Abstract
Reagents for evaluating non-clade B HIV-specific T cell responses are uncommon. Peptides based on highly conserved HIV-1 consensus group M sequences that are phylogenetically closer to most circulating strains may provide potential alternative reagents in populations with diverse infections, and may be relevant for vaccine design. Recognition of such reagents in clade A1-and D-infected populations has not been previously evaluated. Interferon (IFN)-γ ELISpot assay was used to evaluate T cell recognition of Gag and Nef peptides based on consensus group M sequences in 50 treatment-naive adults predominantly infected with HIV-1 clades A1 and D. Gag-induced T cell responses were correlated with gag sequence diversity. Infecting clades were determined from gag sequences for 45 of the 50 subjects as 40% clade A1 (18/45), 45% clade D (20/45), 2% clade C (1/45), 2% A1/C recombinant (1/45), 2% A1/D (1/45), 7% CRF10_CD (3/45), and 2% U (unclassifiable) (1/45). The mean genetic divergence and diversity of clade A and D gag region compared to group M consensus sequences at synonymous and nonsynonymous nucleotide and amino acid levels were not always significant. Gag peptides were targeted at significantly higher frequency [88% (44/50)] than Nef [64% (32/50)]; p=0.014, although their mean IFN-γ magnitudes were comparable ([3703 (95% CI 2567–4839)] vs. [2120 (95% CI 478–3762)]), respectively. Measurable virus-induced IFN-γ responses were detected in 96% (48/50) individuals, primarily targeting the more conserved Gag p24 and Nef central core regions. Use of these reagents to screen for HIV-specific IFN-γ responses may mitigate the challenge of viral diversity; although this targeting is apparently biased toward a few highly conserved epitopes.
Introduction
Group M viruses dominate the global HIV-1 epidemic and comprise 13 circulating subtypes and subsubtypes (A1–A4, B, C, D, F1–F2, G, H, J, and K), over 49 CRFs, in addition to various URFs (http://www.hiv.lanl.gov/content/sequence/HIV/CRFs/CRFs.html, Feb 2011); these occur in distinct geographic locations.1–4 Although the bulk of HIV-1 infection and diversity occurs in sub-Saharan Africa,2,5 detailed virus characterizations have focused on clade B viruses, which predominate in Europe and North America, possibly due to the scarcity of non-clade B virus reagents. Potential immunogens like centralized sequences,6–10 polyvalent mosaic sequences,11–15 or highly conserved sequences16 have been pursued, and in some cases these increased the breadths and magnitudes of responses.11,12 Such prospective immunogens may also be useful for global vaccine design and evaluation.
Most HIV-1-infected individuals have circulating T cells that target epitopes within the Gag and Nef proteins. Use of peptides based on consensus group M sequences to identify such epitopes may circumvent the scarcity of reagents required for use in non-clade B-infected populations. Here, we report findings for the T cell recognition of these reagents by 50 untreated subjects largely infected with HIV-1 clades A1 and D.
Materials and Methods
Study population
Fifty prevalent HIV-infected antiretroviral treatment (ART)-naive consenting adults, consecutively recruited from attendees of The AIDS Support Organisation (TASO) clinic Entebbe, were evaluated at a single time point between August and September 2007. Eligibility was determined from CD4 T cell counts enumerated within 6 months prior to sample collection. All study subjects had CD4 T cell counts ≥350 cells/μl, and provided written informed consent for collection and subsequent analysis of their specimens. The study was approved by the Uganda Virus Research Institute Ethics Review Board and the Uganda National Council of Science and Technology.
Plasma viral load, CD4 T cell count, and HLA analyses
CD4+ T cell counts and HLA class I typing were determined as previously described.17 Plasma viral loads were determined by HIV RNA copy quantification using the Roche Amplicor MONITOR 1.5 protocol (Roche Molecular Systems, Roche, Nutley, NJ). The HIV RNA assay lower detection limit was 400 HIV-1 RNA copies/ml.
HIV peptides and preparation of pools
Peptides were 15 amino acids long, overlapping by 11 amino acids and spanning HIV-1 Gag (#11057; 129 peptides) and Nef (#11055; 53 peptides) consensus group M sequences (https://www.aidsreagent.org/Index.cfm). Up to 10 distinct peptides were pooled at 2 μg/ml per peptide so that each peptide appeared twice in both a protein and a matrix pool to allow for subsequent single peptide mapping. Duplicate wells containing 100,000 peripheral blood mononuclear cells (PBMCs) per well were incubated with peptide pools at 37°C in 5% CO2 in air for 18 h. ELISpot plates were developed using Nova-Red substrate (Vector, Burlingame, CA) according to the manufacturer's protocols. Test wells inducing interferon (IFN)-γ responses in both pools that shared a peptide were considered positive, and were confirmed by retesting. Assay consistency was monitored using cryopreserved PBMCs of known reactivity to human cytomegalovirus, Epstein–Barr virus, and influenza virus (CEF) control peptide pools (NIH AIDS Reference Reagent Repository, #9808) at 1 μg/ml per peptide. Positive controls were two wells containing phytohemagglutinin (PHA)-treated PBMCs. Background response was determined from four wells containing cells in media alone and two wells with media only. Spots were counted using the AID ELISpot Reader system (GMBH, Germany). Virus-induced IFN-γ-secreting T cells were enumerated as spot-forming units per million PBMCs (SFU/106 PBMCs). The test acceptance criteria were ≥300 spots per PHA well, ≤100 spots in all six background wells, and ≤5 spots in the two wells containing media only. Test wells with net response ≥100 SFU/106 PBMCs were considered positive. All ELISpot data are presented as net SFU/106 PBMCs after subtracting three times the mean of the background wells. The magnitude of IFN-γ was defined as the total SFU/106 PBMCs from all responding peptides constituting the evaluated protein. Breadth was defined as the total number of peptides recognized.
DNA extraction and polymerase chain reaction amplification
Viral DNA was extracted from 200 μl of whole blood using the QIAmp DNA Mini Kit protocol (Qiagen Inc, Chatsworth, CA). Gag-specific primers were used in a nested PCR to amplify a 1.7-kb pair gag gene encompassing the p17, p24, and p15 regions, as described elsewhere.18 The amplified products were purified with a QIAquick PCR purification kit (Qiagen, Valencia, CA) and sequenced in house using a Beckman CEQ 8000 automated capillary DNA sequencer. The sequence data were assembled and manually edited using Sequencher 4.7 software (Gene Codes Corporation, Ann Arbor, MI).
Phylogenetic analysis and assignment of infecting clades
Viroblast19 was used to blast nucleotide sequences against the HIV-1 database. Clade classification and recombinant patterns were determined using the REGA subtyping tool20 and further confirmed using phylogenetic analysis. Maximum likelihood phylogenetic reconstruction was performed using PhyML based on the General Time-Reversible model with gamma distributed rate variation among sites. Bootstrap support values were obtained using 500 replicates. The similarity plots were obtained using SimPlot (v3.5.1: S. Ray, Johns Hopkins University, Baltimore, MD) as previously described,21 with a window of 500 nt and steps of 50 nt. Subtype reference sequences at the Los Alamos Database were used to automatically align the generated sequences using MUSCLE v3.722 and were manually refined in MacClade v4.08 software (Sinauer Associates, Inc., Sunderland, MA).
Statistical analysis
Medians with interquartile ranges (IQR), proportions, and correlations were compared using the Mann–Whitney rank, Fisher's exact, and Spearman's rank correlation coefficient tests, respectively. Magnitudes and breadths of Gag (129 peptides) or Nef (53 peptides) recognition were evaluated as means in order to adjust for differences in the number of peptides constituting the protein. Genetic divergence from consensus amino acid sequences was compared using Wilcoxon rank sums tests. Graph Pad 5.0 was used for all the graphical presentations of the data. All analyses were performed using Stata V 10.0 (Stata Corp, TX).
Results
Cohort characteristics and infecting HIV gag clades
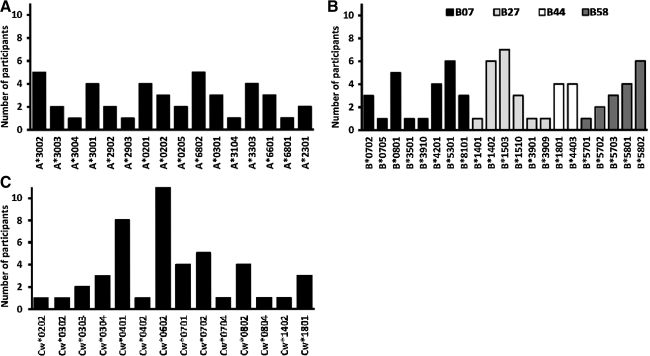
Fourteen males (28%) and 36 females (72%) with median (IQR) enrollment age 37 (31–41) years, CD4 counts 538 (484–647) cells/μl, and plasma viral loads of 15,550 (3450–52,400) RNA copies/ml were evaluated. Subject CD4 counts for clade A1 [551; IQR 510–1046 cells/μl] and D infection [640; IQR 566–816 cells/μl] were comparable. The WHO clinical stages of HIV infection were 24% (12/50), 64% (32/50), and 12% (6/50) stage I, II, and III, respectively. The study population comprised a broad range of host HLA alleles including 16 HLA-A, 21 HLA-B, and 14 HLA-C alleles (Fig. 1A, B, and C), respectively.
FIG. 1.
Distribution of host HLA alleles in the study population. The frequency and distribution of HLA A (A), HLA B (B), and HLA C (C) alleles presenting interferon (IFN)-γ responses in this population is illustrated. HLA B alleles are further classified according to known HLA supertypes B07, B27, B44, and B58.
Five gag specimens failed to amplify; thus, infecting clades were deduced for 45 of the 50 subjects as 40% clade A1 (18/45), 45% clade D (20/45), 2% clade C (1/45), 2% A1/C recombinant (1/45), 2% A1/D recombinant (1/45), 7% CRF10_CD (3/45), and 2% U (unclassifiable). The HIV-1 gag gene DNA sequences from this study were submitted to GenBank under accession numbers HQ702686–HQ702731. Clades A1 and D gag sequence mean divergence and diversity were not significantly different from that of consensus group M sequences at synonymous, nonsynonymous, and amino acid levels. Overall, genetic variation within clade A1 against consensus group M sequences was 8.4% while variation within clade D was 10% (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/aid). These data implied that the clade A1 sequences were phylogenetically closer to the consensus group M gag sequences compared to clade D.
Consensus group M Gag-induced IFN-γ responses
The frequency, magnitudes, and breadths of IFN-γ responses induced by the 129 Gag peptides traversing p17 (34 peptides), p24 (60 peptides), and p15 (35 peptides) sequences were evaluated. HIV-specific IFN-γ responses were detected in all the diverse infecting clades. Gag was targeted by 90% subjects (45/50) at median magnitude 2420 (IQR 580–6445 SFU/106 PBMCs) and breadth of three peptides (IQR 2–5). Measurable IFN-γ responses were detected in 39% (50/129) of the Gag peptides comprising 35.3% (12/34) p17, 62% (31/60) p24, and 20% (7/35) p15 peptides.
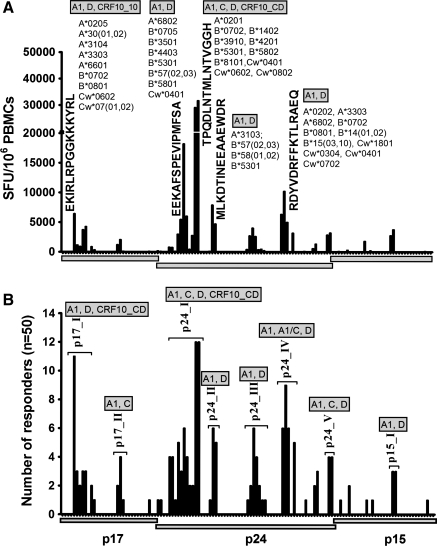
The highest cumulative magnitudes were induced by five peptides: one p17 peptide, Gag17–31 EKIRLRPGGKKKYRL (6,435 SFU/106 PBMCs), and four p24 peptides, Gag160–174 EEKAFSPEVIPMFSA (18,220 SFU/106 PBMCs), Gag180–194 TPQDLNTMLNTVGGH (31,675 SFU/106 PBMCs, Gag200–214 MLKDTINEEAAEWDR (7880 SFU/106 PBMCs), and Gag294–304 RDYVDRFFKTLRAEQ (10,205 SFU/106 PBMCs). Thirty-five subjects targeted at least one of the five peptides; these comprised 18 clade A1, 13 clade D, one clade C, and two CRF10_CD-infected subjects. All five peptides were recognized by clade A and D infection; clade C targeted only peptide Gag180–194 TPQDLNTMLNTVGGH, CRF10_CD infection targeted EKIRLRPGGKKKYRL and TPQDLNTMLNTVGGH peptides, while A1/C infection did not target any of the five peptides (Fig. 2A). These data implied that targeting of the consensus group sequences depended on how well these sequences match the circulating strain.
FIG. 2.
Consensus group M Gag-induced IFN-γ responses. The profile of consensus group M Gag-induced T cell recognition is shown (n=50) illustrating cumulative IFN-γ magnitudes (SFU/106 PBMCs) to individual Gag peptides, sequences of the dominantly targeted peptides, and the circulating subject HLA alleles (A). Recognition of consensus group M Gag peptides clusters into eight regions: Gag17–47 (p17-I), Gag74–95 (p17-II), Gag140–194 (p24-I), Gag196–218 (p24-II), Gag245–283 (p24-III), Gag290–381 (p24-IV), Gag290–381 (p24-V), and Gag429–447 (p15-I) and the proportion of individuals targeting peptides within the Gag clusters are illustrated (B).
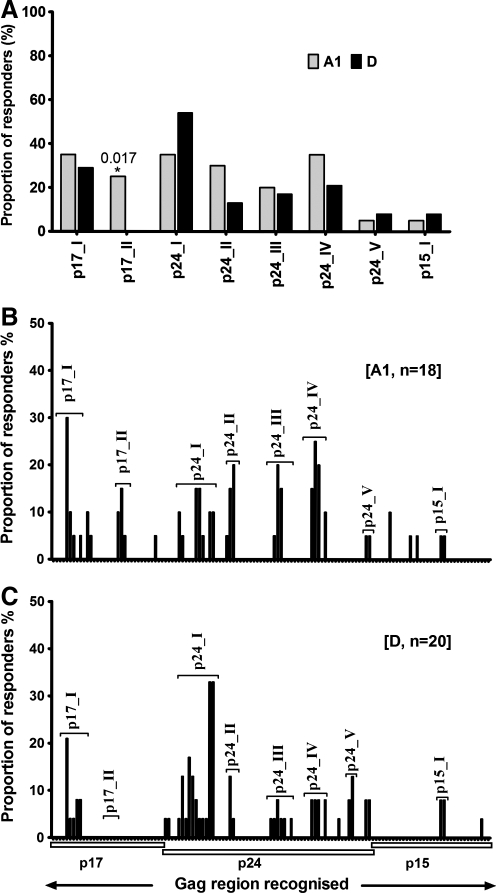
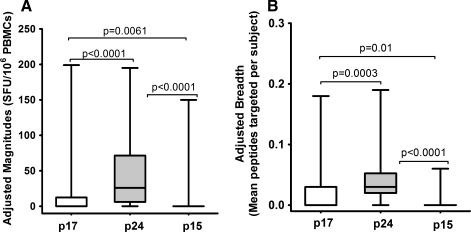
Gag-induced responses clustered in eight regions: Gag17–47 (p17-I), Gag74–95 (p17-II), Gag140–194 (p24-I), Gag196–218 (p24-II), Gag245–283 (p24-III), Gag290–381 (p24-IV), Gag290–381 (p24-V), and Gag429–447 (p15-I) (Fig. 2B and Table 1). Targeting of these clusters did not significantly differ by clade except the p17-II cluster, which was exclusively targeted by clade A1-infected individuals [A1 (5/18) vs. D (0/20)]; p=0.017, Fisher's exact test (Fig. 3A). In line with this, data from multiple alignments of consensus M sequences against A1 and consensus M against D suggested that clade A1 was more similar to group M in the p17 region compared to clade D (Supplementary Tables S2 and S3). In line with this, we observed differential recognition of individual peptides within clusters in both clade A1- and D-infected individuals (Fig. 3B and C), reflecting differences in similarities between clade A1 and D sequences and the group M sequences. Gag p24 induced significantly higher IFN-γ magnitudes (Fig. 4A) and breadths (Fig. 4B) compared to either p17 or p15. Responses were frequently higher when the consensus M peptide sequences matched the autologous sequences (data not shown). These data demonstrated the ability of consensus group M Gag peptides to induce high frequencies of HIV-specific IFN-γ responses in this population, principally focusing on the Gag p24 region.
Table 1.
Recognition of Consensus Group M Gag Clusters by Clade A1 and D Infection
|
Clade A1 (n=18) |
Clade D (n=20) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p17_I (5) | p17_II (3) | p24_I (11) | p24_II (3) | p24_III (7) | p24_IV (5) | p24_V (2) | p15_I (2) | p17_I (5) | p17_II (3) | p24_I (11) | p24_II (3) | p24_III (7) | p24_IV (5) | p24_V (2) | p15_I (2) |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 0 |
| 0 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 1 | 2 | 0 |
| 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 4 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 2 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | ||||||||
| 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | ||||||||
| 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
The table illustrates recognition of the eight gag clusters by clade A1- and D-infected individuals. The numbers of peptides contained in each cluster are indicated in parentheses. Each cell represents the number of peptides targeted within the respective cluster.
FIG. 3.
Gag targeting and infecting HIV-1 clade. The frequency of recognition of consensus group M Gag peptides is compared in clade A (n=20) and D (n=24) infection. Gag T cell recognition clustered in eight regions: Gag17–47 (p17-I), Gag74–95 (p17-II), Gag140–194 (p24-I), Gag196–218 (p24-II), Gag245–283 (p24-III), Gag290–381 (p24-IV), Gag290–381 (p24-V), and Gag429–447 (p15-I). Overall recognition of Gag clusters (A) and recognition by clade A1- (B) and D-infected (C) individuals are illustrated.
FIG. 4.
Gag-induced IFN-γ magnitudes and breadths. The magnitudes and breadths of IFN-γ responses induced by the three major Gag regions p17, p24, and p15 are illustrated. To fairly compare these magnitudes and breadths, the data are presented as means to account for differences in the number of peptides evaluated for Gag p17 (n=34), p24 (n=62), and p15 (n=34). Adjusted magnitudes (A) and adjusted breadths (B) of consensus group M Gag peptide-induced IFN-γ responses are compared. Floating bars represent minima and maxima while the horizontal lines represent medians.
Consensus group M Nef peptide IFN-γ responses and relationship with Gag responses
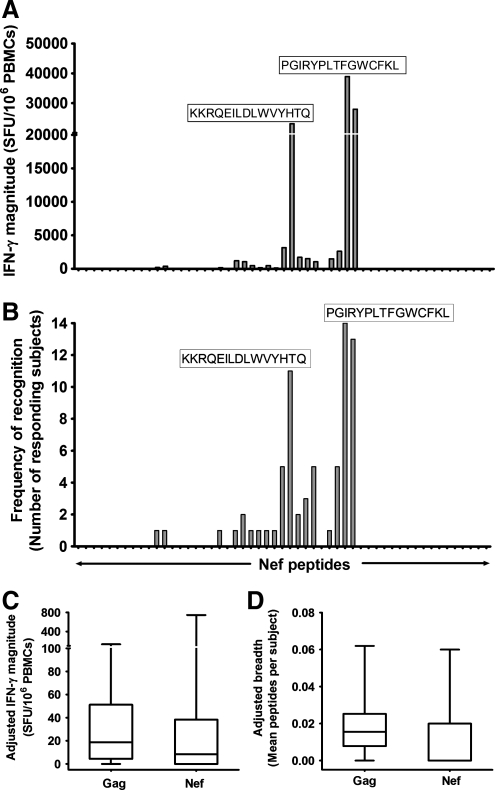
The frequency, magnitudes, and breadths of IFN-γ responses induced by the 53 consensus group M Nef peptides were also evaluated. Measurable Nef-specific IFN-γ responses were detected to 34% (18/53) of Nef peptides in 64% (32/50) of subjects at median magnitude 453 (0–2000 SFU/106 PBMCs) and breadth of 1 peptide (IQR 0–2). The responses focused on the Nef core region and were primarily contributed by two peptides: Nef131–145 PGIRYPLTFGWCFKL [24% (12/50 subjects); cumulative magnitude 38,990 SFU/106 PBMCs] and Nef104–118 KKRQEILDLWVYHTQ [32% (16/50 subjects); cumulative magnitude 23,115 SFU/106 PBMCs] (Fig. 5A and B).
FIG. 5.
Nef-induced IFN-γ responses (A, B) and their relationship to Gag IFN-γ responses (C, D). Cumulative magnitudes (SFU/106 ΠBMX) (A) and frequency of T cell recognition (B) of consensus group M Nef peptides are illustrated (n=50). Individual Nef peptides recognized are shown; sequences of dominant peptides targeted are indicated. To reasonably compare magnitudes and breadths induced by Gag (129 peptides) and Nef (53 peptides), the data were adjusted and presented as means to adjust for differences in the number of peptides evaluated per protein. Adjusted magnitudes (C) and breadths (D) of IFN-γ responses induced by Gag and Nef are shown. Floating bars represent minima and maxima while the horizontal lines represent medians.
Overall, measurable HIV-specific IFN-γ responses to Gag, Nef, or both proteins were induced in 96% of the evaluated subjects. Gag was more frequently targeted than Nef (45/50 vs. 32/50); p=0.01, Fisher's exact test, although the induced magnitudes and breadths did not significantly differ (Fig. 5C and D).
Discussion
HIV-1 genetic variability is a challenge to vaccine development and to the evaluation of virus-induced immune responses. Sub-Saharan Africa bears an inordinate 68% of the HIV-1 disease23 with its circulating clades A, C, D, and the two CRFs, CRF01-AE and CRF02_AG, jointly contributing up to 75% of the global burden.5 Despite this, characterization of non-clade B viruses is limited partly due to the general scarcity of non-clade B reagents. HIV-1 diversity influences rates of virus transmission24 and disease progression,25 highlighting the importance of elucidating clade-associated properties. This possibly paved the way for evaluating HIV-1 vaccine concepts based on geographic region26 and global approaches.10,27 Induction and subsequent detection of virus-induced immune responses are major objectives of HIV vaccine formulations. However, the resulting data may differ with type of reagent used given the high variability of HIV-1 group M viruses.2,28 Here, we used an IFN-γ ELISpot assay to assess recognition of peptides based on HIV-1 group M consensus sequences by HIV-1 clades A1- and D-infected subjects. We sought to determine whether use of these reagents could alleviate challenges of viral diversity and mitigate the scarcity of non-clade B reagents. We demonstrated that the evaluated group M peptide sets detected high frequencies of virus-specific IFN-γ; both Gag and Nef were recognized although Gag peptides and were more frequently targeted. The frequency of recognition of these reagents was comparable in both clade A- and D-infected subjects in line with the fact that the mean divergence and diversity between clades were not significantly different. However, clade A- and D-infected subjects demonstrated differential recognition of consensus group M sequences. This finding was in line with the fact that clade A1 sequences were genetically closer to the group M sequences compared to clade D, and could partly explain the observed limitation in intraclade T cell cross-reactivity among the clade D-infected individuals. These findings are in line with others that used these reagents to demonstrate superior T cell recognition of the more conserved Gag29 and lesser recognition of the more variable Env30 in clade B- and C-infected populations, respectively.
Recognition of the Gag and Nef peptide sets was principally attributed to targeting very few highly conserved peptides encompassing the Gag p24 and Nef core regions, respectively. This is not surprising given that both these regions are reasonably conserved.16,31 Accordingly, these peptide sets were not necessarily better at detecting HIV-specific T cell responses; rather, recognition depended on how well they matched the circulating virus strains. These data support the concept that while a global vaccine is the ideal goal, development of multiple locally representative HIV-1 vaccines may be more practical. Although successful vaccines were developed using conserved antigens, such strategies are challenged by extensive antigenic variability. Conventional HIV-1 vaccine attempts failed to prevent infection or to reduce postinfection viral loads,32,33 although hope was raised by the modest efficacy elicited by the RV144 vaccine.34 Immune pressure on conserved HIV-1 regions induces CTL escape mutations that impair virus fitness and replication35; this paves way for exploration of global vaccine approaches targeting highly conserved epitopes.16,36 Outstanding hurdles still exist, for example, while the STEP trial incorporated highly conserved regions in Gag, Pol, and Nef, it induced responses that were biased toward the less conserved epitopes37 suggesting that the development of several regional vaccines may be more attainable. Consequently, strategies to improve immunological coverage of regionally circulating strains are concurrently under exploration.11,12
Despite such setbacks, T cell HIV-1 vaccine development is crucial given the protective role of T cells remarkably demonstrated in infected individuals that efficiently control viral loads.38 This elite control has been attributed to enrichment with protective HLA class I alleles39 and induction of robust HIV Gag T cell responses.40 Further support is demonstrated by the coinciding of acute infection peak T cell responses with temporally reduction in viremia,41 impacting of early immune pressure on viral set point,42 the association between polyfunctional CD8+ T cells and slow disease progression,43 and the promising data demonstrated in nonhuman primates.44 Though the RV1444 trial failed to induce CD8+ T cells and suggests an antibody correlate, robust CD4 T cell responses were elicited,34 and these are critical for providing help for a durable effective antibody response. However, despite this body of evidence, immune correlates of protection remain unclear given that polyfunctional T cells have not always conferred protection in vaccinees.45 Studies to elucidate valuable correlates of immune protection possibly through systems biology approaches are needed. Monitoring other aspects of the induced T cell responses such as differentiation status, migration patterns, proliferative, and survival capacity as well better understanding of their interplay with transmission routes, host factors, and viral fitness are necessary.
Our study had some unavoidable limitations: first, we did not sequence the nef and therefore could not interpret the relationship between HIV Nef diversity and induction of virus-induced T cell recognition. Second, the T cell recognition we report here possibly reflected an experimental selection of conserved epitopes since it is established that use of autologous sequences detects up to 30% more responses compared to consensus sequences.46 We did not use autologous peptides because their use would have been very costly and impractical, and could not be translated into population strategies for HIV vaccine development. Third, direct comparison of these reagents to consensus clades A, B, C, and D would have allowed for an improved evaluation of the usefulness of the group M peptide sets in screening for HIV-specific T cell responses in this population. Lastly, we used IFN-γ ELISpot to demonstrate T cell cross recognition of these peptide sets. While IFN-γ ELISpot offers a quick screening tool for the detection of activated T cells, IFN-γ responses failed to predict the nonprotective effect of the STEP trial47 and in our study lacked any correlations with viral loads (data not shown). Future studies would need to monitor other parameters of the induced T cell responses likely to predict protection.
Taken together, these data demonstrate that despite the ever-increasing diversity of HIV-1, peptides based on consensus HIV-1 group M sequences can be used to detect high frequencies of HIV-specific IFN-γ responses in a population predominantly infected with HIV-1 clades A1, D, and their associated recombinants. These findings also highlight the limitations of the use of these reagents in that they are biased toward detecting highly conserved epitopes.
Supplementary Material
Acknowledgments
We thank all the study participants and the dedicated clinical and field staff at TASO Entebbe. We thank the Medical Research Council/Uganda Virus Research Institute (MRC/UVRI) Uganda Research Unit on AIDS for enabling access to the study participants of the MRC/UVRI Entebbe cohort. The peptides: HIV-1 Group M Consensus Gag Peptides Set (#11057) and HIV-1 Group M Consensus Nef Peptides Set (#11055) were obtained through the NIH AIDS Research and Reference reagent program, Division of AIDS, NIAID, NIH: HIV-1 Gag Peptides Complete Set and HIV-1 Group M Consensus Nef Peptides Set. The study was funded by the International Atomic Energy Agency, project code RAF6/029, through a grant to the WHO/African AIDS Vaccine programme, with cofunding from the Medical Research Council (MRC UK). This work was also supported in part by a grant from the U.S. public health service to the Computational Biology Core of the University of Washington Center for AIDS research (AI 27757).
This manuscript has not been published in its current form or a substantially similar form. Part of this work was presented as an abstract at the AIDS Vaccine Conference, Paris, 19–22 October 2009. The abstract from this presentation was published as Serwanga J et al., Persistence of robust cross-reactive group M consensus T cell responses in a chronic HIV-1 clade A1 and D-infected Ugandan population. Retrovirology 2009;6(Suppl 3):232 (22 October 2009).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lynch RM. Shen T. Gnanakaran S. Derdeyn CA. Appreciating HIV type 1 diversity: Subtype differences in Env. AIDS Res Hum Retroviruses. 2009;25(3):237–248. doi: 10.1089/aid.2008.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCutchan FE. Global epidemiology of HIV. J Med Virol. 2006;78(Suppl 1):S7–S12. doi: 10.1002/jmv.20599. [DOI] [PubMed] [Google Scholar]
- 3.Taylor BS. Sobieszczyk ME. McCutchan FE. Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;358(April 10):1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi U. Sood V. Banerjee S, et al. Global HIV-1 molecular epidemiology with special reference to genetic analysis of HIV-1 subtypes circulating in North India: Functional and pathogenic implications of genetic variation. Indian J Exp Biol. 2009;47(6):424–431. [PubMed] [Google Scholar]
- 5.Hemelaar J. Gouws E. Ghys PD. Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS. 2011;25(5):679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brander C. Frahm N. Walker BD. The challenges of host and viral diversity in HIV vaccine design. Curr Opin Immunol. 2006;18(4):430–437. doi: 10.1016/j.coi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Fischer W. Liao HX. Haynes BF. Letvin NL. Korber B. Coping with viral diversity in HIV vaccine design: A response to Nickle et al. PLoS Comput Biol. 2008;4(1):e15. doi: 10.1371/journal.pcbi.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F. Weaver EA. Lu Z, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol. 2005;79(2):1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickle DC. Rolland M. Jensen MA, et al. Coping with viral diversity in HIV vaccine design. PLoS Comput Biol. 2007;3(4):e75. doi: 10.1371/journal.pcbi.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver EA. Lu Z. Camacho ZT, et al. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. J Virol. 2006;80(14):6745–6756. doi: 10.1128/JVI.02484-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH. O'Brien KL. Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16(3):319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santra S. Liao HX. Zhang R, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16(3):324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolland M. Jensen MA. Nickle DC, et al. Reconstruction and function of ancestral center-of-tree human immunodeficiency virus type 1 proteins. J Virol. 2007;81(16):8507–8514. doi: 10.1128/JVI.02683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F. Korber BT. Weaver E, et al. Centralized immunogens as a vaccine strategy to overcome HIV-1 diversity. Expert Rev Vaccines. 2004;3(4 Suppl):S161–168. doi: 10.1586/14760584.3.4.s161. [DOI] [PubMed] [Google Scholar]
- 15.Kothe DL. Li Y. Decker JM, et al. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology. 2006;352(2):438–449. doi: 10.1016/j.virol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Rolland M. Nickle DC. Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3(11):e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serwanga J. Shafer LA. Pimego E, et al. Host HLA B* allele-associated multi-clade Gag T-cell recognition correlates with slow HIV-1 disease progression in antiretroviral therapy-naive Ugandans. PLoS One. 2009;4(1):e4188. doi: 10.1371/journal.pone.0004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngandu NG. Bredell H. Gray CM. Williamson C. Seoighe C. CTL response to HIV type 1 subtype C is poorly predicted by known epitope motifs. AIDS Res Hum Retroviruses. 2007;23(8):1033–1041. doi: 10.1089/aid.2007.0024. [DOI] [PubMed] [Google Scholar]
- 19.Deng W. Nickle DC. Learn GH. Maust B. Mullins JI. ViroBLAST: A stand-alone BLAST web server for flexible queries of multiple databases and user's datasets. Bioinformatics. 2007;23(17):2334–2336. doi: 10.1093/bioinformatics/btm331. [DOI] [PubMed] [Google Scholar]
- 20.de Oliveira T. Deforche K. Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–3800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- 21.Lole KS. Bollinger RC. Paranjape RS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73(1):152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS report on the Global epidemic. 2010.
- 24.Kiwanuka N. Laeyendecker O. Quinn TC, et al. HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. AIDS. 2009;23(18):2479–2484. doi: 10.1097/QAD.0b013e328330cc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiwanuka N. Laeyendecker O. Robb M, et al. Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197(5):707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 26.McKnight A. Aasa-Chapman MM. Clade specific neutralising vaccines for HIV: An appropriate target? Curr HIV Res. 2007;5(6):554–560. doi: 10.2174/157016207782418524. [DOI] [PubMed] [Google Scholar]
- 27.Gao F. Liao HX. Hahn BH, et al. Centralized HIV-1 envelope immunogens and neutralizing antibodies. Curr HIV Res. 2007;5(6):572–577. doi: 10.2174/157016207782418498. [DOI] [PubMed] [Google Scholar]
- 28.Frahm N. Nickle DC. Linde CH, et al. Increased detection of HIV-specific T cell responses by combination of central sequences with comparable immunogenicity. AIDS. 2008;22(4):447–456. doi: 10.1097/QAD.0b013e3282f42412. [DOI] [PubMed] [Google Scholar]
- 29.Bansal A. Gough E. Ritter D, et al. Group M-based HIV-1 Gag peptides are frequently targeted by T cells in chronically infected US and Zambian patients. AIDS. 2006;20(3):353–360. doi: 10.1097/01.aids.0000206501.16783.67. [DOI] [PubMed] [Google Scholar]
- 30.Rutebemberwa A. Currier JR. Jagodzinski L, et al. HIV-1 MN Env 15-mer peptides better detect HIV-1 specific CD8 T cell responses compared with consensus subtypes B and M group 15-mer peptides. AIDS. 2005;19(11):1165–1172. doi: 10.1097/01.aids.0000176216.02743.98. [DOI] [PubMed] [Google Scholar]
- 31.Yang OO. Candidate vaccine sequences to represent intra- and inter-clade HIV-1 variation. PLoS One. 2009;4(10):e7388. doi: 10.1371/journal.pone.0007388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455(7213):613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston MI. An HIV vaccine-evolving concepts. N Engl J Med. 2007;356(20):2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 34.Rerks-Ngarm S. Pitisuttithum P. Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 35.Wang YE. Li B. Carlson JM, et al. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J Virol. 2009;83(4):1845–1855. doi: 10.1128/JVI.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letourneau S. Im EJ. Mashishi T, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007;2(10):e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F. Finnefrock AC. Dubey SA, et al. Mapping HIV-1 vaccine induced T-cell responses: Bias towards less-conserved regions and potential impact on vaccine efficacy in the Step Study. PLoS One. 2011;6(6):e20479. doi: 10.1371/journal.pone.0020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker BM. Block BL. Rothchild AC. Walker BD. Elite control of HIV infection: Implications for vaccine design. Expert Opin Biol Ther. 2009;9(1):55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emu B. Sinclair E. Hatano H, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82(11):5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura T. Brockman MA. Schneidewind A, et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol. 2009;83(6):2743–2755. doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koup RA. Ho DD. Shutting down HIV. Nature. 1994;370(6489):416. doi: 10.1038/370416a0. [DOI] [PubMed] [Google Scholar]
- 42.Brockman MA. Brumme ZL. Brumme CJ, et al. Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated for in chronic infection. J Virol. 2010;84(22):11937–11949. doi: 10.1128/JVI.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betts MR. Nason MC. West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen SG. Vieville C. Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts MR. Exley B. Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci USA. 2005;102(12):4512–4517. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altfeld M. Addo MM. Shankarappa R, et al. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J Virol. 2003;77(13):7330–7340. doi: 10.1128/JVI.77.13.7330-7340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchbinder SP. Mehrotra DV. Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert PB. Rossini AJ. Shankarappa R. Two sample tests for comparing intra-individual genetic sequence diversity between populations. Biometrics. 2005;61(1):106–117. doi: 10.1111/j.0006-341X.2005.020719.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.