Abstract
Background
Mentha piperita L. (Labiatae) is an herbaceous plant, used in folk medicine for the treatment of several medical disorders.
Methods and Results
In the present study, the aqueous extract of Mentha piperita leaf, at the i.p doses 200 and 400 mg/kg, showed significant analgesic effects against both acetic acid-induced writhing and hot plate-induced thermal stimulation in mice, with protection values of 51.79% and 20.21% respectively. On the contrary, the Mentha piperita leaf aqueous extract did not exhibit anti-inflammatory activity against carrageenan induced paw oedema.
Conclusion
These findings indicate that Mentha piperita has a potential analgesic effect that may possibly have mediated centrally and peripherally, as well as providing a pharmacological evidence for its traditional use as a pain reliever.
Keywords: Mentha piperita, traditional medicine, analgesic activity, mice
Mentha piperita Linn (family: Labiatae) is an herbaceous plant of small size with greenish-feathery leaves. Worldwide M. piperita leaves are used in various forms of preparations (as a tea preparation, alcohol extracts, a tincture or as an oil (1). The plant contains volatile oils, resins and flavonoids (2, 3). In Mediterranean region, M. piperita has been reported to possess diverse medicinal properties. Traditionally, it is used in treatment of various conditions including flatulence, carminative and as a local antiseptic (4). In addition, M. piperita is reported to have analgesic activity and its leaves are used to reduce tooth and abdominal pains (5). Besides, this plant has been indicated to relieve inflammatory disorders. Topically M. piperita essential oil is employed as an analgesic compound for diseases of the pharynx and in the relief of tension headache and migraines (6). In mouthwashes, it is utilized for oral hygiene, possibly due to its antimicrobial activities (3). In vitro, several studies have shown that M. piperita has antibacterial effects, strong antioxidant and antitumor action, and antiallergenic potential (2, 7, 8). Additionally, in animal studies, it was clearly demonstrated in the Ames test that M. piperita has antimutagenic effects once it significantly inhibited the shamma—a tobacco product—induced oral carcinogenesis in the hamster cheek pouch (9).
The genus Mentha L. comprises more than 20 species, mainly perennial herbs growing wildly in damp places throughout temperate regions of Eurasia, Australia, and Africa. Hence, the pharmacological activities of M. piperita (2, 10) and other species of the family Labiatae (11–13) was the subjects of certain studies, antinociceptive and anti-inflammatory activities included (10, 13, 14). Animal model studies have demonstrated that Mentha suaveolens (13) and Mentha microphylla (10) could be able to protect mice from peripheral pains. Furthermore, Atta and Alkofahi have shown that the ethanol extracts of M. piperita induced in addition to a dose-dependent pain-reducing protective activity anti-inflammatory effects against both exudative and proliferative inflammation (14). Keeping this in view, the present study aimed to evaluate the analgesic and the anti-inflammatory activities of the aqueous extract of M. piperita leaf in mice.
Materials and methods
Plant material
Samples of peppermint fresh green leaves, M. piperita plant, were purchased in November 2009 from local Al-Karaymia public market located at the south-western part of the capital, Tripoli, Libya. After identification, by Dr F. Elrateeb (Department of Botany, faculty of Science, Tripoli University, Libya), a voucher specimen was deposited at the Herbarium of the same University.
Aqueous extracts
M. piperita leaves were cleaned and air-dried in the open away from direct sun light. The dried powdered leaves (5 g) were extracted with 150 ml boiling water (decoction) for 10 min. The liquid extracts were filtered, combined, and concentrated almost to dryness using a water bath at 60°C for 3 h (the yields; 6.4%). Thereafter, the concentrated aqueous extract was stored in a refrigerator for subsequent evaluation of its pharmacological activities.
Animals
Inbred Albino mice of either sex (weight 15–40 g, n = 8 each group) reared in the local animal house, faculty of Pharmacy, Tripoli University, Tripoli, Libya, were used. Mice were kept under normal environmental condition and fed with standard diet with free access to water. Animal care and use were in strict compliance with the standard guidelines (Canadian Council). The experimental protocol, dated October 04, 2009; (Ref. No. Pharm/04/10/2009/2010), was approved by the Ethics Committee for Research Programs, faculty of Pharmacy, Tripoli University.
Drugs and chemicals
The following drugs were used: acetylsalicylic acid, Indomethacin, Lambda-carrageenan Type IV (Sigma Chemical Co., St. Louis, USA) and morphine (Lavoisier, France). A bent blunted 25G needle connected to a 1 ml syringe was used for the i.p. administration.
Acetic acid-induced abdominal constrictions in mice
This method was used to evaluate the possible peripheral effects of the plant as analgesic substance. The writhing acetic acid test (abdominal constriction test) was performed as previously described (15, 16). Briefly, M. piperita leaf aqueous extracts (200 and 400 mg/kg), acetyl salicylic acid (100 mg/kg), and equivalent volume of saline (as a negative control) were injected i.p., 30 min prior to the injection of 10 ml/kg of 0.6% v/v acetic acid. Acetyl salicylic acid is a well-known peripheral analgesic drug, and it was used as a positive control. Mice (n = 8 each group) were then placed in an observation box, and the number of writhing movements (constriction of the abdominal muscles together with stretching of the hind limbs) was counted for each mouse for a period of 20 min after acetic acid injection. Antinociceptive activity was expressed as the number of abdominal constrictions between saline treated control and animals pre-treated with M. piperita leaf aqueous extracts or acetyl salicylic acid. Percentage inhibition of writhing was calculated using the formula:
Thermally-induced pain in mice
The hot plate test was employed for preferential assessment of possible centrally mediated analgesic effects of the M. piperita leaf aqueous extract. This test was used to measure response latencies according to the method previously described (17, 18). Briefly, for two consecutive days preceding the experiments, mice (n = 8 each group) were placed kindly on a plate (Model-DS37) maintained at room temperature for 10 min each day. On third day each mouse was placed gently on 55±1°C hot plate for a maximum time of 25 sec. Latency to exhibit nociceptive responses such as licking forepaws, shaking or jumping off the plate was measured twice before distraction (basal) in 10 min interval (Tb = average basal responses). The M. piperita leaf aqueous extracts was given by i.p. injection at doses 200 and 400 mg/kg. The central analgesic drug, morphine, as a positive control was administered s.c. at a dose of 4.6 mg/kg. Control mice received 10 ml/kg normal saline. After 30 min distraction latency time was measured (Ta). For each mouse, the mean latency of nociceptive responses was calculated (a latency period of 25 sec was defined as complete analgesia), permitting us to express the percentage of analgesia using the following formula:
Inflammatory paw oedema in mice
The anti-inflammatory activity was evaluated by the use of carrageenan-induced paw oedema test as described in rats (19, 20) with modification in mice (21). Briefly, 0.02 ml of freshly prepared 1% carrageenan (phlogistic agent) suspension in isotonic saline was injected subplantarly into the right hind paw of each mouse (Eight animals per group were tested). M. piperita leaf aqueous extracts at 200 and 400 mg/kgs were i.p. administered to mice 30 min before carrageenan injection. Control mice received normal saline while a reference group received indomethacin (10 mg/kg, i.p.). Mouse paw thickness was measured immediately before and then at 1 h intervals for 5 h after carrageenan injection, using micrometer-apparatus (Mitutoyo, Japan) (22). The thickness of hind paws was taken as an index of the increase in paw's dimension, and as the measure of acute inflammations. The increase in paw thickness (measured in mm) 3 h after carrageenan injection was taken as thickness of oedema. The inhibition percentage of the inflammatory reaction was determined for each mouse by comparison with control and calculated by the following formula:
Statistical analysis
Data are expressed as a mean response ± SEM. In the study of analgesic properties, comparisons were made on the individual algic reactions obtained for treated and control animals, in the writhing test, and between the individual response times obtained before and after each treatment, in the hotplate test. For the anti-inflammatory test, comparisons were made on the individual differences calculated between the paw thickness obtained after 3 h and those obtained before treatment. The statistical comparisons between the groups were assessed by one-way analysis of variance (ANOVA). When F ratios were significant (p < 0.05), Tukey's post hoc test between groups was done using GraphPad Prism (GraphPad Software Inc., version 3.0, San Diego, USA).
Results
The i.p administration of M. piperita leaf aqueous extracts at 200 and 400 mg/kg showed significant inhibitions of acetic acid-induced abdominal constrictions in mice (46.67% and 51.79% protection, respectively; p < 0.01, n = 8 each, Table 1). Besides, there was no important difference in analgesic effects between the two doses. The reference drug, acetyl salicylic acid, at 100 mg/kg produced 94.87% significant protective effect towards the acetic acid induced pain in this nociception model (p < 0.001, Table 1).
Table 1.
Antinociceptive effect of M. piperita leaf aqueous extract in the acetic acid-induced writhing test
| Treatment | Dose (mg/kg, i.p)a | Writhingb | Inhibition (%) |
|---|---|---|---|
| control | 10 ml/kg | 39 ± 5.6 | – |
| M. piperita | 200 | 20.80 ± 6.15** | 46.67 |
| M. piperita | 400 | 18.80 ± 8.36** | 51.79 |
| Acetyl salicylic acid | 100 | 2 ± 5.6*** | 94.87 |
Administered 30 min before 0.6% v/v acetic acid administration (10 ml/kg, i.p.).
Counted for 20 min, starting 5 min after acetic acid administration; values are mean (8 mice per group) ± SEM.
p < 0.05
p < 0.001 vs. control, ANOVA followed by Tukey's test.
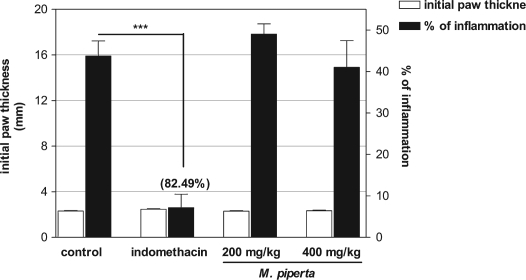
Using the hot-plate test, it was also shown that administration of M. piperita leaf aqueous extract (200 and 400 mg/kg) produced a significant increase in the latency to a response to thermal stimulation compared to control mice (20.21% and 17.13% protection, respectively, p < 0.001, n = 8 each, Table 2). In this model, the reference drug morphine significantly increased the response time of mice to pain (p < 0.001, Table 2). In different experiment, the aqueous extract of M. piperita leaf at 200 and 400 mg/kg failed to protect mice from carrageenan induced acute inflammation assessed by increased paw oedema (Fig. 1). On the other hand, the reference drug indomethacin significantly inhibited the keen inflammation by 82.49% (p < 0.001, Fig. 1) compared to control mice.
Table 2.
Antinociceptive effect of M. piperita leaf aqueous extract in the hot-plate test
| Treatment | Dose (mg/kg, i.p)a | Response timeb (sec) | Analgesia (%) |
|---|---|---|---|
| Control | 10 ml/kg | 0.65 ± 1.36 | - |
| M. piperita | 200 | 4.92 ± 1.29** | 20.21 |
| M. piperita | 400 | 4.17 ± 1.08** | 17.13 |
| Morphine c | 4.6 | 13.28 ± 2.53*** | 54.53 |
Administered 30 min before thermal stimuli at 55 ± 1°C.
Values are mean (8 mice per group) ± SEM.
Administered s.c
p < 0.05
p < 0.001 vs. control, ANOVA followed by Tukey's test.
Fig. 1.
Effects of the i.p. administration of M. piperita leaf aqueous extract and indomethacin on the mouse paw oedema-induced by intraplantar injection of carrageenan at 3rd h. Results are expressed as mean ± SEM. of paw oedema thickness. Eight mice were tested per group. Significant difference from control is shown as ***p < 0.001.
Discussion
In the present study, three animal models for investigation of the antinociceptive and anti-inflammatory effects of the M. piperita leaf aqueous extract were used. The hot plate thermal stimulation and the acetic acid induced writhes in mice were respectively selected to evaluate the central and peripheral antinociceptive activities. Carrageenan-induced paw oedema in mice was selected to represent a model of acute (exudative phase) inflammation.
The present study showed that the aqueous extract of M. piperita leaf has protective effects against the nociception to the intraperitoneally injected acetic acid-induced writhes, and to thermal pain stimuli. These findings are in agreement with the earlier observations by others (10, 13) who did evaluate the pain-reducing activities of other species of the family Labiatae. Studies by Moreno et al., (13) have shown that the methanolic extract of Mentha suaveolens displayed analgesic effects against chemical and mechanical but not thermal stimulation, indicating the induction of only peripheral analgesic effects. Whereas, the studies by Atta and Abo (10) on nociception have demonstrated both central and peripheral analgesic effects for Mentha microphylla in mice treated systemically with merely large doses of methanol extract. Nonetheless, the discrepancies in mechanisms of actions seen in this study and others (10, 13) indicate that even though the genus Mentha exhibited antinociceptive activities, these effects are species and extract-form dependent.
In contrast, the present study showed that the aqueous extract of M. piperita leaf failed to exert anti-oedematogenic effect on the increase in paw thickness induced by carrageenan injection indicating a lack of peripheral anti-phlogestic activity. Hence, it is assumed that this extract has a non-inflammatory pain reliever activity. These data are not completely in line with the previous findings by Atta and Alkofahi, who demonstrated that M. piperita produced systemic anti-inflammatory activities (14). One likely explanation for this discrepancy may be that Atta and Alkofahi (14) used ethanolic extract whereas we used an aqueous extract.
On this basis, our present findings indicate that the extract evaluated has analgesic effect arising from both CNS and peripheral actions since it showed significant effects on both thermal and chemical pain stimuli. Such an efficacy on these two stimuli is characteristic of central analgesics, such as morphine, which inhibits inflammatory and non-inflammatory pains (23). Our data also indicate that the active constituents, which produced analgesic but not anti-inflammatory effects, in this extract are relatively polar compounds. In addition, since several studies have shown that the broad spectrum of bioactivity of this plant has usually been ascribed to the components of its essential oil, menthol and flavonoids (2, 10, 13) it seems likely that the M. piperita analgesic effect, observed in the present mice study, attributed to one of the/these active constituents.
Thus, as far as the analgesic effects are concerned our results strongly support the traditional indication of M. piperita as pain-reducing medicinal plant in folk medicine. Furthermore, these activities affirm the presence of biologically active compounds in the plant. The pharmacological mechanisms that could contribute to this antinociceptive activity need further studies.
Acknowledgements
S. Shalabi is acknowledged for excellent technical assistance. This work was supported by the research program for UGS (Ministry of Higher education) in Tripoli University.
Conflict of interest and funding
The author has declared that there is no conflict of interest.
References
- 1.Nair B. Final report on the safety assessment of Mentha Piperita (Peppermint) Oil, Mentha Piperita (Peppermint) Leaf Extract, Mentha Piperita (Peppermint) Leaf, and Mentha Piperita (Peppermint) Leaf Water. Int J Toxicol. 2001;20(Suppl. 3):61–73. [PubMed] [Google Scholar]
- 2.Inoue T, Sugimoto Y, Masuda H, Kamei C. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol Pharm Bull. 2002;25:256–9. doi: 10.1248/bpb.25.256. [DOI] [PubMed] [Google Scholar]
- 3.Iscan G, Kirimer N, Kurkcuoglu M, Baser KH, Demirci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem. 2002;50:3943–6. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- 4.Kotob FT. Libya: Dar Arabia Lilketab; 1979. Medicinal plants: plantation and contents; p. 288. [Google Scholar]
- 5.El gadi AA. Tripoli: Dar Al-Hekma; 1994. Usage of some plants in Libyan Folk-medicine, part two. 2nd ed; p. 128. [Google Scholar]
- 6.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20:619–33. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 7.Bassolé IH, Lamien-Meda A, Bayala B, Tirogo S, Franz C, Novak J, et al. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules. 2010;15:7825–39. doi: 10.3390/molecules15117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edris A. Pharmaceutical and therapeutic potentials of essentials oils and their individual volatile constituents: a review.x. Phytother Res. 2006;20:1–16. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 9.Samman MA, Bowen ID, Taiba K, Antonius J, Hannan MA. Mint prevents shamma-induced carcinogenesis in hamster cheek pouch. Carcinogenesis. 1998;19:1795–801. doi: 10.1093/carcin/19.10.1795. [DOI] [PubMed] [Google Scholar]
- 10.Atta AH, Abo E-SK. The antinociceptive effect of some Egyptian medicinal plant extracts. J Ethnopharmacol. 2004;95:235–8. doi: 10.1016/j.jep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, et al. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103(4):1449–56. [Google Scholar]
- 12.Jagetia GC, Baliga MS. Influence of the leaf extract of Mentha arvensis Linn. (mint) on the survival of mice exposed to different doses of gamma radiation. Strahlenther Onkol. 2002;178:91–8. doi: 10.1007/s00066-002-0841-y. [DOI] [PubMed] [Google Scholar]
- 13.Moreno L, Bello R, Primo-Yufera E, Esplugues J. Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother Res. 2002;16(Suppl. 1):S10–3. doi: 10.1002/ptr.744. [DOI] [PubMed] [Google Scholar]
- 14.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 15.Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–6. [Google Scholar]
- 16.Okpo SO, Fatokun F, Adeyemi OO. Analgesic and anti-inflammatory activity of Crinum glaucum aqueous extract. J Ethnopharmacol. 2001;78:207–11. doi: 10.1016/s0378-8741(01)00318-x. [DOI] [PubMed] [Google Scholar]
- 17.Lanhers MC, Fleurentin J, Dorfman P, Mortier F, Pelt JM. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991;57:225–31. doi: 10.1055/s-2006-960079. [DOI] [PubMed] [Google Scholar]
- 18.Woolfe G, MacDonald AD. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) J Pharmacol Exp Ther. 1944;80:300–7. [Google Scholar]
- 19.Neto AG, Costa JM, Belati CC, Vinholis AH, Possebom LS, Da Silva Filho AA, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen . J Ethnopharmacol. 2005;96:87–91. doi: 10.1016/j.jep.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 21.Samud AM, Asmawi MZ, Sharma JN, Yusof AP. Anti-inflammatory activity of Crinum asiaticum plant and its effect on bradykinin-induced contractions on isolated uterus. Immunopharmacology. 1999;43:311–6. doi: 10.1016/s0162-3109(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 22.Sharma JN, Samud AM, Asmawi MZ. Comparison between plethysmometer and micrometer methods to measure acute paw oedema for screening anti-inflammatory activity in mice. Inflammopharmacology. 2004;12:89–94. doi: 10.1163/156856004773121400. [DOI] [PubMed] [Google Scholar]
- 23.Kolesnikov YA, Jain S, Wilson R, Pasternak GW. Peripheral morphine analgesia: synergy with central sites and a target of morphine tolerance. J Pharmacol Exp Ther. 1996;279:502–6. [PubMed] [Google Scholar]