Abstract
Human T lymphotropic virus type 1 (HTLV-1) requires regulated gene expression from unspliced and alternatively spliced transcripts for efficient replication and persistence. HTLV-1 Rex is known to facilitate cytoplasmic export of unspliced, gag/pol and incompletely spliced env mRNAs, but its contribution to the expression of other viral transcripts has not been experimentally assessed. In this study, we utilized HTLV-1 proviral clones, cellular fractionation, and real-time reverse transcriptase PCR to determine the role of Rex on the expression and export of all viral mRNAs. Our results indicate that the steady-state levels of the different viral mRNAs are modulated by Rex, which we attribute to a redistribution of completely spliced mRNAs toward incompletely spliced mRNAs. Furthermore, we confirmed the positive effect of Rex on the unspliced gag/pol mRNA and singly spliced env mRNA, resulting in increased cytoplasmic expression. However, the cytoplasmic export of the alternatively spliced HTLV-1 mRNAs encoding the accessory proteins and the antisense Hbz mRNA are independent of direct Rex regulation. This is consistent with the conclusion that viral mRNAs that contain the cis-acting repressive sequence (CRS) and/or a fully functional splice donor site require a Rex/RxRE interaction for efficient cytoplasmic expression.
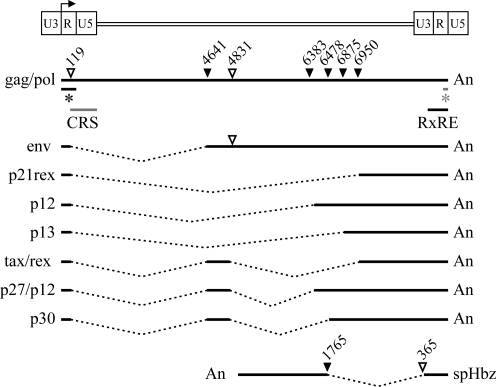
A unique feature of retroviruses, including human T lymphotropic virus type 1 (HTLV-1), is the ability to export intron-containing mRNAs to the cytoplasm for subsequent translation. In HTLV-1, the 27-kDa Rex nuclear phosphoprotein is responsible for this function.1–8 Two cis-acting sequences have been identified in the viral genome that are important for Rex regulation (Fig. 1): the Rex-response element (RxRE) functionally maps to u3/r sequences and is present at the 3′ end of all positive sense transcripts; the cis-acting repressive sequence (CRS) functionally maps to r/U5 sequences and is present only at the 5′ end of the full-length gag/pol transcript.9–12 Since HTLV-1, like all retroviruses, has direct long terminal repeats (LTRs) located at the 5′ and 3′ end of their proviral genome, processes including transcription initiation, splicing, and polyadenylation also result in these same transcripts containing partial RxRE sequences at their 5′ end and partial CRS at their 3′ end [Fig. 1 denoted by an asterisk (*)]. The antisense transcript that encodes HBZ does not contain either RxRE sequences or the CRS. The proposed working model for Rex is that the CRS retains the incompletely spliced mRNA in the nucleus and either prevents it from degradation until spliced or delays splicing until multiple molecules of Rex bind the RxRE forming an RNA/Rex/CRM-1/Ran-GTP complex.10,13–15 This interaction overcomes the inhibitory effect of the CRS and facilitates CRM-1-dependent mRNA export to the cytoplasm.16,17 Rex also actively inhibits the splicing machinery by binding in vitro to pre-mRNA splicing factors such as SF2/ASF.11,18–20 It has also been reported that the HTLV-1 p30 accessory protein when bound to its target tax/rex mRNA efficiently interacts with Rex.21 It has been proposed that this complex interaction represses tax/rex mRNA nuclear export further modulating viral replication.22
FIG. 1.
Provirus genome of human T lymphotropic virus type 1 (HTLV-1) and its unspliced, singly spliced, and doubly spliced mRNAs. The genomic unspliced mRNA encodes the Gag, Pol, and Pro proteins. Four singly spliced mRNA species are the result of splicing of exon 1 (nt 1–119) to major splice acceptors at positions 4641 (Env), 6383 or 6478 (p12), 6950 (p21rex), and 6875 (p13). The three doubly spliced mRNAs include exon 1, exon 2 (4641–4831), and a third exon that starts at position 6950 (Tax/Rex), 6478 (p30), or 6383 (p27/p12). The Hbz singly spliced major antisense transcript initiates at multiple sites in the 3′ LTR and utilizes a splice donor site at position 365 and a splice acceptor site at nt position 1765 (Hbz unspliced and minor spliced transcripts are not shown). Nucleotide numbering starts at the beginning of the R region for the positive sense transcripts and the last nucleotide of U5 in the 3′ LTR for the antisense transcript. Black lines designate exons and dotted lines designate introns. Major utilized splice donor (open triangles) and splice acceptor (closed triangles) sites are indicated above the unspliced mRNA. The gray line below the unspliced transcript at the 5′ end shows the location of the mapped CRS and the black line at the 3′ end shows the location of the mapped RxRE sequences.9–12 Due to direct repeats (identical LTRs) located at the 5′ and 3′ end of the genome, transcription initiation, splicing, and polyadenylation transcripts will also contain truncated RxRE sequences at the 5′ end (black line*) and partial CRS at their 3′ end (gray line*).
In the context of the entire provirus, Rex has been found to increase the levels of full-length gag/pol and singly spliced env mRNAs, and concomitantly decrease the level of the doubly spliced tax/rex message.5,11 To date, the role of Rex on the expression and export of alternatively spliced HTLV-1 mRNAs that encode the accessory proteins has been poorly investigated partially due to their complex splicing pattern and low abundance. HTLV-1 accessory proteins, although dispensable in cell culture, have been shown to play an important role in viral replication and persistence in animal models.23–27 Studies designed to determine the precise regulation pattern of viral gene expression are essential to understand viral pathogenesis. The goal of this study is to utilize full-length proviral molecular clones and real-time reverse transcriptase polymerase chain reaction (RT-PCR) to determine the role of Rex in the regulation of gene expression and cytoplasmic export of all major HTLV-1 transcripts.
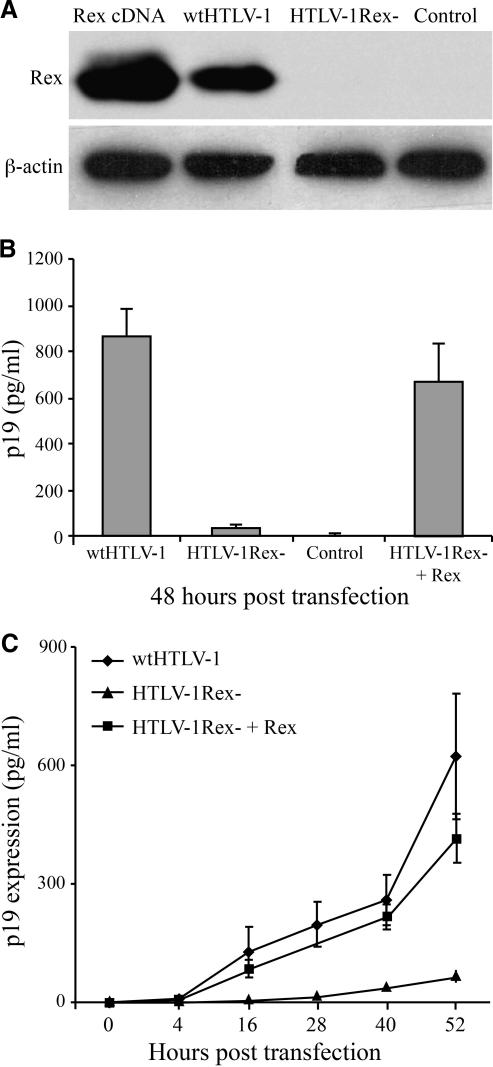
This study utilized the well-characterized wild-type HTLV-1 proviral clone (ACH)28 and a Rex-deficient derivative proviral plasmid termed HTLV-1Rex–.29 HTLV-1Rex– is identical to wtHTLV-1 except that the N-terminal coding sequence of Rex 5121GCATGCCCAAG5131 (based on Ach proviral sequence) was mutated to GCATGTCCTAG, so that the codon AAG of the third amino acid lysine was mutated to a stop codon TAG and the SphI restriction enzyme site GCATGC at the beginning of the Rex coding sequence was destroyed to facilitate diagnostic analyses. Previous phenotypic characterization of the HTLV-1Rex– virus supported the conclusion that Rex and its function to modulate viral gene expression and virion production was not required for in vitro immortalization of primary T-lymphocytes by HTLV-1. However, Rex expression was critical for efficient infection of cells and persistence in a rabbit animal model.29 We first revisited the expression of Rex and p19 Gag production in 293T cells 48 h after Lipofectamine (Invitrogen, Carlsbad, CA) transfection of these proviral clones. Western blot analysis using rabbit anti-Rex-1 polyclonal antisera (1:1000)30 demonstrated that Rex was expressed from wtHTLV-1 but not from the HTLV-1Rex– proviral plasmid (Fig. 2A). β-Actin detected with rabbit anti-β-actin monoclonal antibody (1:1000, Abcam, Cambridge, MA) was used as a loading control. Similarly, p19 Gag ELISA (Zeptometrix Corporation, Buffalo, NY) revealed high levels of p19 in supernatants from wtHTLV-1 transfected cells, and background levels in HTLV-1Rex– transfected cells (Fig. 2B). Moreover, p19 expression could be rescued from HTLV-1Rex– by cotransfection of the cDNA Rex expression plasmid (Fig. 2B). Next, a time-course transfection experiment (4, 16, 28, 44, and 52 h) was performed to determine the effect of Rex on the kinetics of p19 Gag production. Cells transfected with the wtHTLV-1 clone produced p19 Gag starting at 4 h posttransfection, which increased to a concentration of approximately 600 pg/ml at 52 h (Fig. 2C). In contrast, p19 Gag production in cells transfected with the HTLV-1Rex– clone was at background levels (<25 pg/ml) throughout the time course. Thus, HTLV-1Rex– did not express Rex or measurable p19 Gag protein. However, background levels of p19 Gag do not rule out low-level virion production or the capacity to transmit a transforming virus by cell-to-cell contact, which we reported previously with the HTLV-1Rex– virus.29 As expected, cotransfection of the Rex cDNA expression plasmid and HTLV-1Rex– rescued the kinetic expression of p19 to nearly wild-type levels (Fig. 2C).
FIG. 2.
Rex and p19 Gag protein expression in transiently transfected 293T cells. (A) 293T cells were transfected with wtHTLV-1, HTLV-1Rex–, Rex cDNA (SE356 CMV expression vector), or control (BC12) plasmids. Rex protein was detected by Western blot analysis using a rabbit anti Rex-1 polyclonal antibody. (B) p19 Gag production was tested 48 h after transfection of wtHTLV-1, HTLV-1Rex–, and HTLV-1Rex–+Rex into 293T cells. BC12 was transfected as a negative control. Data shown are the average of three independent transfections. (C) Kinetics of p19 Gag production in 293T cells transfected with wtHTLV-1, HTLV-1Rex–, or HTLV-1Rex–+Rex after 4 h, 16 h, 28 h, 44 h, and 52 h of incubation. Data shown are the average of three independent transfections.
Although Rex has been shown previously to facilitate export of the gag/pol and env mRNAs to the cytoplasm, it is not known whether Rex also influences the expression and export of other viral mRNAs encoding the accessory proteins. To determine the role of Rex on the expression level of individual viral mRNAs, wtHTLV-1 or HTLV-1Rex– plasmids were transfected into 293T cells and each viral transcript was quantified 48 h posttransfection. RNA was extracted from these cells using the RNeasy kit (Qiagen, Valencia, CA) and treated three times with RNase-free DNase. Equivalent amounts of purified RNA were subjected to cDNA synthesis using the SuperScript First-Strand Synthesis kit (Invitrogen Corp., Carlsbad, CA) with random hexamers. Taqman real-time RT-PCR was performed for each of the HTLV-1 mRNAs using specific primers and probes as described previously.31 Reactions were performed in the presence and absence of RT; none of the latter generated amplification signals, thus ruling out DNA contamination. RNA copy number was normalized to transfection efficiency and 1×106 mRNA copies of the cellular gene, GAPDH.
Table 1 shows the effect of Rex on the steady-state levels of HTLV-1 mRNAs. The data are presented as the ratio of a specific viral mRNA level in HTLV-1Rex– over its level in wtHTLV-1-transfected cells. Thus, <1 indicates a reduction and >1 indicates an increase in a specific mRNA in the absence of Rex. The total amount of viral mRNA detected increased approximately 2-fold in the absence of Rex. However, the amounts of mRNAs encoding Gag/Pol and Env were reduced 50% and 30%, respectively, in the absence of Rex, whereas the doubly spliced mRNA encoding Tax and Rex regulatory proteins was increased approximately 2-fold (2.1). Western blot analysis revealed that the 2-fold increase in the doubly spliced tax/rex mRNA in the absence of Rex translated into an approximately 5- to 7-fold increase in Tax protein expression (data not shown). HTLV-1 transcripts encoding accessory proteins (p21Rex, p12, p27, p13, p30) and antisense mRNA encoding HBZ were increased approximately 1.5- to 4-fold (1.4–4.4) in the absence of Rex. Any alteration in the expression of Tax would be expected to directly influence 5′ LTR-mediated viral transcription and the quantitative comparison of the transcripts. To account for this, we normalized the mRNA expression of all the transcripts to the level of tax/rex mRNA (Table 1). The Gag/Pol and env mRNA ratios remained significantly reduced at 0.2 and 0.3, respectively. However, with the exception of the p21Rex transcript (2-fold) we found no significant difference in the relative ratio of these accessory protein coding transcripts in the absence of Rex. Thus, the absence of Rex increased the abundance of the alternatively spliced mRNAs that encode pX region containing open reading frames (ORFs) at the expense of decreasing the gag/pol and env mRNAs, indicating that Rex is essential for overall gene regulation. However, this regulation appears to reflect only a redistribution of these transcripts rather than particularly affecting the expression of these genes as shown here and by Princler et al.32 In addition, it is important to note that in the absence of Rex, the 50–60% reduction of gag/pol mRNA translated to a much more significant reduction in p19 Gag (Fig. 2B). This is consistent with a previous report providing evidence that Rex also facilitates translation of Gag in addition to the regulation of gag/pol mRNA levels.5
Table 1.
Effect of Rex on Steady-State Levels of Human T Lymphotropic Virus Type 1 mRNAs
| |
HTLV-1Rex−/wtHTLV-1 |
|
|---|---|---|
| Viral transcripts | Actuala | Normalized to tax/rex mRNAb |
| gag/pol | 0.5 | 0.2 |
| env | 0.7 | 0.3 |
| tax/rex | 2.1 | 1.0 |
| p21Rex | 4.4 | 2.0 |
| p12 | 1.4 | 0.7 |
| p27 | 2.0 | 1.0 |
| p13 | 2.1 | 1.0 |
| p30 | 1.6 | 0.8 |
| Hbz | 1.5 | 0.7 |
| Total | 2.2 | 1.0 |
Ratio of HTLV-1Rex− over wtHTLV-1.
The mRNA quantities were normalized to tax/rex mRNA and then the ratios were calculated.
HTLV-1, human T lymphotropic virus type 1.
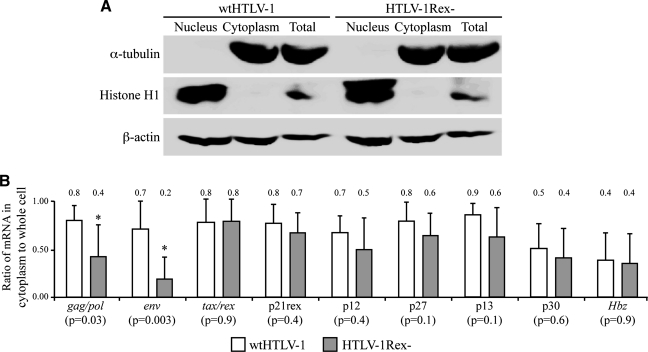
Although Rex has been reported to facilitate the export of the gag/pol and env mRNAs at the expense of the doubly spliced tax/rex mRNA, no studies have evaluated whether Rex mediates the export of the alternatively spliced mRNAs that code for the accessory proteins and the antisense HBZ. To determine whether Rex affects the subcellular distribution of HTLV-1 mRNAs, we quantitated nuclear and cytoplasmic viral mRNAs from wtHTLV-1 or HTLV-1Rex– in 293T cells at 48 h posttransfection. The cells were harvested and lysed in 450 μl hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT) containing 50 μl of 5% NP-40 solution, vortexed at maximum speed for 30 s and placed on ice for 10 min. The suspension was centrifuged at 3000 rpm for 3 min. The supernatant was recentrifuged to remove any residual nuclear fraction. The final supernatant was collected as the cytoplasmic fraction. The nuclear pellet was washed twice with 500 μl of hypotonic buffer. The purity of our nuclear and cytoplasmic fractions was confirmed by Western blot against α-tubulin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), histone H1 (1:1000, Abcam, Cambridge, MA), and β-actin. RNAs prepared from these fractions were quantitated by our HTLV-1 real-time RT-PCR detection assay.31 We detected α-tubulin in cytoplasmic and total cell fractions, histone H1 in nuclear and total cell fractions, and β-actin (loading control) in all the fractions, confirming the purity of the nuclear and cytoplasmic fractionations (Fig. 3A). The ratio of cytoplasmic mRNA over total cellular mRNA (nuclear+cytoplasmic) for each of the viral transcripts was graphed and the ratios of HTLV-1 transfected cells to HTLV-1Rex– transfected cells were compared to determine the role of Rex on cytoplasmic export of each viral transcript. Consistent with previous reports, we showed that the export of gag/pol and env mRNAs was significantly reduced in the absence of Rex (p<0.05), whereas tax/rex mRNA export was not affected. In addition, our results showed no significant effect of Rex on the export of the alternatively spliced viral mRNA encoding accessory proteins p21Rex, p12, p27, p13, p30, and the spliced antisense transcript encoding HBZ. It is important to note that although p12 and p13 mRNA showed a slight reduction in cytoplasmic levels in the absence of Rex, this was not statistically significant. Thus, Rex facilitated the nuclear-cytoplasmic transport of the gag/pol and env viral mRNAs as shown here and by others,5,11 and had no significant effect on the export of alternatively spliced viral mRNAs, including the antisense Hbz mRNA.
FIG. 3.
Cytoplasmic export of viral mRNA in the presence and absence of Rex. (A) 293T cells were transfected with wtHTLV-1 or HTLV-1Rex– proviral clones. Equal numbers of cells were processed for nuclear and cytoplasmic fractionation. The purity of the fractionation was shown by Western blot against α-tubulin, histone H1, and β-actin. Sixty micrograms of protein according to the Bradford assay from each fraction was loaded per lane. (B) RNA was extracted from nuclear and cytoplasmic fractions and quantitated by real-time RT-PCR. Bars represent the percentage of each viral mRNA exported to the cytoplasm in wtHTLV-1 transfected cells (white bar) and HTLV-1Rex– transfected cells (gray bar). Numbers above the bars represent the average value from four independent experiments, each with duplicate transfections. Error bars denote standard deviations. Statistical significance was determined by the analysis of variance (ANOVA) test. *Statistically significant p value. All p values are given in parentheses below each of the mRNAs denoted on the x-axis.
HTLV-1, like other viruses, has evolved alternate splicing mechanisms to generate multiple proteins from the single primary viral transcript. Thus, appropriate regulation of this mechanism is critical for the virus to generate the necessary levels of structural, regulatory, and/or accessory proteins required for active replication as well as immune evasion and persistence. Previous studies showed that both Rex-1 and Rex-2 similarly stabilized the full-length gag/pol and singly spliced env mRNAs and also positively regulated their nuclear-cytoplasmic transport by inhibiting the splicing machinery and facilitating translation, which in turn resulted in efficient structural and enzymatic protein expression and virion production.4,5,11 Therefore, Rex is considered a positive posttranscriptional regulator that controls the switch between early/latent and late/productive viral infection.17 However, the field lacked clear knowledge regarding the regulation of the expression of the accessory genes that are critical for viral persistence in vivo (although dispensable in vitro).24–27 Here our data indicate that unlike the gag/pol and env transcripts encoding the structural and enzymatic proteins, the efficient expression and cytoplasmic export of the alternatively spliced regulatory and accessory transcripts including Hbz (transcribed from the antisense strand of the genome) are not directly dependent on Rex.
All the HTLV-1 transcripts except Hbz contain the RxRE and partial CRS at their 3′ end, while in addition the gag/pol transcript carries a complete CRS and partial RxRE at its 5′ end.10,16,33 It was previously proposed that the CRS retains the intron-containing transcripts in the nucleus until Rex multimerizes on the RxRE and facilitates their cytoplasmic export.13–15 It has also been shown that Rex binding to the RxRE requires an RNA stem bulge-loop secondary structure and an intact upstream splice donor site.33 Both these critical sequences are present only in the unspliced gag/pol mRNA at the 5′ end (r region) and the intron-containing env mRNA, which still contains a highly active and functional splice donor site (nt 4831, Fig. 1). Taking these together, we propose that the gag/pol and env mRNAs are held in the nucleus by the CRS/splice donor requiring Rex-RxRE-dependent cytoplasmic transport. The alternatively spliced regulatory and accessory transcripts, including Hbz, are Rex independent because they lack a known CRS as in the case of Hbz or are devoid of a CRS or an active splice donor site.
The HTLV-1 p30 accessory protein has been shown to suppress viral gene expression and replication by two mechanisms that include (1) the inhibition of Tax-mediated LTR transcriptional activation,34,35 and (2) by interacting with and retaining tax/rex mRNA in the nucleus,30,36 resulting in down-regulation of both positive regulators, Tax and Rex. One recent study also indicated that the p30 forms a complex with Rex and this interaction is enhanced by the presence of tax/rex mRNA.21 In this study we see no significant suppressive affect on Tax expression with the loss of Rex, revealing no apparent increase in p30 suppressive activity. One possible explanation for this is the level of p30 expression. It is known that p30 expression is extremely low in provirus-transfected and virus-infected cells31; thus it is possible that a threshold level of p30 is required before its repressive activity can be detected.
In summary, we provide direct evidence that the expression and cytoplasmic export of the alternatively spliced HTLV-1 mRNAs encoding the accessory proteins and the antisense Hbz mRNA are independent of direct regulation by Rex. This work opens venues for the exploration of Rex-independent regulatory mechanisms of the accessory genes, which play an important role in viral persistence, immune evasion, and clinical latency. Future mutagenesis studies will also be required to determine the precise role of the 3′ RxRE and CRS on the regulation and export of the alternatively spliced mRNAs.
Acknowledgments
We thank Kate Hayes for editorial comments on the manuscript and Tim Vojt for figure preparation. This work was supported by a grant from the National Institutes of Health (CA100730) to P.L.G.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ciminale V. D'Agostino DM. Zotti L, et al. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology. 1995;209:445–456. doi: 10.1006/viro.1995.1277. [DOI] [PubMed] [Google Scholar]
- 2.Ciminale V. Zotti L. D'agostino DM. Chieco-Bianchi L. Inhibition of human T-cell leukemia virus type 2 Rex function by truncated forms of Rex encoded in alternately spliced mRNAs. J Virol. 1997;71:2810–2818. doi: 10.1128/jvi.71.4.2810-2818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidaka M. Inoue J. Yoshida M. Seiki M. Post transcriptional regulator (rex) of HTLV-I initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988;7:519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue J. Itoh M. Akizawa T. Toyoshima H. Yoshida M. HTLV-1 Rex protein accumulates unspliced RNA in the nucleus as well as in cytoplasm. Oncogene. 1991;6:1753–1757. [PubMed] [Google Scholar]
- 5.Kusuhara K. Anderson M. Pettiford SM. Green PL. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J Virol. 1999;73:8112–8119. doi: 10.1128/jvi.73.10.8112-8119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan M. Younis I. D'Agostino DM. Green PL. Functional domain structure of human T-cell leukemia virus type 2 Rex. J Virol. 2003;77:12829–12840. doi: 10.1128/JVI.77.23.12829-12840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nosaka T. Miyazaki Y. Takamatsu T, et al. The post-transcriptional regulator Rex of the human T-cell leukemia virus type I is present as nucleolar speckles in infected cells. Exp Cell Res. 1995;219:122–129. doi: 10.1006/excr.1995.1212. [DOI] [PubMed] [Google Scholar]
- 8.Nosaka T. Siomi H. Adachi Y, et al. Nucleolar targeting signal of human T-cell leukemia virus type I Rex-encoded protein is essential for cytoplasmic accumulation of unspliced viral mRNA. Proc Natl Acad Sci USA. 1989;86:9798–9802. doi: 10.1073/pnas.86.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiki M. Hikikoshi A. Yoshida M. The U5 sequence is a cis-acting repressive element for genomic RNA expression of human T cell leukemia virus type I. Virology. 1990;176:81–86. doi: 10.1016/0042-6822(90)90232-g. [DOI] [PubMed] [Google Scholar]
- 10.King JA. Bridger JM. Lochelt M, et al. Nucleocytoplasmic transport of HTLV-1 RNA is regulated by two independent LTR encoded nuclear retention elements. Oncogene. 1998;16:3309–3316. doi: 10.1038/sj.onc.1201884. [DOI] [PubMed] [Google Scholar]
- 11.Gröne M. Koch C. Grassmann R. The HTLV-1 Rex protein induces nuclear accumulation of unspliced viral RNA by avoiding intron excision and degradation. Virology. 1996;218:316–325. doi: 10.1006/viro.1996.0200. [DOI] [PubMed] [Google Scholar]
- 12.Askjaer P. Jensen TH. Nilsson J. Englmeier L. Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 13.Hakata Y. Yamada M. Shida H. A multifunctional domain in human CRM1 (exportin 1) mediates RanBP3 binding and multimerization of human T-cell leukemia virus type 1 Rex protein. Mol Cell Biol. 2003;23:8751–8761. doi: 10.1128/MCB.23.23.8751-8761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay ME. Holaska JM. Welch K. Paschal BM. Macara IG. Ran-binding protein 3 is a cofactor for Crm1-mediated nuclear protein export. J Cell Biol. 2001;153:1391–1402. doi: 10.1083/jcb.153.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taura T. Krebber H. Silver PA. A member of the Ran-binding protein family, Yrb2p, is involved in nuclear protein export. Proc Natl Acad Sci USA. 1998;95:7427–7432. doi: 10.1073/pnas.95.13.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black AC. Chen IS. Arrigo S, et al. Regulation of HTLV-II gene expression by Rex involves positive and negative cis-acting elements in the 5′long terminal repeat. Virology. 1991;181:433–444. doi: 10.1016/0042-6822(91)90875-c. [DOI] [PubMed] [Google Scholar]
- 17.Younis I. Green PL. The human T-cell leukemia virus Rex protein. Front Biosci. 2005;10:431–445. doi: 10.2741/1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker ALX. Ruland CT. Stephens DW. Black AC. Rosenblatt JD. Human T-cell leukemia virus type 2 Rex inhibits pre-mRNA splicing in vitro at an early stage of spliceosome formation. J Virol. 1996;70:5511–5518. doi: 10.1128/jvi.70.8.5511-5518.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahler AM. Lane WS. Stolk JA. Roth MB. SR proteins: A conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 20.Zahler AM. Neugebauer KM. Lane WS. Roth MB. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- 21.Sinha-Datta U. Datta A. Ghorbel S. Dodon MD. Nicot C. Human T-cell lymphotrophic virus type I Rex and p30 interactions govern the switch between virus latency and replication. J Biol Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 22.Bai XT. Baydoun HH. Nicot C. HTLV-1 p30: A versatile protein modulating virus replication and pathogenesis. Mol Aspects Med. 2010;31:344–349. doi: 10.1016/j.mam.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman LR. Phipps AJ. Montgomery A. Ratner L. Lairmore MD. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: Evidence of in vivo reversion. J Virol. 2004;78:3837–3845. doi: 10.1128/JVI.78.8.3837-3845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold J. Yamamoto B. Li M, et al. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107:3976–3982. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins ND. Newbound GC. Albrecht B, et al. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 26.Hiraragi H. Kim SJ. Phipps AJ, et al. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo. J Virol. 2006;80:3469–3476. doi: 10.1128/JVI.80.7.3469-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valeri VW. Hryniewicz A. Andresen V, et al. Requirement of the human T-cell leukemia virus p12 and p30 genes for infectivity of human dendritic cells and macaques but not rabbits. Blood. 2010;116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimata JT. Wong FH. Wang JJ. Ratner L. Construction and characterization of infectious human T-cell leukemia virus type I molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 29.Ye J. Sileverman L. Lairmore MD. Green PL. HTLV-1 Rex is required for viral spread and persistence in vivo but is dispensable for cellular immortalization in vitro. Blood. 2003;102:3963–3969. doi: 10.1182/blood-2003-05-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younis I. Khair L. Dundr M, et al. Repression of human T-cell leukemia virus type 1 and 2 replication by a viral mRNA-encoded posttranscriptional regulator. J Virol. 2004;78:11077–11083. doi: 10.1128/JVI.78.20.11077-11083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M. Green PL. Detection and quantitation of HTLV-1 and HTLV-2 mRNA species by real-time RT-PCR. J Virol Methods. 2007;142:159–168. doi: 10.1016/j.jviromet.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Princler GL. Julias JG. Hughes SH. Derse D. Roles of viral and cellular proteins in the expression of alternatively spliced HTLV-1 pX mRNAs. Virology. 2003;317:136–145. doi: 10.1016/j.virol.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Black AC. Ruland CT. Yip MT, et al. Human T-cell leukemia virus type II Rex binding and activity requires an intact splice donor site and a specific RNA secondary structure. J Virol. 1991;65:6545–6653. doi: 10.1128/jvi.65.12.6645-6653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael B. Nair AM. Datta A, et al. Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity. Virology. 2006;354:225–239. doi: 10.1016/j.virol.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W. Nisbet JW. Albrecht B, et al. Human T-lymphotropic virus type 1 p30II regulates gene transcription by binding CREB binding protein/p300. J Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicot C. Dundr JM. Johnson JR, et al. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]