Abstract
Epidemiological studies show association between sleep duration and lipid metabolism. In addition, inactivation of circadian genes induces insulin resistance and hyperlipidemia. We hypothesized that sleep length and lipid metabolism are partially controlled by the same genes. We studied the association of total sleep time (TST) with 60 genetic variants that had previously been associated with lipids. The analyses were performed in a Finnish population-based sample (N=6334) and replicated in 2189 twins. Finally, RNA expression from mononuclear leucocytes was measured in 10 healthy volunteers before and after sleep restriction. The genetic analysis identified two variants near TRIB1 gene that independently contributed to both blood lipid levels and to TST (rs17321515, P=8.92*10−5, Bonferroni corrected P=0.0053, β=0.081 h per allele; rs2954029, P=0.00025, corrected P=0.015, β=0.076; P<0.001 for both variants after adjusting for blood lipid levels or body mass index). The finding was replicated in the twin sample (rs17321515, P=0.022, β=0.063; meta-analysis of both samples P=8.1*10−6, β=0.073). After the experimentally induced sleep restriction period TRIB1 expression increased 1.6-fold and decreased in recovery phase (P=0.006). In addition, a negative correlation between TRIB1 expression and slow wave sleep was observed in recovery from sleep restriction. These results show that allelic variants of TRIB1 are independently involved in regulation of lipid metabolism and sleep. The findings give evidence for the pleiotropic nature of TRIB1 and may reflect the shared roots of sleep and metabolism. The shared genetic background may at least partially explain the mechanism behind the well-established connection between diseases with disrupted metabolism and sleep.
Keywords: epidemiology, genetics, lipids, metabolism, sleep, SNP
Introduction
A previously unknown association between sleep and lipid metabolism has started to emerge, based on epidemiological, human experimental, and human genetics data as well as data from animal models. Epidemiological studies show that deviation from mid-range sleep length (7–8 h per night) associates to several metabolic diseases with changed lipid profile1 and even to increased mortality.2 Experimental sleep restriction studies on humans demonstrate that shortening of sleep reduces the utilization of lipids as energy source.3
Genome-wide association studies (GWAS) on blood lipid levels, obesity and type 2 diabetes have identified risk variants from genes that have traditionally been related to psychiatric traits and sleep regulation, such as melatonin receptor 1 B (MTNR1B),4 brain-derived neurotrophic factor (BDNF)5 and a circadian pace maker gene cryptochrome 2 (CRY2).6 Mice lacking circadian genes CLOCK or BMAL1 manifest altered triglyceride and cholesterol metabolism and develop diabetes, obesity and metabolic syndrome.7, 8, 9, 10 Furthermore, in Drosophila, knocking out one of the key circadian genes, cycle, leads to increased triglyceride levels, increased waking and intolerance for sleep deprivation, demonstrating that sleep homeostasis has a role in the regulation of lipid metabolism in this species.11
Sleep length and blood lipid levels are regulated by a strong genetic component. Electroencephalography spectra measured at 8–16 Hz frequency range during sleep shows heritability of 96%,12 and self- reported sleep length has been estimated to have heritability of 44% in a Finnish study of 6783 twin pairs.13 Recent GWAS studies have revealed that known genetic variants explain 25–30% of the individual differences in blood lipid levels at population level.14 Some of these variants belong to genes that have previously been related to lipid metabolism, such as apolipoproteins or LDL-C receptor genes, whereas others are less obvious and are also expressed in the central nervous system. The latter group includes variants in the TRIB1 gene, which mediates the MAPK signaling cascades,15 which is activated during inflammation16 and participates in the regulation of cell cycle progression.17 Most of the variants affecting blood lipids have now been replicated and established in several populations, even though the effect size of an individual variant alone is usually relatively small. The question whether the same gene variants that affect blood lipid levels would also have an effect on sleep duration has remained unexplored.
The aim of the present study was to elucidate the potentially shared genetic component in sleep regulation and metabolism. We examined the association of 60 previously identified lipid gene variants18, 19, 20, 21 with sleep length in a population-based sample. We also addressed the question whether the association between lipid trait variants and total sleep time (TST) would be mediated through blood lipid levels. In order to study the functional role of the identified gene variant in regulation of sleep homeostasis, we measured the expression of TRIB1 in healthy volunteers after they had been exposed to partial sleep restriction (4 h per night) for 5 days. The analyses revealed that variants of the TRIB1 gene were independently associated with sleep length and lipid metabolism. Restriction of sleep increased the gene expression of TRIB1 indicating that it may be involved in regulation of sleep homeostasis.
Materials and methods
Study design
The association of TST with four lipid traits, triglycerides (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C) and total cholesterol (TC) was tested in a Finnish population-based sample set. After revealing a connection between lipid levels and TST, SNPs that had previously been shown to associate with TG, HDL-C, LDL-C or TC were selected for the genetic association analysis. We correlated these SNPs with TST and other sleep-related traits in our Finnish population-based samples and aimed for replication of our most significant finding in an independent Finnish twin sample. Finally, the expression levels of the gene highlighted in the genetic association analysis was assessed in a sample of healthy individuals that were subjected to cumulative sleep loss.
Study participants
A description of the cohorts is presented in Table 1. The study was performed by combining data from two population-based cohorts from Finland. The first cohort, Health 2000 study (http://www.terveys2000.fi), is a nationwide interview and health examination study performed from 2000–2001. The participants of the Health 2000 study were selected from the national population register so that they would reflect the main demographic distributions of the Finnish population. The participants were over 30 years of age and were characterized for their health by home interview including questions on their quality and duration of sleep and questions concerning mental and general health. A health examination, including laboratory examinations, was conducted at a local health center, where blood samples for DNA extraction and measurements of lipid levels were taken. Altogether, Health 2000 comprised 8028 individuals, of which 6269 returned the questionnaire containing questions about sleep length.
Table 1. Study subjects of Finnish samples.
| Cohort | N | N (males) | N (females) | Age (years±SD) | TST (h±SD) | TC (mmol l–1±SD) | HDL-C (mmol l–1±SD) | LDL-C (mmol l–1±SD) | TG (mmol l–1±SD) |
|---|---|---|---|---|---|---|---|---|---|
| Health 2000 | 5948a | 2630 | 3318 | 54.22±16.2 | 7.49±1.22 | 5.93±1.12 | 1.32±0.38 | 3.77±1.19 | 1.62±1.05 |
| FINRISK2007 | 6092b | 2846 | 3246 | 50.80±14.3 | 7.64±1.21 | 5.25±1.00 | 1.44±0.37 | 3.16±0.87 | 1.44±0.95 |
| Finnish twin sample | 2189 | 1024 | 893 | 44.08±6.09 | 7.96±3.67 | NA | NA | NA | NA |
Abbreviations: HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TC, total cholesterol; TG, triglyceride; TST, total sleep time.
All individuals included in the genetic analysis.
Overall, 635 individuals included in genetic analysis.
The second cohort was the nationwide interview and health examination study FINRISK 2007 collected in 2007 (http://www.ktl.fi/finriski). For this study a random sample of adults in five regions of Finland was drawn from the Finnish national population register.22 This study comprised altogether 7993 individuals aged 25–74 years. In this sample, genotype information was derived from a GWA study carried out for 635 participants from the Helsinki area and described in Inouye et al.23
The Finnish Twin Cohort, here used as replication sample, was compiled from the Finnish national population register and consisted of virtually all same-sex twin pairs born in Finland before 1958 and with both co-twins alive in 1974. Among them we identified 2189 individuals of 20–69 years of age with both phenotype and genotype information (with phenotype assessment from questionnaires given in 1975 or 1981).24
The experimental study consisted of 19 healthy men (age 19–29) with habitual sleep duration of 7–9 h. They were subjected to sleep restriction of 4 h of time in bed per night for 5 nights. Nine of them were treated as controls with 8 h of sleep per night during the whole study. After the fifth day of the study the sleep-deprived subjects were allowed to recover from the restriction for 2 nights with 8 h of time in bed.
The ethics committee of Helsinki and Uusimaa Hospital District approved the study protocol and informed consent was obtained from all the participants. All participants provided written informed consent for the collection of samples and subsequent analysis.
Measures
For the metabolic analysis in the Finrisk sample, TC was measured with the CHODPAP-assay (Abbott Laboratories, Abbott Park, IL, USA), HDL-C with a direct enzymatic assay (Abbott Laboratories) and TG with the enzymatic GPO assay (Abbott Laboratories). In the Health 2000 sample HDL-C, TC and TG were measured using enzymatic assays (Roche Diagnostics, Mannheim, Germany, for HDL-C; Olympus System Reagent, Hamburg, Germany, for TC and TG). Sleep length was evaluated with a similar question in all three cohorts: ‘How many hours do you sleep per day?'. In addition to TST, we assessed the chronotypes of the individuals using questions 4, 7, 9, 15,17 and 19 derived from the morningness–eveningness questionnaire.25 In the twin sample, chronotype was assessed by asking ‘Try to estimate, whether you are a morning- or evening-type person' and the answers were given as clearly morning-type, a bit morning-type, a bit evening-type, clearly evening-type. The individuals were assigned as morning-type, evening-type or neither of the extreme types (N=635, in FINRISK and N=2089 in Twin sample in the year 1981 questionnaire). The analysis was performed so that the morning or evening types were compared with the neither type group. Daytime dozing was measured in Health 2000 sample using the Finnish version of Epworth sleepiness scale (ESS),26 which comprised eight questions: How easily you fall asleep when (1) sitting and reading (2) watching television (3) sitting inactive in a public place (4) as a passenger for an hour in a car (5) lying down to rest in the afternoon (6) when talking with someone (7) when the car is stopping at traffic lights (N=5981, 2706 males, 3275 females). Fatigue was assessed from the same individuals in Health 2000 with a question: ‘Are you more tired during the day than other people of your age?'
Genotyping and SNP selection
The SNPs were selected based on their previous association from GWAS either with TC, HDL-C-C, LDL-C-C or TG (Supplementary Table 1).18, 19, 20, 21 The genotype data from Health 2000 and FINRISK2007 projects were obtained from the respective projects and were originally genotyped using Sequenom Massarray technology (San Diego, CA, USA) or Illumina 610 K platform (Illumina, San Diego, CA, USA). In quality-control SNPs showing a success rate over 95%, minor allele frequency over 0.1 and Hardy–Weinberg equilibrium P>1*10−3 were kept in the analysis. Two variants were excluded in the quality-control phase due to low Hardy–Weinberg P-value (rs150908 and rs1289742).
Statistical analysis
In the epidemiological analysis the individuals were divided into six groups based on TST (⩽5 h, 6 h, 7 h, 8 h, 9 h and ⩾10 h). The association with TST and lipid traits was tested using general linear model. The analyses were performed using PASW Statistics 18 and all analyses were adjusted for relevant covariates. These included age, gender, lipid medication, hypnotics, body mass index (BMI) and cohort.
In the genetic association analysis TST, Epworth sleepiness scale and the lipid variables TC, HDL-C, LDL-C and TG were analyzed as quantitative traits using a linear regression model. For categorical variables (fatigue and morningness–eveningness scale) a logistic regression model was applied. In addition, short sleep (⩽6 h sleep) was analyzed against the 7–8 h sleep group. All individuals with information of their sleep length and genotype were kept in the analysis. All analyses were adjusted for age and gender. The lipid variables and ESS values were squared in order to obtain normal distribution. The genetic association analyzes were conducted using PLINK27 version 1.07 from http://pngu.mgh.harvard.edu/purcell/plink/ (Boston, MA, USA). The twin sample was analyzed using PLINK's qfam option with 1000 permutations. We judged the association by P-value and β and the results were considered significant when Bonferroni-corrected P-value for each SNP was P<0.05.
Sleep restriction and RNA expression analysis
The RNA expression analysis was performed as part of another study.28 In short, the participants with a regular sleep-wake schedule and habitual sleep duration of 7–9 h participated in the study. During the experiment the experimental group spent 8 h in bed for the first 2 nights (baseline (from 11 PM to 7 AM)), followed by 5 nights where they rested for only 4 h in bed (sleep restriction (from 3 AM to 7 AM)) and, finally, again 2 nights of 8 h in bed (recovery). Continuous electroencephalography recordings and a continuously present investigator served to monitor that the participants did not nap during the experiment. Meals were standardized and energy-balanced based on the current national recommendations, provided at fixed times and consumed by all participants throughout the experiment. The study design was approved by the ethics committee of Helsinki University Central Hospital and a written informed consent was obtained from participants. The experiment was conducted at the Brain and Work Research Centre of the Finnish Institute of Occupational Health.
RNA was collected from blood mononuclear leucocytes in the morning after baseline, restriction and in recovery phases. The RNA expression levels were tested using Affymetrix U133 Plus 2.0 human genome expression arrays (Affymetrix, Santa Clara, CA, USA). Student's t-test was used for calculating significance values between the time points, repeated measures ANOVA was used to detect changes during the whole sleep restriction experiment and fold changes were used for estimating the magnitude of changes in expression levels. Slow wave sleep (SWS) was scored manually. The values from sleep restriction and recovery time points were normalized to baseline values. Linear regression model was applied to calculating the significance between baseline-normalized TRIB1 RNA expression levels and baseline-normalized SWS values in deprivation and recovery time points.
Results
Sleep length and blood lipid levels
The distributions of the lipid levels across self-reported sleep duration are shown in Table 2. Individuals having extremely short (⩽5 h per day) or long sleep (⩾10 h per day) had the most unfavorable lipid profiles as TG and TC levels were higher and HDL-C levels were lower than in other groups. However, LDL-C levels were lowest in the long sleepers. The unadjusted and adjusted associations of sleep duration with lipid levels were significant in general linear models P<0.001 (Table 3). However, sleep length explained only a small percentage of the variation within the studied traits (Table 3).
Table 2. Mean±SD values of laboratoric measures of four different lipid traits in the sleep length groups.
| Total sleep time (hours) | N | TG mmol l–1±SD | TC mmol l–1±SD | HDL-C mmol l–1±SD | LDL-C mmol l–1±SD |
|---|---|---|---|---|---|
| ⩽5 | 370 | 1.63±1.04a | 5.77±1.08b | 1.36±0.41 | 3.61±1.145a |
| 6 | 1327 | 1.57±1.04a | 5.65±1.12a | 1.38±0.39 | 3.51±1.11 |
| 7 | 4101 | 1.48±0.97b | 5.61±1.11b | 1.39±0.38 | 3.5±1.08b |
| 8 | 4472 | 1.50±0.96 | 5.57±1.11b | 1.39±0.38 | 3.45±1.09b |
| 9 | 1208 | 1.53±1.00 | 5.48±1.07c | 1.39±0.37 | 3.37±1.01c |
| ⩾10 | 562 | 1.79±1.28c | 5.57±1.20 | 1.31±0.39c | 3.36±1.17a |
| Total | 12 040 | 1.52±1.00 | 5.59±1.11 | 1.38±0.38 | 3.47±1.09 |
Abbreviations: HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
ANOVA P-value <0.05 indicates significant association with sleep duration when comparing the group to all other sleep duration groups within the lipid variable.
ANOVA P-value <0.01 indicates significant association with sleep duration when comparing the group to all other sleep duration groups within the lipid variable.
ANOVA P-value <0.001 indicates significant association with sleep duration when comparing the group to all other sleep duration groups within the lipid variable.
Table 3. GLM model of TST and lipid levels.
| Trait | N with data | Model unadjusted | Model adjusted for age and gendera |
Model 3b |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted effect of sleep duration | The whole model | Independent effect of sleep duration | The whole model | Independent effect of sleep duration | |||||||||||||||
| df | F | P | R2 | N with data | df | F | P | R2 | F | P | N with data | df | F | P | R2 | F | P | ||
| TC | 12 040 | 5 | 5.626 | <0.001 | 0.002 | 12 040 | 9 | 65.489 | <0.001 | 0.036 | 5.409 | <0.001 | 9763 | 11 | 156.25 | <0.001 | 0.149 | 2.428 | 0.033 |
| HDL-C | 12 040 | 5 | 5.461 | <0.001 | 0.002 | 12 040 | 9 | 205.99 | <0.001 | 0.106 | 6.29 | <0.001 | 9763 | 11 | 160.96 | <0.001 | 0.153 | 4.082 | 0.001 |
| LDL-C | 12 039 | 5 | 5.589 | <0.001 | 0.002 | 12 039 | 9 | 53.146 | <0.001 | 0.029 | 7.166 | <0.001 | 9762 | 11 | 144.59 | <0.001 | 0.139 | 2.523 | 0.027 |
| TG | 12 040 | 5 | 12.028 | <0.001 | 0.005 | 12 040 | 9 | 94.015 | <0.001 | 0.051 | 9.258 | <0.001 | 9763 | 11 | 77.202 | <0.001 | 0.079 | 4.34 | 0.001 |
Abbreviations: HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Model 2 adjusted for age, sex.
Model 3 adjusted for age, sex, BMI cohort, lipid medication and hypnotics.
Association of lipid trait SNPs with TST, replication and meta-analysis
We then elucidated the role of 60 SNPs previously defined as significant genetic determinants for blood lipid levels by GWAS18, 19, 20, 21 and associated them with sleep duration. Two significant association signals downstream of TRIB1 gene were observed with TST (rs17321515, P=8.92*10−5, β=0.081; rs2954029, P=0.00025, β=0.076, corrected P=0.0053 and P=0.015, respectively) with the protective minor allele ‘G' and ‘T'. The percentages of genotypes for rs17321515 in the TST groups are presented in Table 4.
Table 4. Genotype frequencies of rs17321515 in sleep length groups (N=6334).
| Sleep length (hours) | AA (%) | AG (%) | GG (%) |
|---|---|---|---|
| ⩽5 | 3.93 | 3.53 | 3.68 |
| 6 | 13.58 | 10.92 | 10.83 |
| 7 | 36.21 | 36.18 | 33.16 |
| 8 | 35.14 | 36.86 | 38.47 |
| 9 | 7.74 | 8.71 | 9.65 |
| ⩾10 | 3.39 | 3.80 | 4.20 |
In addition, the same variants associated significantly with blood lipid traits and both TRIB1 SNPs showed the strongest association with blood TC levels (rs17321515, P=1.45*10−5, β=−1.004 and rs2954029, P=4.62*10−5, β=−0.95). The variant rs2954029 that originally associated with blood TG levels was only nominally significant here (P=0.029, β=−0.29). However, the associations of both SNPs with TST remained significant after adjustment for TG, TC, HDL-C, LDL-C levels or BMI (for all analyses P<0.05). Similarly, the association of TRIB1 SNPs with TC remained significant after adjustment for sleep duration (TC P<0.05 for both variants), whereas the signal of rs2954029 with TG was lost when adjusted with TST. The effect size in healthy individuals was largest among those having 6 h or shorter sleep duration. The findings are in line with a recent publication where the heritability for fat mass index and BMI were higher in individuals having short sleep duration, suggesting that the genetic component of lipid metabolism is largest among those having short sleep duration.29 The original P-values for all tested SNPs including suggestive significant variants are listed in Supplementary Table 1 and the association analysis of 17321515 and rs2954029 with lipid levels in different TST groups are presented in Supplementary Table 2.
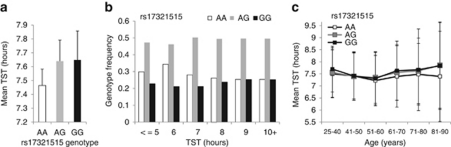
The risk allele ‘A' of rs17321515, originally associated with higher blood TC in GWAS for blood lipid levels19 was found to associate with shorter sleep length (P=0.04, OR=1.109, 95% CI=1.004–1.22 in dichotomous analysis). In addition, individuals having genotype major/major AA of rs17321515 had the shortest mean sleep length (Figure 1a) and the frequency of this genotype was highest in the individuals sleeping <7 h (Figure 1b). Furthermore, the major/major AA individuals had the shortest sleep duration in all age groups throughout the data when compared with individuals carrying AG or GG genotypes (Figure 1c). The tightly linked variants rs17321515 and rs2954029 in TRIB1 (D′=0.998 and R-Sq=0.957) were possibly tagging the same position. There was no interaction of gender or age and TST with either of the TRIB1 variants (analysis of interaction with gender and age P>0.05). In order to study the variants in healthy individuals without major sleep problems and high cholesterol, we excluded individuals taking hypnotics or lipid medication and performed the association analysis with the two significant TRIB1 variants. We observed that the association was still significant and the effect was greater (rs17321515, P=0.00067, β=0.13 and rs2954029, P=0.00076, β=0.13).
Figure 1.
Mean TST of the different genotypes of Trib1 SNP rs17321515 with error bars representing s.d. are presented in panel a and genotype frequencies of the same genotypes are presented in panel b N=6334 with both genotype and phenotype information. The mean total sleep length by genotype (AA, AG and GG) are shown in panel c. TST, total sleep time.
The finding on TRIB1 variant rs17321515 was replicated in the twin sample with 2189 adult Finnish twins, where we saw allelic replication (P=0.022, β=0.063, permuted P=0.031). The meta-analysis combining the population cohort and twin sample further strengthened the original association (P=8.1*10−6, β=0.073).
Association with other sleep-related traits
As a secondary analysis, we examined the association of TRIB1 variants with other sleep-related traits. We found evidence for association with sleepiness (rs17321515, P=0.034, β=−1.45, rs2954029, P>0.05 to ESS) and eveningness chronotype (rs17321515, P=0.030, OR=0.61, 95% CI=0.39–0.96, rs2954029, P=0.026, OR=0.60, 95% CI=0.38–0.94) but no association with fatigue (P>0.05). The association signal with ESS also remained significant when adjusted for blood lipids (P<0.05). Individuals with the minor allele for either variant were less evening-type, whereas the risk alleles increased the eveningness chronotype. However, we could not replicate the finding on eveningness chronotype in the twin sample (P>0.05).
TRIB1 RNA levels in sleep restriction
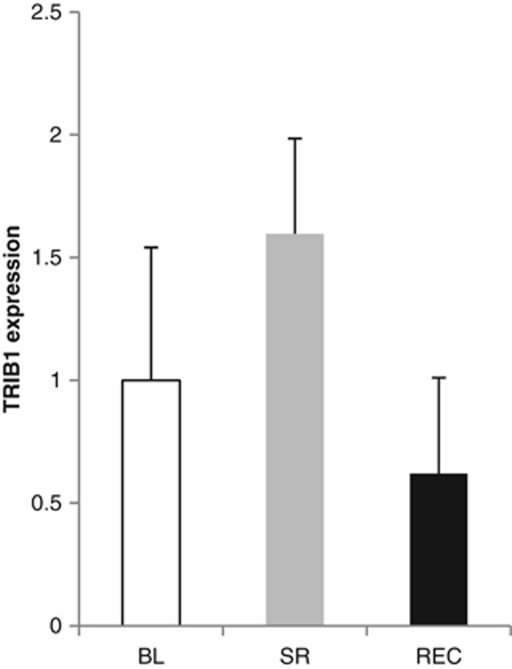
In order to elucidate the role of TRIB1 in regulation of sleep homeostasis, we studied the reaction of TRIB1 RNA levels to sleep restriction (Figure 2). We found that the TRIB1 expression levels were significantly higher in individuals subjected to sleep restriction (TRIB1 fold increase of 1.6, P<0.05). The expression levels returned back to baseline in the recovery phase (TRIB1 fold decrease from deprivation to recovery 2.6, P<0.001) and the overall change was significant based on repeated measures ANOVA (P=0.006). Finally, we performed analysis of TRIB1 expression levels and SWS, where the amount of SWS and Trib1 expression was normalized to baseline levels. No association was seen in baseline vs deprivation but a significant association with TRIB1 expression and SWS was detected in the baseline to recovery phase (P=0.022, β=−1.05), suggesting a role for Trib1 in recovery sleep.
Figure 2.
Mean intensities of TRIB1 expression for baseline (BL), sleep restriction (SR) and recovery (REC) with error bars representing SEM.
Discussion
We found independent association of two genetic variants near TRIB1 gene with blood lipid levels and TST in 6334 adults from a population-based sample. The best finding, rs17321515, was replicated in an independent sample of 2189 Finnish adult twins and meta-analysis of these two cohorts further strengthened the association. We found association of the risk allele ‘A' with increased short sleep and increased TC and TG levels. The association was strongest among individuals that did not take hypnotics or lipid medication, suggesting a role for TRIB1 in physiological sleep regulation. Furthermore, an experimental sleep restriction study gave functional evidence for the role of TRIB1 also in homeostatic sleep regulation in healthy individuals as TRIB1 was upregulated upon sleep restriction. The TRIB1 variants were earlier reported to associate with TC and TG levels18, 19, 20, 21 and we were able to confirm such an association. Recently, it was shown that Trib1 regulates TG and VLDL levels also in mice.30
Surprisingly, the associations with sleep length and lipid levels were independent from each other, and adjusting the analysis of TST for lipid traits, or vice versa did not abolish the association with the TRIB1 variants with either TST or lipid traits. Here, the risk variants were related to shorter TST. In addition to shorter sleep length, the individuals carrying the risk allele of either of the studied variants scored higher on sleepiness. They also scored higher in questions on the eveningness chronotype, which has been related to many adverse health-related phenomena such as smoking, alcohol use, physical inactivity or depressiveness,31, 32, 33 as well as to increased heart rate or blood pressure both at rest and during stress.34 However, the finding on the eveningness chronotype could not be replicated, which was possibly due to the single item used to assess eveningness in the replication data set whereas the original data set consisted of seven questions measuring the chronotype or due to a relatively weak association with eveningness in the original dataset, which would have required a larger replication sample.
The risk variants associated with higher TC and earlier TRIB1 variants have been associated with coronary artery disease35 and metabolic syndrome.36 Short sleep length is also a known risk factor for developing cardiovascular diseases.37, 38, 39, 40 The findings suggest that common genetic factors may contribute both to short sleep and to diseases with disrupted metabolic regulation. To our knowledge, this is the first study to show that the same genetic variants are associated with both sleep duration and with plasma lipid levels.
Earlier studies show significant correlation between sleep duration and diseases with disrupted metabolism such as cardiovascular diseases, diabetes and obesity.37, 38, 39, 40, 41 Short sleep is also a predisposing factor for developing these diseases. In concert with these earlier studies, we observed a significant association between self-reported sleep length and with all measured lipid traits: TG, TC, HDL-C and LDL-C after adjusting for several confounders including age, sex, BMI, study cohort, lipid medication and hypnotics. However, sleep length explained only a small proportion of the total variation in lipid levels. Our findings are in line with previous studies and suggest that the previously described association of short and long sleep length with cardiovascular diseases41 and mortality2 might be carried out by prolonged exposure to high TG and low HDL-C levels. However, there may be also other factors contributing to this association and this hypothesis would need to be studied in longitudinal setting.
Finally, we studied the reaction of TRIB1 RNA levels to sleep restriction. We observed an upregulation of TRIB1 RNA levels after sleep restriction. The expression levels returned back to baseline after the recovery phase. In addition, the relative decrease in the Trib1 expression level in recovery was associated with the relative increase in SWS in recovery phase further suggesting a role for TRIB1 in the regulation of sleep homeostasis. Interestingly, another protein from the same family, TRIB2, has been found to have a role in the pathogenesis of narcolepsy, a sleep disorder with symptoms of daytime sleepiness.42 In addition, TRIB2 autoabtibodies have been found in uveitis.43 TRIB1 and TRIB2 are part of the same protein family and regulate similar signaling cascades related to inflammation and Akt and MAPK signaling.15, 44, 45 Even though TRIB1 and TRIB2 share molecular signaling properties, they are found in different physiological traits. TRIB1 is related to energy metabolism and has been found in lipid trait and coronary heart disease GWAS, whereas TRIB2 is related to inflammatory reactions and autoantibody production in narcolepsy and uveitis. Furthermore, Trib1 and Trib2 are found in different cellular locations. Trib1 protein is detected in the nucleus, whereas Trib2 is cytoplasmic protein.45, 46 In addition, TRIB2 has been shown to be a negative regulator for innate immune reactions and monocyte activation that have an important role in autoimmune disorders.42, 47 Interestingly, innate immune have also been found to be activated upon sleep restriction.28
Trib1 is ubiquitously expressed with relatively high expression values in liver, blood leucocytes and brain.30 Studies in Trib1 over expressing mice show that higher hepatic Trib1 expression lowers TG, VLDL, HDL-C and LDL-C levels and APOB secretion, whereas in Trib1 knockout animals an opposite effect is seen. The action of Trib1 on lipids in the hepatic cells has been shown to be mediated through the modulation of genes involved in lipid metabolism such as acetyl-CoA carboxylase 1 (Acc1), fatty acid synthase (Fasn), and stearoyl-coenzyme A desaturase1.30 Our results suggest that the effect of Trib1 on TST is not directly dependent on lipid levels. However, TST associates with cholesterol and triacylglycerols in epidemiological studies, and experimental sleep deprivation experiments in mice have shown that transcription of genes regulating cholesterol synthesis and lipid transport is increased during sleep.48 Recently, a sleep deprivation study in Drosophila found a connection between lipid metabolism and tolerance to sleep deprivation, suggesting that alterations in lipid metabolism enzymes altered the response to sleep deprivation.11 It is thus possible that the association between Trib1, sleep and lipid levels is mediated at least partially through the same genes. Another possible mechanism linking Trib1 with sleep regulation is MAPK signaling. This pathway is one of the key signaling cascades affecting sleep and circadian rhythms49 and Trib1 has also been shown to regulate MAPK signaling.15 The mechanism could also involve the (inhibitory) effect of Trib on Akt signaling43 and, subsequently, the circadian pacemaker and the sleep–wake cycle, on sleep homeostasis.50 It is plausible that Trib1 association with TST is mediated through the interconnected regulation of sleep and metabolism. Elucidation of the molecular mechanisms of Trib1 on sleep regulation will require further studies applying both experimental animal models and sleep studies in humans.
In this study, we have included a large sample from Finland for both epidemiologic and genetic analysis. Compared with other European populations, Finns are genetically a relatively homogenous group,51 which makes them ideal for genetic studies. A limitation of the study is that sleep was assessed solely with questionnaires. Some studies have suggested that self-reported sleep duration may somewhat overestimate the amount of physiological sleep52, 53 although this overestimation may decline with longer sleep.52
To conclude, our results suggest that TRIB1 is a novel factor regulating sleep duration and homeostasis. TRIB1 has a pleiotropic role in metabolic regulation as it associates with several metabolic traits.36 Here, we observed its independent association with both blood lipid levels and with TST in a population-based sample from Finland, suggesting a pleiotropic nature for TRIB1 not only in metabolism but also in the physiological mechanism of sleep regulation. The association to TST was replicated in another population-based sample of twins. After experimental sleep restriction of 5 nights in healthy individuals, expression of TRIB1 was found to be significantly increased and returned back to baseline in recovery phase. In addition, the relative decrease in TRIB1 expression in recovery phase associated with the increase in SWS further suggesting a role for TRIB1 in homeostatic sleep regulation. To our knowledge, this is the first study to describe a human metabolic gene variant that regulates both sleep and metabolism. The finding may reflect the common roots of these physiological processes. Furthermore, their shared genetic background may at least partially explain the well-established connection between diseases involving disrupted metabolic processes, such as cardiovascular disease, obesity and type 2 diabetes mellitus, and sleep.
Acknowledgments
We thank Johanna Suhonen for technical assistance. We thank Amy Sanders and Mackenzie Lind for English proofreading. This work was funded by the Academy of Finland (Grant # 124404 for TP and #129494 for VS), Sigrid Juselius Foundation for TP, Helsinki University Hospital (TYH2010306). Emil Aaltonen foundation, Jalmari and Rauha Ahokas Foundation, Biomedicum Helsinki Foundation and the Helsinki Graduate Program in Biotechnology and Molecular Biology. The twin study has been supported by the Academy of Finland Center of Excellence in Complex Disease Genetics.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S, Brodsky J, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan MS, Suzuki Y, Seugnet L, Gottschalk L, Shaw PJ. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 2010;8:e1000466. doi: 10.1371/journal.pbio.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–185. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- Sung HY, Francis SE, Crossman DC, Kiss-Toth E. Regulation of expression and signalling modulator function of mammalian tribbles is cell-type specific. Immunol Lett. 2006;104:171–177. doi: 10.1016/j.imlet.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Musunuru K, Orho-Melander M. Defining the spectrum of alleles that contribute to blood lipid concentrations in humans. Curr Opin Lipidol. 2008;19:122–127. doi: 10.1097/MOL.0b013e3282f70296. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Manning AK, Demissie S, D'Agostino RB, Surti A, Guiducci C, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1:S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Mannisto S, Sundvall J, et al. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39:504–518. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- Inouye M, Silander K, Hamalainen E, Salomaa V, Harald K, Jousilahti P, et al. An immune response network associated with blood lipid levels. PLoS Genet. 2010;6:e1001113. doi: 10.1371/journal.pgen.1001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu X, Arguelles LM, Patwari PP, Zee PC, Chervin RD, et al. A population-based twin study on sleep duration and body composition. Obesity (Silver Spring) 2012 Jan;20 (1:192–199. doi: 10.1038/oby.2011.274. [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Toh SA, Lagor WR, Birkeland A, Levin M, Li X, et al. Trib1 is a lipid- and myocardial infarction-associated gene that regulates hepatic lipogenesis and VLDL production in mice. J Clin Invest. 2010;120:4410–4414. doi: 10.1172/JCI44213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009;63:283–290. doi: 10.1111/j.1440-1819.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Randler C. Association between morningness-eveningness and mental and physical health in adolescents. Psychol Health Med. 2011;16:29–38. doi: 10.1080/13548506.2010.521564. [DOI] [PubMed] [Google Scholar]
- Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011;28:238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis TA, O'Connor DB, Smith L. The influence of morningness-eveningness on anxiety and cardiovascular responses to stress. Physiol Behav. 2005;85:125–133. doi: 10.1016/j.physbeh.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraja AT, Vaidya D, Pankow JS, Goodarzi MO, Assimes TL, Kullo IJ, et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011;60:1329–1339. doi: 10.2337/db10-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–719. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Davis JL, Li W. Identification of tribbles homolog 2 as an autoantigen in autoimmune uveitis by phage display. Mol Immunol. 2005;42:1275–1281. doi: 10.1016/j.molimm.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Kiss-Toth E, Wyllie DH, Holland K, Marsden L, Jozsa V, Oxley KM, et al. Functional mapping and identification of novel regulators for the Toll/Interleukin-1 signalling network by transcription expression cloning. Cell Signal. 2006;18:202–214. doi: 10.1016/j.cellsig.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell Signal. 2007;19:238–250. doi: 10.1016/j.cellsig.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Eder K, Guan H, Sung HY, Ward J, Angyal A, Janas M, et al. Tribbles-2 is a novel regulator of inflammatory activation of monocytes. Int Immunol. 2008;20:1543–1550. doi: 10.1093/intimm/dxn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Giebultowicz J, Kapahi P. Circadian clocks and metabolism: the nutrient-sensing AKT and TOR pathways make the link. Curr Biol. 2010;20:R608–R609. doi: 10.1016/j.cub.2010.05.052. [DOI] [PubMed] [Google Scholar]
- Service S, DeYoung J, Karayiorgou M, Roos JL, Pretorious H, Bedoya G, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38:556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they. Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsleben JA, Kapur VK, Newman AB, Shahar E, Bootzin RR, Rosenberg CE, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.