Abstract
Our objective was to dynamically observe changes in peripheral blood Th17, Treg cells, and interleukin (IL)-17 levels in HIV-1/AIDS patients before and after highly active antiretroviral therapy (HAART). The study design consisted of a randomized case-controlled study. A total of 33 HIV-1/AIDS patients were chosen to receive a HAART regimen and 30 healthy volunteers were assigned as controls. Peripheral blood Th17 and Treg cells were measured by flow cytometry before or 6 and 12 months after HAART therapy. The plasma IL-17 level was determined by ELISA. The percentage of Th17 cells to total CD4+ cells was 1.2±0.37% in HIV/AIDS patients before treatment, which was significantly lower than that in uninfected controls (4.7±1.43%). After HAART therapy for 6 or 12 months, the Th17 percentage increased to 2.5±1.03% and 3.7±1.56%, respectively. The percentage of Treg cells to CD4+ cells is 9.16±3.33% in HIV/AIDS patients, which was significantly elevated compared to controls (4.43±0.97%). HAART therapy for 6 and 12 months significantly decreased Treg cell percentage (7.19±2.91% and 5.53±1.88%, respectively). Interestingly, the ratio of Th17/Treg cells was significantly decreased in HIV/AIDS patients before treatment, while HAART treatment partially normalized the Th17/Treg ratio. IL-17 levels were 5.3±2.5 and 17.7±6.60 pg/ml in HIV/AIDS patients and controls, respectively; the HAART regimen increased the IL-17 level to 7.7±2.4 and 10.4±3.1 pg/ml at 6 and 12 months, respectively. The percentage of Th17 cells correlated with IL-17 level, but both negatively correlated with viral load before treatment, whereas the percentage of Treg cells positively correlated with viral load before HAART therapy. The imbalance of peripheral blood Th17 and Treg cells may play a crucial role in the pathogenesis of AIDS. HAART can restore the balance of Th17 and Treg cells as well as the IL-17 level, which may gradually rebuild the immune equilibrium in HIV/AIDS patients.
Introduction
The deletion of CD4+ T cells is a leading pathological characteristic of human immunodeficiency virus (HIV) infection. In the acute phase of HIV infection that covers the first few weeks to several months, high levels of viral replication lead to infection and deletion of CD4+ T cells in lymphoid tissue.1,2 Acute HIV infection usually progresses over time to clinically latent HIV infection, early symptomatic HIV infection, and finally to AIDS.3,4 In the chronic symptomatic phase, HIV also leads to chronic immune injury (acquired immunodeficiency syndrome), which directly and indirectly destroys a large number of CD4+ T cells. At the same time, immune injury is generally activated, which progressively destroys the immune system, reduces its regenerative capacity, and ultimately leads to failure of the immune system and the occurrence of opportunistic infections and tumors.5
Helper T cell 17 (Th17) and regulatory T cell (Treg) were two subsets of CD4+ T cells. Th17 cells produce interleukin (IL)-17, IL-21, and IL-22, which are important in the maintenance of intact epithelium and host defense functions against extracellular bacteria and fungi infection.6 For example, in mice, IL-17 has been shown to reduce systemic dissemination of bacterial infection from the intestine,7 while IL-22 induces the production of antibacterial defensins.3 Recent studies have indicated that Th17 cells are preferentially deleted during SIV infection, which may be associated with disease progression.8 Also, Th17 cells may play a role in HIV pathogenesis and antiretroviral therapy-induced immune reconstitution in the gut mucosa.4
Recently, the proportions of both circulating and lymphoid Treg cells were found to correlate with the viral load of AIDS patients.1,9 In vitro studies further demonstrated that Treg cells could contribute to HIV/SIV pathogenesis by suppressing HIV-specific immune responses.5,10 It was suggested that the direct interaction between HIV and Treg cells would drive the accumulation of Treg cells in lymphoid tissues and facilitate disease progression, though conflicting results were reported.2,11 The Th17 and Treg cells are closely related to each other in both cell development and differentiation.12 However, whether the balance between Th17 and Treg correlates with the progression and prognosis of HIV infection has not been fully addressed.
There is currently no vaccine available for HIV. The only known methods of prevention are based on avoiding exposure to the virus. In the event an individual is significantly exposed to the virus, an antiretroviral treatment will be given directly. Highly active antiretroviral therapy (HAART) is currently the most effective treatment to control AIDS progression. Conventional regimens consist of two nucleoside analogue reverse transcriptase inhibitors (NARTIs or NRTIs) plus either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor (NNRTI). HAART treatment functions by controlling viral replication and reducing viral load, preventing worsening symptoms of immune deficiency, slowing disease progression, and ultimately decreasing opportunistic infections and tumors.13,14 We therefore hypothesized that the therapeutic effect of HAART is involved in the regulation of the immune system through the balance of Th17 and Treg cells.
In this study, we observed the dynamic changes in percentages of peripheral blood Th17 and Treg cells, viral load, and IL-17 level in HAART-treated HIV/AIDS patients before therapy or 6 and 12 months after therapy. We found that an imbalance of systemic Th17 and Treg cells may contribute to the pathogenesis of AIDS while HAART treatment can help restore the balance.
Materials and Methods
Case selection
Patients
Thirty-three HIV-1/AIDS patients (13 females and 20 males) with an average age of 40±12 years were randomly recruited at Hunan Provincial Center for Disease Control and Prevention from January 2008 to January 2009. Thirty healthy volunteers who were screened as HIV negative (13 females and 17 males) with an average age of 33±15 years were recruited during the same period as uninfected controls. All of the AIDS patients were diagnosed according to the “AIDS Diagnose and Treatment Guidelines” (National Ministry of Health, China, 2007). All patients were confirmed as HIV-1 positive by Western blot. All of the recruited HIV/AIDS patients were antiviral drug naive at the acute phase to early symptomatic HIV infection. AIDS patients and controls with a history of high blood pressure, coronary heart disease, diabetes, stroke, infection, or tumor were excluded from the study. All of the 33 AIDS patients were treated with the conventional HAART regimen: zidovudine, 0.3 g, twice per day; lamivudine, 0.3 g, once per day; and nevirapine, 0.2 g, twice per day. The study was approved by the Ethics Committee of the Central South University. Signed informed consent forms were obtained from all subjects who participated in the study.
Follow-up visits
Patients were scheduled to visit the clinic routinely. The examination of peripheral blood viral load, Th17 and Treg amount, and IL-17 level took place every 6 months up to a year.
Endpoints
The primary endpoints are the amount of peripheral blood CD4+ T cells, the percentages of Th17 and Treg cells, the level of peripheral blood IL-17, and viral load.
Laboratory tests
Sample collection
Eight milliliters of fasting blood was collected in an anticoagulant tube from each subject. Plasma and peripheral blood mononuclear cells (PBMCs) were separated with lymphocyte separation reagents. Cell morphology was observed and the number was counted under a microscope. Plasma was frozen at –80°C.
CD4+ T and CD8+ T cells and CD4+ subsets sorting
The absolute counts (cells/μl) of mature human T lymphocytes (CD3+), helper/inducer (CD3+CD4+) T lymphocytes, and suppressor/cytotoxic (CD3+CD8+) T lymphocytes in erythrocytosed whole blood were identified and determined using the CD3/CD4/CD8 Tritest kit (BD Biosciences, CA) by following the manufacturer's manual.
Th17 and Treg cells were sorted by flow cytometry. To sort Th17 cell, 106 cells were mixed with 2.5 μg of Jiarufobo acetate (PMA), 1 μg of ionomycin, and 0.7 pg of monensin, followed by incubation with FITC antihuman CD3 monoclonal antibody (mAb) and phycoerythrin (PE) antihuman CD4 mAb at 37°C for 5 h in the dark. After the cells were rinsed with phosphate-buffered saline (PBS) three times, 100 μl of fixative solution (4% polymerisatum) and 1 ml of permeabilization buffer (R&D systems China Co. Ltd., Shanghai, China) were added to the cells followed by incubation at 37°C for 1 h in the dark, respectively. Cells were then incubated with Alexa Fluor 647-labeled antihuman IL-17 mAb for 2 h at room temperature followed by washing with permeabilization buffer three times and suspension in 300 μl of fixative solution.
To sort Treg cells, each group of 106 cells was incubated with FITC antihuman CD4 mAb and APC antihuman CD25 mAb for 1 h in the dark, and then rinsed with PBS and incubated with fixative and permeabilization solutions as described above. After the cells were incubated with PE antihuman Foxp3 mAb for 2 h at room temperature, they were rinsed with permeabilization solution and suspended in 200 μl of fixative solution. Th17 or Treg cells were sorted with MoFlo flow cytometry (Dako, CA) and data were analyzed by FACS Express 3.0 software (De Novo Software, FL).
ELISA detection of IL-17
The plasma IL-17 level was detected by an ELISA kit (Bio-Rad Laboratories, CA). Sample absorbance was obtained with a microplate reader, and data were analyzed with CurveExpert1.3 software (Microsoft, WA).
Plasma viral load
Plasma viral load was detected with an HIV-RNA quantitative PCR kit (PG Biotech, Shenzhen, China) by following the factory manual. The sensitivity of this kit makes it possible to detect about 500 virus copies. Real-time PCR was performed with a Light Cycler PCR System (Roche R&D Center Ltd., Shanghai, China).
Statistical analysis
All data were analyzed using SPSS 17.0 software. Results were expressed as mean±SD. Differences between two groups were analyzed by t-test and correlations were analyzed by Spearman rank test. A p<0.05 was considered statistically significant.
Results
Dynamic changes in HIV viral load
All of the recruited subjects finished the study. Results from the real-time PCR showed that the average HIV viral load in HIV/AIDS patients was 4.62±1.09×106 before HAART treatment. The viral load fell rapidly below the detection limit of the kit after HAART treatment at 6 and 12 months. No HIV infection was detected in the control subjects.
Changes of peripheral blood T cells and subsets in HIV/AIDS patients
Before treatment, the amount of peripheral blood CD4+ T cells was significantly lower in HIV/AIDS patients compared to the uninfected controls. In contrast, the amount of CD8+ T cells was relatively higher in AIDS patients, but without statistical difference. CD4+ T cells were significantly increased at 6 and 12 months after HAART treatment, but were still lower compared to controls (Table 1). The blood CD4+ T cells negatively correlated with HIV viral load in HIV/AIDS patients before treatments (R=–0.502, p=0.001).
Table 1.
Comparison of T Cell Subsets in Peripheral Blood 
| Groups | CD4+T cells | p value | CD8+T cells | p value | ||
|---|---|---|---|---|---|---|
| Uninfected control | 775±150 | 512±195 | ||||
| HIV/AIDS | 201±94 | <0.001* | 851±327 | >0.05* | ||
| HAART 6 month | 298±137 | <0.001* | <0.001† | 783±294 | >0.05* | >0.05† |
| HAART 12 month | 384±384 | 0.033* | 0.001‡ | 745±232 | >0.05* | >0.05‡ |
Cell count per μl.
*p vs. uninfected control; †p vs. HIV/AIDS; ‡p vs. HAART 6 month.
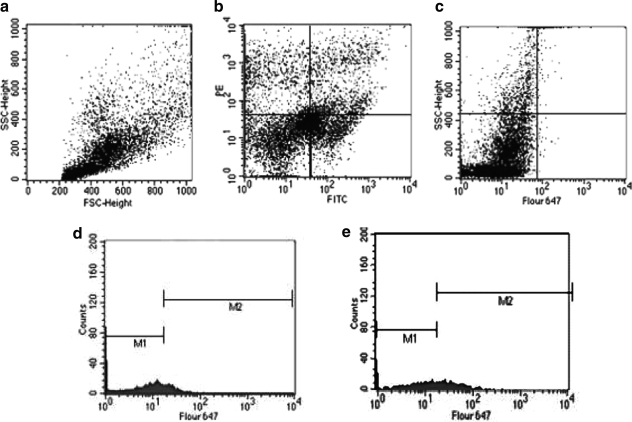
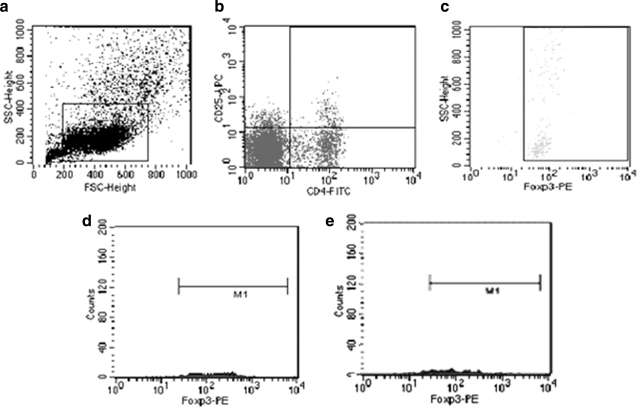
Peripheral blood Th17 cells were sorted by FACS, and the percentage of Th17 cells to CD4+ T cells was significantly reduced in HIV/AIDS patients compared to controls (Fig. 1). After 6 or 12 months of HAART therapy, Th17 cells were significantly increased (Table 2). Peripheral blood Treg cells were sorted by FACS, and the percentage of Treg cells to CD4+ T cells was significantly elevated in HIV/AIDS patients compared to controls (Fig. 2). HAART gradually decreased Treg cell percentage in patients at 6 and 12 months (Table 3). The percentage of Th17 cells was negatively associated with viral load (R=–0.32, p=0.038), but the percentage of Treg cells positively correlated with viral load before treatments (R=0.56, p=0.019).
FIG. 1.
Representative FACS analysis of Th17 cells. (a) Flow cytometric dot plots of blood lymphocytes. (b) Flow cytometric dot plots of CD3-FITC+ and CD4-PE+ cells. (c) Dot plots of IL-17-Fluor 647 on gated CD3-FITC+ and CD4-PE+ cells. (d) Histogram shows IL-17-Fluor 647 staining on gated cells from AIDS patient (d) and uninfected control (e).
Table 2.
Comparison of Percentage of Th17 Cells in Peripheral Blood 
| Groups | Th17 cells (%) | p value | |
|---|---|---|---|
| Uninfected control | 4.7±1.43 | ||
| HIV/AIDS | 1.2±0.37 | <0.001* | |
| HAART 6 month | 2.5±1.03 | <0.001* | <0.001† |
| HAART 12 month | 3.7±1.56 | 0.033* | 0.001‡ |
p vs. uninfected control; †p vs. HIV/AIDS; ‡p vs. HAART 6 month.
FIG. 2.
Representative FACS analysis of Treg cells. (a) Flow cytometric dot plots of blood lymphocytes. (b) Flow cytometric dot plots of CD4+-FITC and CD25+-APC cells. (c) Dot plots of Foxp3-PE+ cells on gated CD4+-FITC and CD25+-APC cells. Histogram shows Foxp3-PE+ staining of gated cells from AIDS patient (d) and uninfected control (e).
Table 3.
Comparison of Percentage of Treg Cells in Peripheral Blood 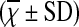
| Groups | Treg cells (%) | p value | |
|---|---|---|---|
| Uninfected control | 4.43±0.97 | ||
| HIV/AIDS | 9.16±3.33 | <0.001* | |
| HAART 6 month | 7.19±2.91 | <0.001* | 0.041† |
| HAART 12 month | 5.53±1.88 | 0.018* | 0.021‡ |
p vs. uninfected control; †p vs. HIV/AIDS; ‡p vs. HAART 6 month.
To reflect the changes in the balance between Th17 and Treg cells, we further calculated the ratio of Th17/Treg cells (Table 4). The ratio of Th17 to Treg in uninfected subjects and HIV/AIDS patients is 1.91±0.33 and 0.20±0.04, respectively. HIV/AIDS infection resulted in about a 10-fold decrease in the ratio of Th17 to Treg cells. The ratio was significantly restored at 6 or 12 months (0.47±0.05 and 0.77±0.10, respectively) post-HAART treatment. The Th17 to Treg ratio significantly correlated with the viral load in HIV/AIDS patients before treatment (R=0.611, p<0.0001).
Table 4.
Comparison of Peripheral Blood Th17/Treg Ratio 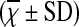
| Groups | Th17/Treg | p value | |
|---|---|---|---|
| Uninfected control | 1.91±0.33 | ||
| HIV/AIDS | 0.20±0.04 | <0.001* | |
| HAART 6 month | 0.47±0.05 | <0.001* | <0.001† |
| HAART 12 month | 0.77±0.10 | 0.042* | 0.002‡ |
p vs. uninfected control; †p vs. HIV/AIDS; ‡p vs. HAART 6 month.
Dynamic changes in plasma IL-17 level
As shown in Table 5, plasma IL-17 levels were significantly decreased in HIV/AIDS patients compared to controls before treatment. HAART therapy caused significant increases in the IL-17 level 6 or 12 months after treatment (Table 4). The plasma IL-17 level positively correlated with the percentage of peripheral blood Th17 cells in HIV/AIDS patients (R=0.670, p=0.037 before treatment; R=0.872, p=0.027 6 months after HAART; R=0.554, p=0.019 12 months after HAART).
Table 5.
Comparison of Serum IL-17 Concentration 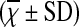
| Groups | IL-17 (pg/ml) | p value | |
|---|---|---|---|
| Uninfected control | 17.7±6.60 | ||
| HIV/AIDS | 5.3±2.5 | <0.001* | |
| HAART 6 month | 7.7±2.4 | <0.001* | <0.001† |
| HAART 12 month | 10.4±3.1 | <0.001* | <0.001‡ |
p vs. uninfected control; †p vs. HIV/AIDS; ‡p vs. HAART 6 month.
The correlation between peripheral blood Th17, Treg, and CD4+ T cells
In HIV/AIDS patients, the percentage of peripheral blood Th17 cells positively correlated with CD4+ T cells, whereas the percentage of Treg cells negatively correlated with CD4+ T cells before treatment or 6 and 12 months after HAART therapy. Also, Th17 cells negatively correlated with Treg cells before treatment or 6 and 12 months after HAART regimens (Table 6).
Table 6.
Correlation Between Accounts of Th17, Treg, and CD4+ T Cells
| |
Th17 and Treg |
Th17 and CD4+ |
Treg and CD4+ |
|||
|---|---|---|---|---|---|---|
| Group | R value | p value | R value | p value | R value | p value |
| HIV/AIDS | −0.298 | 0.007 | 0.543 | 0.031 | −0.397 | 0.048 |
| HAART 6 month | −0.520 | <0.001 | 0.506 | 0.044 | −0.558 | 0.035 |
| HAART 12 month | −0.750 | 0.029 | 0.660 | 0.030 | −0.640 | 0.026 |
Discussion
It is well established that HIV destroys CD4+ T cells, which progressively damages the immune system. Recent studies have suggested that an imbalance between Th17 and Treg subsets of CD4+ T cells is involved in infections, cancers, and autoimmune diseases.15 Loss of balance between Th17 and Treg cells was also observed in chronic HIV infection.16 However, there is a lack of evidence on whether regulation of Th17/Treg balance could be a target of HIV treatment. HAART is currently the most effective treatment to control AIDS progression. However, no report addresses whether HAART treatment works by regulating the balance of Th17/Treg cells. In particular, the dynamic changes of CD4+ cell subsets after HAART therapy have been rarely reported. In this study, we found that HIV infection causes an imbalance of Th17/Treg cells at the acute phase to early symptomatic HIV infection, while HAART can restore the balance. The correlation of dynamic restoration of Th17/Treg balance with a significant decrease in viral load after HAART treatment suggests that regulation of Th17/Treg balance could be a target of AIDS treatment.
How Th17 cells are selectively deleted during pathogenic HIV infection is not currently fully understood. As the preferential Th17 deletion occurs quite quickly after acute SIV infection in nonhuman primates, this strongly suggests that destruction of existing Th17 cells must occur.17,18 Cecchinato et al. first reported that Th17 cells were infected by SIV and that deletion of Th17 cells in mucosal tissues strongly correlated with viral load.19 The following studies further demonstrated that resting systemic CD4+ T cells expressing high levels of α4β7 integrin, a gut homing marker that acts as a coreceptor for HIV, were preferentially infected and deleted during SIV infection.20 Moreover, peripheral blood memory CD4+ T cells expressing chemokine receptor 6 (CCR6) displayed increased susceptibility to viral infection21 and HIV infection causes decreased cell surface expression of CCR5 on Th17 cells.22 Our study also demonstrated that the Th17 population significantly correlated with the viral load before HAART treatment and the viral load fell rapidly below the detection limit after therapy. These facts suggested that a direct destruction of HIV-infected Thl7 cells might be the main reason for the reduced Th17 population in AIDS patients.
In addition to the direct destruction by HIV infection, Th17 cells could be deleted by viral and/or nonviral bystander mechanisms, such as changes in environmental cytokines. Chronic immune activation and inflammation during HIV disease progression could lead to differentiation of Th17 to Treg and apoptosis of noninfected Th17 cells.23 Th17 cells are proinflammatory cells and are critical in the defense against bacteria and fungi, and also contribute to the homeostasis of enterocytes.24 In addition, IL-17 is the cytokine mainly secreted by Th17 cells and serves to maintain the integrity of the mucosal barrier.25 Our study revealed that the plasma IL-17 level positively correlated with the percentage of Th17 cells and negatively correlated with viral load in HIV/AIDS patients. Deletion of Thl7 cells and deficiency of IL-17 reduce the host cells' ability to defend against bacteria, resulting in destruction of the mucosal barrier, leading to local immune activation and accelerating virus replication, and ultimately accelerating the deletion of Th17 and CD4+ T cells followed by the collapse of the immune system.16,23
Regulatory T (Treg) cells were reported to suppress the effector T cell response, the function of APCs, and CD4+ and CD8+ effector T cells in chronic infection.26 In addition, it was suggested that they suppress a protective anti-HIV role by mediating the suppression of HIV-specific CD4+ T cell proliferation and the cytolytic activity of CD8+ T cells.27 Accompanying the profound loss of Th17 cells, Treg cells are observed to gradually decline during AIDS progression.28,29 This implies that Treg cells exhibit a survival advantage over Th17 cells underlying AIDS progression. A recent study suggested that the balance between Th17 and Treg may be critical in HIV pathogenesis.16 Interestingly, HIV-1-infected long-term nonprogressors showed Th17 and cycling subsets comparable to uninfected controls.4 This suggests that a stable level of Th17 cells and Treg cells is beneficial to the control of HIV progression.
In this study, the percentage of Treg in total CD4+ T cells was significantly increased in HIV/AIDS patients before treatment. Accompanied by a significant decrease in viral load after HAART therapy, the percentage of Treg cells was also significantly reduced. In addition, the ratio of Th17 to Treg cells was significantly decreased in HIV/AIDS patients and HAART treatment significantly restored the ratio. The Th17 to Treg ratio correlated with viral load before HAART therapy. Therefore, we propose that the Th17 to Treg ratio might more accurately reflect the balance between Th17 and Treg cells. Importantly, peripheral blood Th17 cells negatively correlated with Treg cells before treatment or 6 and 12 months after HAART therapy. Therefore, the balance of peripheral blood Th17 and Treg cells may be a critical parameter for the prognosis of HIV infection.
HAART therapy can suppress viral replication, lower the viral load, protect CD4+ T cells from destruction, and gradually restore the immune system.13,30 In this study, we found that HAART therapy gradually increased the percentage of peripheral blood Th17 cells in CD4+ T cells and gradually decreased the percentage of Treg cells. The mechanism of how HAART regulates Treg and Th17 cell levels is not clear. We hypothesize that antiviral treatment decreases the viral load, which reduces the direct destruction of Th17 cells by HIV. In addition, HIV/AIDS infection increased peripheral blood CD8+ T cell count (Table 1). Cytolytic CD8+ T cells may destroy the HIV-infected Th17 cells. HAART therapy reduced CD8+ T cells, which may be associated with the restoration of Th17 cells after HAART therapy. Also, HAART may alter the cellular environment to favor the differentiation of CD4+ T cells to Th17 cells and inhibit the generation of Treg cells. IL-17 is a proinflammatory cytokine mainly produced by Th17 cells. However, other cells, such as Vdelta1 T cells, can also make IL-17 in vivo during HIV infection. In this study, the IL-17 levels significantly correlated with the level of systemic Th17 cells. HAART therapy significantly increased IL-17 levels 6 and 12 months after treatment. This suggests that the preinflammatory environment was improved and cytokines, such as IL-17, may stimulate Th17, but inhibit Treg cell proliferation.31,32 Our study suggests that HIV/AIDS patients' immune imbalance can be gradually restored by HAART therapy.
In conclusion, we found a significant loss of CD4+ T cells and an imbalance between Th17 cells and Treg cells in the peripheral blood of HIV/AIDS patients, suggesting that the imbalance of Treg cells and Th17 cells plays an important role in HIV/AIDS pathogenesis. HAART helped to restore the Th17/Treg balance as well as IL-17 level in the patients. The balance of Th17/Treg could be a target for the development of new therapeutic strategies and vaccines for HIV/AIDS treatment.
Acknowledgments
This work was supported by the National Key Science Foundation of China (Grant 2008ZX10005-003).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lim A. Tan D. Price P. Kamarulzaman A. Tan HY. James I, et al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS. 2007;21:1525–1534. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 2.Mason RD. De Rose R. Kent SJ. CD4+ T-cell subsets: What really counts in preventing HIV disease? Expert Rev Vaccines. 2008;7:155–158. doi: 10.1586/14760584.7.2.155. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang W. Kolls JK. Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccone EJ. Greenwald JH. Lee PI. Biancotto A. Read SW. Yao MA, et al. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1 infected long-term non-progressors. J Virol. 2011 Apr 6; doi: 10.1128/JVI.02643-10. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinter AL. Horak R. Sion M. Riggin L. McNally J. Lin Y, et al. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res Hum. Retroviruses. 2007;23:438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 6.Miossec P. Korn T. Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 7.Raffatellu M. Santos RL. Verhoeven DE. George MD. Wilson RP. Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecchinato V. Trindade CJ. Laurence A. Heraud JM. Brenchley JM. Ferrari MG, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson J. Boasso A. Nilsson J. Zhang R. Shire NJ. Lindback S, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 10.Kinter A. McNally J. Riggin L. Jackson R. Roby G. Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA. 2007;104:3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson J. Boasso A. Velilla PA. Zhang R. Vaccari M. Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver CT. Harrington LE. Mangan PR. Gavrieli M. Murphy KM. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhou HY. Zheng YH. He Y. Chen Z. Liu M. Yin W. Liu C. Evaluation of a 6-year highly active antiretroviral therapy in Chinese HIV-1-infected patients. Intervirology. 2010;53:240–246. doi: 10.1159/000302762. [DOI] [PubMed] [Google Scholar]
- 14.Grulich AE. Cancer: The effects of HIV and antiretroviral therapy, and implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4:183–187. doi: 10.1097/COH.0b013e328329c5b2. [DOI] [PubMed] [Google Scholar]
- 15.Kryczek I. Wei S. Zou L. Altuwaijri S. Szeliga W. Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 16.Prendergast A. Prado JG. Kang YH. Chen F. Riddell LA. Luzzi G, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 17.Brenchley JM. Paiardini M. Knox KS. Asher AI. Cervasi B. Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favre D. Lederer S. Kanwar B. Ma ZM. Proll S. Kasakow Z, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchinato V. Trindade CJ. Laurence A. Heraud JM. Brenchley JM. Ferrari MG, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kader M. Wang X. Piatak M. Lifson J. Roederer M. Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro P. Gosselin A. Wacleche VS. El-Far M. Said EA. Kared H, et al. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin {beta}7. J Immunol. 2011;186:4618–4630. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 22.El Hed A. Khaitan A. Kozhaya L. Manel N. Daskalakis D. Borkowsky W, et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201:843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanwar B. Favre D. McCune JM. Th17 and regulatory T cells: Implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins SC. Jarnicki AG. Lavelle EC. Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: Role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 25.Ye P. Garvey PB. Zhang P. Nelson S. Bagby G. Summer WR, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson J. Boasso A. Velilla PA. Zhang R. Vaccari M. Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chougnet CA. Shearer GM. Regulatory T cells (Treg) and HIV/AIDS: Summary of the September 7–8, 2006 workshop. AIDS Res Hum Retroviruses. 2007;23:945–952. doi: 10.1089/aid.2006.0259. [DOI] [PubMed] [Google Scholar]
- 28.Oswald-Richter K. Grill SM. Shariat N. Leelawong M. Sundrud MS. Haas DW, et al. HIV Infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggena MP. Barugahare B. Jones N. Okello M. Mutalya S. Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 30.Kader M. Bixler S. Piatak M. Lifson J. Mattapallil JJ. Anti-retroviral therapy fails to restore the severe Th-17: Tc-17 imbalance observed in peripheral blood during simian immunodeficiency virus infection. J Med Primatol. 2009;38:32–38. doi: 10.1111/j.1600-0684.2009.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klatt NR. Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lishomwa C. Ndhlovu Joan M. Snyder-Cappione JE. Pagán M, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS. 2008;22:990–992. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]