpr-xiap thymocytes (line 1 mice) treated with dexamethasone (100 nM) in vitro. All mice were 4 weeks old.
Transgenic lckpr-xiap Thymocytes Resist Dexamethasone and Anti-Fas Initiated Apoptosis in Vivo.
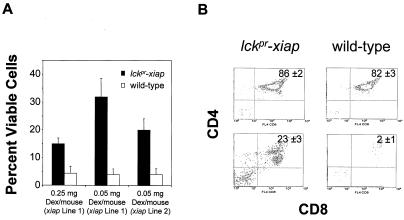
Because lckpr-xiap thymocytes resisted in vitro apoptotic signals, we then questioned whether the transgene might also inhibit apoptosis in vivo. Wild-type thymocytes are quickly depleted when mice are treated with either dexamethasone or anti-Fas antibody. Specifically, treating mice with dexamethasone (from 0.1 to 0.5 mg/mouse, 48 h), results in ≥95% depletion of thymocytes, mostly of the DP CD4+CD8+ thymocyte population (12, 20). Here we treated either lckpr-xiap or wild-type C57BL/6 mice with saline, 0.05 or 0.25 mg of dexamethasone i.p., and examined thymocytes 48 h later. Dexamethasone at 0.05 or 0.25 mg reduced thymocyte numbers by 94 and 96%, respectively, in control mice, relative to saline treatment alone (Fig. 5A). However, thymocytes derived from lckpr-xiap line 1 mice were depleted by only 68–85% with either dose. Flow cytometric analysis of the surviving thymocytes shows that most of the DP population had been eliminated in the control animals (Fig. 5B), whereas ≈23% survived in the lckpr-xiap mice (n = 20, P < 0.01) (Fig. 5B). This effect was dose-dependent, because lower transgene expression levels in line 2 mice correlated with decreased protection (at 0.05 mg of dexamethasone) (Fig. 5A).
Figure 5.
XIAP inhibits dexamethasone-induced apoptotsis in vivo. (A) Total thymocytes recovered 48 h after treatment with 0.05 mg [lckpr-xiap lines 1 (n = 20, P < 0.01) and line 2 (n = 18, P < 0.01)] or 0.25 mg [lckpr-xiap lines 1 (n = 20, P < 0.01)] of dexamethasone i.p. All mice used were between 4 and 5 weeks old. (B) Two-color flow cytometry contour plots of CD4 and CD8 expression on surviving thymocytes from lckpr-xiap line 1 and wild-type mice. The surviving 0.25 mg dexamethasone- or saline-treated thymocytes were doubly stained with anti-CD4-phycoerythrin and CD8a-Cy-Chrome. The percentages of the T cell subpopulations given are the average from all of the mice used in the corresponding experiment.
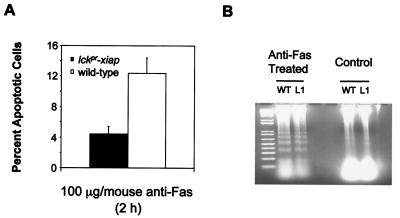
Studies of the mutant mice lymphoproliferation and generalized lymphoproliferative disease (21, 22), which carry loss-of-function mutations in the Fas and FasL genes, respectively, have helped to define an essential role for Fas in development, homeostasis, and self-tolerance of lymphocytes (23). Although both DP and SP thymocytes express Fas in the mouse thymus, the DP population is most sensitive to Fas-triggered apoptosis. To study whether lckpr-xiap thymocytes resist Fas-induced apoptosis in vivo, we analyzed the effects of anti-Fas antibody in lckpr-xiap mice. Anti-Fas antibody (100 μg/mouse) was injected i.p. into control and lckpr-xiap mice, and levels of apoptosis were compared in liver and thymus 2 h later. The extent of apoptosis was assessed by annexin V staining of thymocytes. The thymocytes of mice treated with Jo2 antibody were shown to undergo extensive apoptotic death. As well, i.p. inoculation of this antibody has been previously shown to cause hepatic failure and death of BALB/c and C57BL/6 mice within hours because of Fas-mediated hepatocyte apoptosis (24, 25). Significantly fewer thymocytes from lckpr-xiap mice were undergoing apoptosis than in control mice after treatment with Jo2, 4.5% compared with 12% (Fig. 6A) (n = 24, P < 0.01). Nucleosomal laddering of hepatic DNA demonstrated that extensive nonlymphoid apoptosis occurred in both wild-type and transgenic mice (Fig. 6B).
Figure 6.
XIAP inhibits Fas-induced apoptosis in vivo. Total lckpr-xiap lines 1 (n = 24, P < 0.01) and wild-type thymocytes undergoing apoptosis 2 h after treatment with 100 μg of clone Jo2 anti-Fas antibody i.p. (A). All mice used were between 4 and 5 weeks old. (B) Agarose gel electrophoresis of DNA extracted from the livers of anti-Fas treated wild-type (WT) and lckpr-xiap line 1 mice (100 μg, 2 h) and nontreated saline-injected control mice.
T Cell Homeostasis Is Disrupted in lckpr-xiap Mice.
XIAP-mediated suppression of thymic apoptosis was predicted to have an adverse effect on T cell ontogeny and cell numbers. We therefore counted thymocyte numbers in mice of varying ages, ranging from 1 week to 4 months. As seen in Table 1, the total number of thymocytes was increased by 13–37% at all time points from line 1 mice, whereas only marginally so (5–15%) for line 2 mice. The greatest difference was found in 4-week-old mice, where line 1 lckpr-xiap mice had 37% more thymocytes (n = 80, P < 0.01) than wild-type littermates. The mouse populations used in each total for all mice were ≈50/50 male to female.
Table 1.
| Mouse strain | Age
|
||
|---|---|---|---|
| 1 week | 4 weeks | 4 months | |
| T cells/thymus | |||
| Lckpr-xiap line 1 | 9.6 × 107 ± 0.3 | 1.60 × 108 ± 0.4 | 1.0 × 108 ± 0.2 |
| Wild-type control | 8.2 × 107 ± 0.2 | 1.17 × 108 ± 0.3 | 8.8 × 107 ± 0.2 |
| n value | 26 | 80 | 46 |
| T cells/thymus | |||
| Lckpr-xiap line 2 | 8.6 × 107 ± 0.2 | 1.21 × 108 ± 0.4 | 9.1 × 107 ± 0.3 |
| Wild-type control | 8.1 × 107 ± 0.3 | 1.06 × 108 ± 0.4 | 8.9 × 107 ± 0.4 |
| n value | 12 | 25 | 10 |
Total cell numbers were determined by trypan blue exclusion.
P values for all ages were less than 0.01.
Increased DN (CD4−CD8−) Population.
Because XIAP overexpression altered total thymocyte numbers, we proceeded to determine the effects on subpopulations of maturing thymocytes. When analyzed by flow cytometry, the percentage of DN pro-T cells was 4.5-fold greater (n = 36, P < 0.01) than wild-type littermates (Table 2). The percentage of DP CD4+CD8+ cells was only slightly elevated, whereas the SP population, which represents mature T cells, was normal. However, the CD4+/CD8+ ratio of SP thymocytes in lckpr-xiap transgenic mice was inverted with respect to wild-type littermates (Table 2). Western blot analysis revealed no difference in transgene xiap expression in SP CD4+ vs. CD8+ thymocytes (data not shown). The same inversion had been noted previously in transgenic mice expressing Bcl-2 under the control of the lckpr (12). Our results demonstrate that lckpr-xiap transgenic mice have a moderate increase in the number of thymocytes and a skewing of the T cell population in the thymus, suggesting that overexpression of XIAP interferes with T cell maturation and/or development.
Table 2.
| Mouse strain | Thymic T cell subpopulations at age
4–5 weeks
|
|||
|---|---|---|---|---|
| Thy1.2 | DN | DP | CD4/CD 8 ratio | |
| Lckpr-xiap (line 1) | 96% ± 3 | 2.3% ± 0.5 | 88% ± 2 | 0.44 ± 0.1 |
| Wild-type control | 91% ± 4 | 0.6% ± 0.2 | 83% ± 5 | 2.3 ± 0.7 |
Flow cytometry of cells containing CD4, CD8, or Thy1.2 were analyzed using three-color immunofluorescence on 10,000 cells per sample.
P values for all ages were less than 0.01 and n = 46 for each T cell subpopulation.
Spleen T Cell Counts.
Examination of splenic T cells revealed an even more dramatic increase in cell numbers, which increased with the age of the animal as determined by trypan blue exclusion, and flow cytometric analysis of T cell percentages. The results, in Table 3, suggest that there is an ongoing accumulation of T cells in the spleen. At the 4-month time point, there were 4.5 × 107 splenic T cells from line 1 mice, more than double that of control spleens, 1.9 × 107 (n = 56, P < 0.01).
Table 3.
| Mouse strain | Age
|
||
|---|---|---|---|
| 1 week | 4 weeks | 4 months | |
| T cells/spleen | |||
| Lckpr-xiap, line 1 | 6.6 × 106 ± 0.1 | 3.1 × 107 ± 0.1 | 4.5 × 107 ± 0.2 |
| Wild-type control | 4.0 × 106 ± 0.2 | 1.1 × 107 ± 0.3 | 1.9 × 107 ± 0.5 |
| n value | 22 | 40 | 56 |
Total cell numbers were determined by trypan blue exclusion.
P values for all ages were less than 0.01.
The inverted thymic SP CD4+/CD8+ ratio was also observed within the splenocytes of 4-month-old lckpr-xiap mice [CD4+/CD8+ ratio of 0.65 ± 0.5 (n = 30) compared with 1.7 ± 0.3 (n = 26) for the wild-type controls]. This phenomenon is comparable to what was found with lckpr-bcl-2 transgenics (12), where the SP CD8+ population was elevated relative to SP CD4+.
Discussion
Previous in vivo XIAP antiapoptotic functional studies have used adenovirus overexpression models (13), raising concerns about possible secondary adenovirus-mediated effects such as inflammation. Furthermore, adenovirus expression levels are sufficiently high to raise the issue of the physiological relevance of the effects observed. Here, we assess the effects of XIAP overexpression on the viability of thymocytes and T cells derived from a lckpr-xiap transgenic mice after challenge with various apoptotic triggers.
Overexpression of IAP family members has been shown to block a broad range of apoptotic signals (13) via the inhibition of caspases, specifically caspase-3, -7, and -9. The ability of XIAP to resist a wider spectrum of apoptotic triggers relative to Bcl-2 and Bcl-xL is illustrated with the use of dexamethasone and anti-Fas antibody. Both agents are capable of triggering apoptosis in vivo and in vitro, via distinct pathways (26). Although thymic overexpression of Bcl-2- and Bcl-xL-protected cells from dexamethasone induced apoptosis, Fas-induced apoptosis was unaffected (26). Similarly, thymocytes derived from caspase-9-deficient mice were also resistant to dexamethasone-induced apoptosis but were remarkably sensitive to anti-Fas antibody-induced apoptosis (27). In contrast, we have demonstrated that overexpression of XIAP in thymocytes was able to inhibit both pathways (Figs. 2C and 3B), which is consistent with XIAP functioning at the convergence of these discrete apoptotic pathways and downstream of caspase-9 (27). Although caspase-3 plays a key role in the apoptotic cascade, caspase-3 −/− thymocytes do not display resistance to a variety of triggers (anti-Fas antibody, dexamethasone, C2-ceramide, staurosporin, and γ-irradiation) (28), suggesting compensation by other caspases.
Bcl-2 transgenic mice displayed greater resistance to dexamethasone-induced apoptosis compared with lckpr-xiap thymocytes. We have demonstrated that the level of resistance depends on expression levels, and therefore the superior Bcl-2 mediated protection may be a reflection of higher transgene expression.
Developing T lymphocytes proceed through distinct intervals of relative high and low apoptotic susceptibility. Our results suggest a correlation between MIAP 3 or XIAP transgene levels with thymocyte subpopulation sensitivity or resistance to PCD. The proteolytic removal of MIAP 3 has been previously demonstrated to occur in thymocytes exposed to dexamethasone. Moreover, MIAP 3 removal was relatively specific, whereas the protein levels of Bcl-xL, which is maximally expressed in DP thymocytes (29), were unaffected (19). Although Bcl-2 protein has been shown to be down-regulated in DP thymocytes and at the effector T cell stage (30), thymic overexpression of Bcl-2 did not result in any accumulation of thymocytes or peripheral T cells (12, 30). In contrast, we have established that MIAP 3 was uniformly expressed throughout the mouse thymocyte and T cell subpopulations (Fig. 4A) and that MIAP 3 was eliminated by 2 h postdexamethasone exposure before overt apoptotic death (Fig. 4B). Most importantly, we demonstrate that apoptotic-resistant lckpr-xiap thymocytes, at the same time point, still contain visible levels of transgene encoded XIAP protein (Fig. 4C).
Increased resistance to apoptotic triggers in thymocytes of transgenic mice has resulted in some perturbations of thymocyte maturation and increased the number of cells in both the thymus and spleen. In particular, the proportion of DN pro-T cells was elevated, suggesting that some cells that would normally die by neglect are surviving but are not able to progress to the DP stage. Consequently, maturation of cells that are competent is not disrupted, and the proportion of SP mature cells is unchanged compared with controls. However, lckpr-xiap mice demonstrated an aberrant and preferential maturation of SP CD4−CD8+ over SP CD4+CD8− cells (Table 2), comparable to lckpr-bcl-2 transgenic mice (12). It has been speculated that the observed increase in the number of SP CD4−CD8+ cells is directly attributable to the prolonged survival of the lckpr-bcl-2/-xiap thymocytes compared with wild-type mice (31). The cytoplasmic tail of the CD4 receptor interacts with the signal transduction molecule p56lck with a much higher affinity compared with CD8 (32), which may lead to a reduction in the efficiency of positive selection of SP CD4−CD8+ thymocytes relative to SP CD4+CD8− in normal mice. The protracted survival of the lckpr-bcl-2/xiap thymocytes compared with thymocytes of wild-type mice may compensate for the relatively inefficient selection of SP CD4−CD8+, therefore leading to a relative increase in the number of SP CD4−CD8+ thymocytes (31). Although no increase in SP mature cells was noted in the thymus, T cell numbers in the spleen increased with the age of the mouse.
Overexpression of an antiapoptotic protein results in an outcome analogous to knocking out a proapoptotic gene. Disruption of the Bim gene, a proapoptotic member of the Bcl-2 family, also resulted in phenotypic changes similar to what was observed in the lckpr-xiap transgenic mice (33). Both Bim knockout mice and lckpr-xiap animals displayed a significant increase in DN pro-T cells (Table 1) as well as an ever-increasing splenic T lymphocyte population (Table 3) (33). Knocking out the Bim gene resulted in a disruption in T cell development, and eventually the Bim animals succumbed to autoimmune kidney disease. We are currently investigating the immune response and T cell function of lckpr-xiap animals and whether the overexpression of the transgene-encoded XIAP protein in thymocytes will eventually lead to autoimmunity in aged mice.
Our results suggest that the perturbation of T cell development in the transgenic mice was because of a corresponding in vivo resistance to apoptotic triggers, which was directly related to the overexpression of the antiapoptotic protein, XIAP.
Acknowledgments
We thank L. Kelly and C. McRoberts for technical support. This work was supported by grants to R.G.K. from the Canadian Institutes of Health Research, Networks of Centers of Excellence, Canadian Genetic Diseases Network, and the Howard Hughes Medical Institute (HHMI). R.G.K. is a recipient of a Medical Research Council of Canada Senior Scientist award, a HHMI International Research Scholar, and a Fellow of the Royal Society of Canada.
Abbreviations
- XIAP
X-linked inhibitor of apoptosis, MIAP 3, mouse inhibitor of apoptosis, lckpr, p56 lck tyrosine kinase gene
- PCD
programmed cell death
- DN
double negative
- DP
double positive
- SP
single positive
- TCR
T cell receptor.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vaux D L, Korsmeyer S J. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 4.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 5.Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D. J Biol Chem. 2000;275:31733–31738. doi: 10.1074/jbc.M910231199. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Flanary P L, Altieri D C, Dohlman H G. J Biol Chem. 2000;275:6707–6711. doi: 10.1074/jbc.275.10.6707. [DOI] [PubMed] [Google Scholar]
- 7.Yoon H J, Carbon J. Proc Natl Acad Sci USA. 1999;96:13208–13213. doi: 10.1073/pnas.96.23.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Boehmer H. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 9.Nossal G J. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 10.Maraskovsky E, O'Reilly L A, Teepe M, Corcoran L M, Peschon J J, Strasser A. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 11.Strasser A, Harris A W, Corcoran L M, Cory S. Nature (London) 1994;368:457–460. doi: 10.1038/368457a0. [DOI] [PubMed] [Google Scholar]
- 12.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 13.LaCasse E C, Baird S, Korneluk R G, MacKenzie A E. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 14.Alam A, Cohen L Y, Aouad S, Sekaly R P. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaffin K E, Beals C R, Wilkie T M, Forbush K A, Simon M I, Perlmutter R M. EMBO J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds P J, Lesley J, Trotter J, Schulte R, Hyman R, Sefton B M. Mol Cell Biol. 1990;10:4266–4270. doi: 10.1128/mcb.10.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vacchio M S, Ashwell J D. J Exp Med. 1997;185:2033–2038. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J E, MacKenzie A, Korneluk R G. Nature (London) 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Ashwell J D. J Clin Immunol. 1999;19:337–349. doi: 10.1023/a:1020594531159. [DOI] [PubMed] [Google Scholar]
- 20.Blomgren H, Svedmyr E. Cell Immunol. 1971;2:285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 24.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 25.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Nature (London) 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A, Harris A W, Huang D C, Krammer P H, Cory S. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 28.Kuida K, Zheng T S, Na S, Kuan C Y, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 29.Grillot D A, Merino R, Nunez G. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 31.Tao W, Teh S J, Melhado I, Jirik F, Korsmeyer S J, Teh H S. J Exp Med. 1994;179:145–153. doi: 10.1084/jem.179.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner J M, Brodsky M H, Irving B A, Levin S D, Perlmutter R M, Littman D R. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 33.Bouillet P, Metcalf D, Huang D C, Tarlinton D M, Kay T W, Kontgen F, Adams J M, Strasser A. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]