ABSTRACT
The preponderance of research toward improving embryo development in vitro has focused on manipulation of the chemical soluble environment, including altering basic salt composition, energy substrate concentration, amino acid makeup, and the effect of various growth factors or addition or subtraction of other supplements. In contrast, relatively little work has been done examining the physical requirements of preimplantation embryos and the role culture platforms or devices can play in influencing embryo development within the laboratory. The goal of this review is not to reevaluate the soluble composition of past and current embryo culture media, but rather to consider how other controlled and precise factors such as time, space, mechanical interactions, gradient diffusions, cell movement, and surface interactions might influence embryo development. Novel culture platforms are being developed as a result of interdisciplinary collaborations between biologists and biomedical, material, chemical, and mechanical engineers. These approaches are looking beyond the soluble media composition and examining issues such as media volume and embryo spacing. Furthermore, methods that permit precise and regulated dynamic embryo culture with fluid flow and embryo movement are now available, and novel culture surfaces are being developed and tested. While several factors remain to be investigated to optimize the efficiency of embryo production, manipulation of the embryo culture microenvironment through novel devices and platforms may offer a pathway toward improving embryo development within the laboratory of the future.
Keywords: blastocyst, dynamic culture, embryo culture, microfluidics, surface coating, vibration
New designs of platforms on which embryos are cultured permit use of dynamic fluid conditions, offering a benefit over traditional static culture techniques.
INTRODUCTION
Over the last two decades, arguably the most well-studied variables aimed at improving embryo development in vitro have involved the chemical composition of the culture media. Indeed these approaches have proven extremely beneficial and have undoubtedly contributed largely to improved success rates following assisted reproduction. Both sequential and monoculture media systems have been refined, and the development of high-quality blastocysts in vitro is now common place [1, 2]. However, not only do the chemical requirements of the developing embryo need to be considered, but potential physical requirements may also be important factors in the continuing pursuit of improved in vitro conditions. It is important to remember that progression of the embryo through the female reproductive tract not only results in exposure of the embryo to a changing fluid chemical composition, but also provides gentle mechanical stimulation, which may affect embryo development [3–5]. Furthermore, physical characteristics and parameters of the culture platform may influence chemical composition of the media via regulation of chemical gradients that form around the developing embryo. As our understanding of the preimplantation embryo improves and new analytical approaches and technologies emerge, examination of various novel culture platforms to explore the impact of physical and mechanical modifications on the embryo may assist in further improving in vitro development [6, 7]. Furthermore, these platforms may offer a potential means of improving other common procedures/approaches used within the in vitro fertilization (IVF) laboratory and elsewhere.
STATIC CULTURE PLATFORMS
In the past, mammalian and nonmammalian somatic cell lines, transformed cell lines, gametes, and embryos have been cultivated in, or on, inert surfaces such as glass or plastic polymers. These inert surfaces have taken various configurations, ranging from flat/walled Petri dishes, flasks, and test tubes (Fig. 1). In all of these cell-growth approaches, the culture environment is considered static unless external forces derived from a shaking platform or orbital agitator were employed. These static culture platforms act primarily as fluid-containing barriers and are differentially used based on media volumes and specific laboratory-dependent protocols. Using these static culture platforms for embryo culture can give rise to many different environments by simply altering the volume of media and the number of embryos per volume [8]. In many animal models, increased embryo density has been suggested to improve development, potentially through secretion of autocrine/paracrine factors. Embryos produce and secrete various factors [9–11] that have been suggested to affect embryo homeostasis, growth, and development [12]. This hypothesis is supported by studies using small confined volumes of media that enhance embryo development in comparison to larger volumes [13–16]. It has been speculated that this benefit is obtained because of concentrated biomolecules that support growth. When one considers the culture environment and the varied exposures that embryos experience, one can appreciate that manipulation of the physical environment—volumes, embryo density, and spacing—will also alter the chemical environment. These links between physical and chemical environment are difficult to separate both experimentally and biologically. Some very elegant studies focused on precise embryo spacing during the culture of porcine and bovine embryos have demonstrated that decreasing embryo spacing can be beneficial [17–19]. Additionally, in litter-bearing species such as the mouse, cow, and sheep, researchers have documented the benefit of group culture [14, 20–26]. Collectively these studies would suggest that embryos can communicate through paracrine biomolecules. Additionally, one must consider that embryos of different quality or stage of development may have beneficial or detrimental influence on companion embryos in the same culture environment [27, 28]. Finally, this group effect or spacing effect in non-litter-bearing species such as the human has yet to be fully delineated. Some reports would suggest that human embryos benefit from group culture [29, 30], yet it is recognized that group culture of human embryos is not requisite and that human embryos grown individually produce very efficient pregnancy rates. Future studies on the potential benefit of group culture and/or paracrine/autocrine biomolecule signaling in human embryos are significantly needed.
FIG. 1.
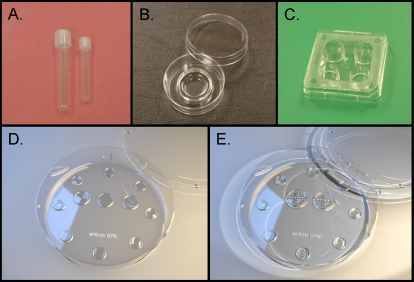
Photographs of numerous platforms used for rodent, domestic animal, nonhuman primate, and human gamete and embryo culture. A) Test tubes. B) Center-well organ culture dish. C) Four-well Nunc culture dish. D) Embryo GPS culture dish. E) Embryo corral culture dish. All of these culture platforms are composed of plastic, many times polystyrene, and provide the ability to retain, confine, and visualize gametes and embryos. Photographs of GPS and embryo corral culture dishes kindly provided by Dr. Donald Rieger and IVFonline, LLC.
As will be mentioned below, many laboratories culture groups of embryos in smaller volumes to obtain this perceived benefit of concentrating growth-promoting autocrine/paracrine biomolecules. It also has to be recognized that the same concentrating effect would occur for metabolic and/or secreted waste products. Additionally, these low volumes, static, high embryo density culture approaches require extreme attention to media properties because shifts in pH and osmolality are common and can have a profound impact on embryo development. Finally, contemporary embryo culture protocols in most species embrace a static environment on inert plastic ware, with limited cell-surface contact, which in reality overlooks the environment that nurtures embryo development in vivo. However, there are static culture platforms in development that reach beyond classic microdrop, center-well, or test-tube embryo cultures.
Specialized Microdrops and Ultralow Volume
Microdrops have long been the approach used to confine embryos to a small area to take advantage of the potential benefit of trophic autocrine/paracrine factors. Traditional volumes of these drops typically range from ∼10 to 50 μl, though some may be slightly less and can be utilized with group as well as individual embryo culture. One drawback to this approach, however, is that drops can fragment and/or coalesce, which can result in embryos being cultured in varying amounts of media and sometimes being difficult to locate and/or track. Specialized microdrop dishes are now available to alleviate this concern of drop/embryo displacement, and they may be beneficial in some laboratories for embryo development by expediting visualization and/or manipulation of the embryos during routine handling [31] (Fig. 1).
Another variation of the microdrop technique entails using extremely small volumes of media to presumably increase the concentration of beneficial embryo-secreted factors. Referred to as the ultramicrodrop approach, it commonly utilizes 1.5–2.0 μl of media to continuously culture groups of embryos for multiple days [15, 32]. Though only tested with human embryos in very small numbers, using sibling embryos in a prospective manner compared to larger 20-μl microdrops, ultramicrodrops has resulted in improved embryo development. A larger data set and more detailed endpoint analysis including pregnancy and implantation rates are required to determine whether the benefits of this approach outweigh the potential risk of working with such small volumes of media, including evaporation, damaging increases in osmolality, potential toxicity due to high embryo density, or embryo loss if media exchange is not performed properly.
A further static culture approach used extremely low submicroliter volumes of media and a culture chip composed of polydimethylsiloxane (PDMS) containing a small vertical channel [33]. When culturing 2-cell mouse embryos, rates of blastocyst development in the vertical channel with submicroliter surrounding volumes were comparable to those found in 20-μl microdrop cultures and were significantly greater than embryos grown in 5-μl microdrop cultures. Thus, this novel device appears to allow embryos to benefit from reduced culture volume and reduced spacing, while avoiding some issues associated with small microdrop volumes; the methodology, however, does include potential detrimental issues of its own, including difficulty in embryo recovery.
Microwells
Another variation of the microdrop that permits culture of embryos in a confined space but grants access to a larger reservoir of media is known as the microwell. This approach attempts to create a microenvironment in the immediate vicinity of individual or small groups of embryos and offers a means of potentially increasing surface area point-of-contact and decreasing spacing with/between embryos (Fig. 2). Furthermore, the approach avoids pitfalls associated with traditional microdrop displacement or merging.
FIG. 2.
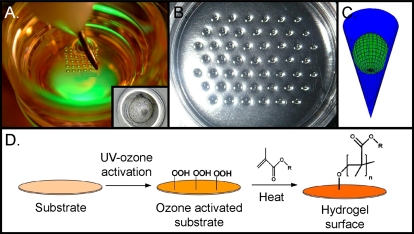
Photographs and schematics representing efforts made toward modification of the embryo culture microenvironment by confinement in microwells. A) Well-of-the-well (WoW) system, whereby curved microwells are produced in the bottom of a polystyrene Petri dish. Insert is micrograph of a blastocyst in a WoW microwell. B) Photograph of microwell imprints in PDMS contained in a Petri dish. C) These microwells can be constructed in a very precise manner as a cone with an opening of 180 μm and a bottom of approximately 10 μm. When embryos are place into these cone-shaped microwells, a microenvironment is formed. D) Schematic representing mechanism of surface modification that can be performed with ultraviolet-ozone activation of the surface and coating with a biomolecule of interest. This forms a uniform hydrogel surface that may have advantages in many types of cell culture [39]. Photograph of WoW kindly provided by Dr. Gabor Vajta.
Perhaps the most well-known microwell approach is the well-of-the-well (WOW) system, as first described by Dr. Vajta [16, 34]. The WOW system has been used successfully with embryos from a variety of species including mouse, pig, cow, and human as well as entails using small impressions, or microwells, of varying sizes and arrangements placed into the bottom of a vessel/dish [16, 34] (Fig. 2). At least one study with porcine embryos indicated the size of the WOW housing the embryo may be important, as 1000 μm wide wells yielded greater blastocyst development than 500 μm wide wells [35]. A similar WOW approach also resulted in differing gene expression levels in bovine embryos compared to microdrop cultured counterparts, perhaps offering further insight into the potential benefits [36].
While many of the WOW culture approaches utilized manual methods of creating the wells in existing Petri dishes, more recently, a WOW system was fabricated in polystyrene using injection molding to allow time-lapse photography of developing bovine embryos. Wells were 287 μm wide by 168 μm deep. Twenty-five wells (five-by-five configuration), each holding an individual embryo, were overlaid with 125 μl of media. Though no differences in blastocyst development (37.2% vs. 36.0%) or cell number (112 vs. 103) were observed in WOWs compared to microdrop controls, WOW-cultured embryos had lower amounts of apoptosis (9.0% vs. 13.5%) and oxygen consumption, closer to in vivo-derived counterparts [37]. Ultimately, these WOW-cultured embryos resulted in significantly higher pregnancy rates at 60 days following transfer (51.7% vs. 21.9%).
An alternate approach that permits a commercial means of utilizing microwells in conjunction with existing dishware in the laboratory involves using microwell inserts consisting of several rows of tiny culture wells composed of PDMS (Fig. 2, B and C). This approach has been developed to allow the culture of multiple individual embryos in the mouse, pig, and cow [38]. Interestingly, these inserts can be produced with isolated wells or with microfluidic channels connecting the wells to allow media exchange and to take advantage of the group culture effect. Such connected inserts could be useful in investigating the impact of autocrine/paracrine compounds versus embryo spacing. These microwells can be constructed in PDMS so finely that the embryos sit in these microwells as a sphere in a cone (Fig. 2C) and form microenvironments that may be beneficial for their development. Additionally, these microwells can be coated with selected biomolecules, such as hyaluronan or poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide [39] (Fig. 2D), as hydrogel surfaces that may influence microenvironments in close proximity to the developing embryos. Whether such hydrogel coatings influence mammalian embryo development remains to be determined; however they have been found to be useful in human embryonic stem cell growth [39].
In a similar, though less refined approach, polyester mesh inserts have also been used to culture porcine and bovine embryos to produce rudimentary microwells [19, 40] (Fig. 3). This mesh allows for easy separation and identification of embryos because each square of the grid houses an individual cell. This approach allows for decreased spacing of embryos and separation of cells at a specific distance. Indeed, use of an ∼220-μm mesh size (embryo spacing of ∼180 μm) yielded increased blastocyst cell number compared to controls or embryos cultured in larger mesh size [19]. Additional mesh sizes remain to be examined to elucidate any additional benefit.
FIG. 3.
Photographs of a mesh insert that can be utilized with traditional static culture dishes to regulate embryo spacing to take advantage of potential autocrine/paracrine factors that may benefit embryo development. Different size meshes can be used to regulate spacing and account for differences in embryo size. A) A mesh insert in a microdrop with a standard 60-mm culture dish. B) Magnification of the microdrop with the mesh insert inside. C) Close-up of embryos developing within a mesh insert. Images courtesy of Dr. Kei Imai.
Microchannels
Initial investigations into microchannel culture of mammalian embryos were described by collaborators in animal science and biomedical engineering. It was initially hypothesized that constraining the amount of media around the embryo in a microchannel as well as increasing the surface area adjacent to the embryo in comparison to a single point-of-contact within a Petri dish might influence embryo development. Raty and colleagues [41, 42] found that 2-cell mouse embryos could be cultured to the blastocyst stage within microchannels under static media conditions. In this experiment, the media volume in the control microdrops (30 μl) and the collective microchannel (10 μl) plus the reservoirs (∼490 μl) system as well as the microenvironment that acted on the developing embryos were different. These researchers found that culture in the microchannels resulted in significantly greater blastocyst formation and hatched blastocyst development at 72 and 96 h, respectively. In this static microchannel system, it was estimated that the effective volume of media surrounding the embryos in the microchannel was approximately 250 nl. However, this calculation is relevant only if one assumes no mixing of the surrounding media in the microchannels and reservoirs that likely occurs through passive diffusion [43]. In a following section, continuation of these studies and devices will be discussed in relation to the microchannel and dynamic media culture environment.
Another approach to performing microchannel culture of embryos was investigated using glass capillary tubes filled with culture media. This system also supports mouse development from the zygote to blastocyst stages [13, 14]. Interestingly, this microchannel system allows embryos to be cultured vertically, which permits increased cell-cell contact. Presently, it is unknown whether this vertical embryo culture platform and the altered spatial relationship between embryos, media, and the capillary walls is beneficial to mammalian embryo development.
In summary, based largely on data from animal models, several new culture approaches that provide reduced media volume and/or confined grouping of embryos may be beneficial for embryo development. Yet, there is little evidence that would allow such conclusions regarding human embryos. Nevertheless, practical utility and ease-of-use benefits of commercially available specialized microdrop dishes are appreciated and provide an alternative to traditional microdrops. Microwell approaches appear promising and have the potential benefit of integrating with real-time microscopic morphometric assessment of embryo development. However, whether microwell devices will provide robust embryo developmental benefit remains to be determined. There are numerous parameters of microwell construction, and the resulting microenvironment they produce, that remain to be tested. Finally, static ultramicrodrop and microchannel culture technologies appear to have benefits but must also be used with caution because of potential alterations that can occur in the stability of the media due to evaporation. Practical issues of placement of embryos into a microchannel and complete fidelity of recovery are of utmost importance in clinical embryology, and its importance cannot be overstated.
DYNAMIC CULTURE PLATFORMS
Over the past decades, nonadherent embryo culture has been modeled after adherent somatic cell culture, where cells are cultured in a relatively large volume of static fluid. It is important to recognize that no current culture system is completely static. Moving dishes or test tubes at any time results in disruption of the static state. For simplicity of discussion in this review, we will consider this static culture and dynamic culture as planned and/or controlled soluble component movement. As pointed out earlier, within the body preimplantation embryos develop in a mechanically and chemically dynamic environment. Again, these mechanical and chemical dynamics are almost impossible to experimentally separate; when a cell undergoes mechanical agitation, this also causes chemical agitation of media substrates and cell waste products, in close proximity to the cell surface, that may be used or secreted by the cell. With this said, there have been recent advancements in developing and testing dynamic culture conditions for preimplantation mammalian embryos.
If we consider that the in vivo state of preimplantation embryo growth in the oviduct and uterus is the ultimate environment to support development, we should be able to justify development of a dynamic culture system. The preimplantation in vivo embryo is continually moving due to muscle contractions and epithelial cell cilia movement. This results in a mechanical influence on the developing embryos. This movement also disrupts cells surface gradients that can form surrounding the embryos in static culture. It has been experimentally demonstrated that gradients exist in culture media under static culture conditions as a result of embryo secretions or depletion of media components. Gradients of potassium, calcium, and oxygen have been measured around mouse embryos [44, 45], and dynamic culture platforms may disrupt these gradients, providing a more homogenous environment that more closely recapitulates the in vivo growth environment.
A dynamic culture system also provides a controlled opportunity to furnish embryos with a continually refreshed supply of new substrates and removal of waste products. As mentioned earlier, degenerating embryos can have an adverse impact on normal appearing companion embryos [27, 28]. In this same line of reasoning, it has been demonstrated that degradation of media components can influence embryo development and also potentially have long-term developmental consequences on fetuses, neonates, and offspring health. For example, amino acids can be broken down at body temperature, releasing ammonia that can compromise normal embryo and fetal development [46–50]. With this said, there are specific substrates that can be used to protect against this degradation product, and many current culture media compositions protect against significant ammonia buildup [51].
There have been significant efforts employed to manually replace and refresh embryo culture media. When employing ultramicrodrops, it was demonstrated that regular manual replacement of media during a 3-day culture window was not beneficial in comparison to nonmedia replacement culture during the same interval [15]. Similar results were reported for the culture of bovine embryos in microdrops with nonmedia replacement in comparison to media replacement at 48-h intervals over a 186-h culture window [52]. These results would appear counterintuitive when one considers the dynamic embryo growth environment in vivo. However, this may be a result of the removal of autocrine/paracrine embryos-secreted factors that support embryo development. Yet, it also could be a result of manual manipulation of embryos, media, and media shifts in pH that occur outside the incubator environment when manual manipulations are performed to replenish the media. The inability to actually discern between these two possibilities with respect to outcome data further emphasizes the utility of an automated precisely controlled dynamic culture system to address basic studies on embryo development and interactions with the culture microenvironment.
Shaking/Rotation
A simple means of providing a dynamic embryo culture environment entails the use of a standard laboratory orbital shaker placed inside the incubator. This approach has been used successfully for mouse fertilization by using 0.5 mL of media overlaid with oil, agitated at 60 rpm [53]. Different volumes of media and times of agitation did not appear to influence the outcome measures. A similar approach has also been utilized with ovarian tissue culture [54]. Additional studies exist using a heated, agitated stage with a Petri dish of microdrops covered in oil to culture mouse or rat embryos [55, 56] though comparisons to adequate static culture controls and demonstration of significant benefit are lacking. Because of its simplicity and ease of implementation, this approach may warrant future investigation though important variables remain to be explored, including the role of the type of rotation (circular vs. linear) as well as rate of agitation. Other concerns exist with the use of electrical equipment in the humidified confines of a laboratory incubator.
Tilting
Investigators have studied the impact of embryo movement through a fixed volume of media using a tilting embryo culture system (TECS; [57]) (Fig. 4). This system employs a motorized tilting platform that can be placed inside an incubator and supports placement of traditional culture dishes on the platform. Both the angle of tilting and the speed of tilting change can be altered through computer-programmed software. Using frozen-thawed 2-cell mouse embryos, these researchers demonstrated that TECS could support blastocyst formation. Although the percentage of embryos reaching the blastocyst stage was not significantly different compared to controls, the number of cells per blastocyst was significantly higher [57]. These investigators also were able to show that TECS could support human embryo development. Recently, these investigators have reported the use of TECS for in vitro maturation of oocytes, in vitro fertilization, and culture of porcine embryos in defined media [58]. Interestingly, the use of this controlled dynamic culture system for in vitro maturation resulted in greater expansion of cumulus-oocyte complexes compared to controls. While the oocyte maturation, fertilization, and blastocyst formation rates were not significantly altered when collectively performed with TECS, the number of cells per blastocyst was significantly elevated compared to controls. Similar studies have been reported using bovine embryos, a titling culture system, and constricted microchannels [59]. Additional work is required to study the benefits and/or limitations of TECS. However, one can appreciate that this system provides a means of investigating controlled dynamic culture of oocytes and embryos.
FIG. 4.
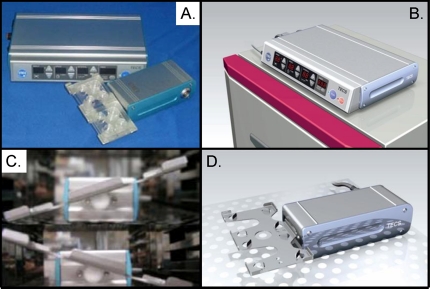
Photographs representing the components and function of the tilting embryo culture system (TECS). This system allows for continual and programmable movement of embryos within existing culture platforms, including Petri, center-well organ, and four-well culture dishes. A) Photographs of the two primary components, a motherboard component that controls the speed, angle, and period of tilting and the mechanical component that drives stage tilting and houses conventional culture dish technologies. B) The placement of the motherboard component on the outside of a tissue culture incubator. C) Two mechanical components within a tissue culture incubator that are at opposing angles of tilt. This mechanical component inside the incubator is attached to the motherboard component outside the incubator by a small cable that allows complete closure of the incubator and separation of the two components. D) Photograph of the mechanical component and its tilted stage. Photographs kindly provided by Dr. Keiji Naruse and STREX Incorporated of Japan.
Vibration
In contrast to other approaches that utilized large-scale media flow or platform movement, more subtle methods to induce dynamic culture conditions may be sufficient, such as vibration. It is estimated that in vivo the embryo is exposed to vibrations of ∼5–20 Hz in response to the ciliated epithelium of the oviduct [3, 4]. A simple vibrating culture platform, on which culture dishes are placed, has been used to mature porcine oocytes and culture the resulting parthenotes. Gentle vibration of oocytes during maturation appeared to improve oocyte developmental competence as a result of subsequent improvement in embryo development [60]. Additionally, vibration of oocytes for various durations yielded different blastocyst formation rates, indicating that differences in extended and/or constant vibration could affect development.
In a similar approach using pulsatile microvibration, immediately after fertilization with IVF or intracytoplasmic sperm injection (ICSI), presumptive human zygotes were cultured in the presence of gentle vibration of 20 Hz for 5 sec, once every hour [61, 62]. Patients were randomly assigned to either vibration or no vibration groups. Fertilization was assessed 18 h later and compared to unagitated controls. Embryo development and pregnancy rates were also compared between patients who had agitated or unagitated embryos. Gentle, short bursts of microvibration immediately following fertilization resulted in no difference in fertilization rates compared to static controls, but did result in significantly greater rates of high quality embryo development on Days 2–5. Vibration yielded higher rates of good quality 4- to 6-cell embryos on Day 2, higher rates of quality 8-cell embryos on Day 3, higher rates of morula formation on Day 4, and higher rates of blastocyst development on Day 5. Additionally, pregnancy rates from embryos exposed to microvibration were significantly higher than static cultured embryos following transfer on Days 3 and 5 [61]. Though ideally embryos from the same patient would have been split between the two treatment groups, these findings certainly suggest that further exploration of gentile vibration may be warranted. Possible causative factors for the benefits of pulsatile microvibration during embryo culture were recently reviewed and include secretion/stimulation of growth factors and activation of various signaling cascades [62].
Taken together, these data indicate that even short periods of gentle vibration in a periodic or pulsative manner during oocyte maturation or early fertilization events may benefit the resulting embryo quality. Future studies examining the impact of additional vibration frequencies as well as more in-depth studies on vibration timing paradigms, examining pulse length and frequency, over the duration of embryo culture may be insightful.
Controlled Fluid Flow
There are numerous ways to generate controlled fluid flow for dynamic cell culture. These can include gravity-driven flow, syringe-pump, or piezo-Braille pin actuation. Independent of the means of generating flow, it is important to recognize that embryos, like many other cell types, can detect the shear stress of fluid flow [63, 64]. Shear stress, in excess of 1.2 dyn/cm2, can cause damage to blastomeres, up-regulation of stress signaling pathway constituents, and embryo degeneration [63, 64]. In fact, as mentioned earlier, some of the initial work on microfluidics, dynamic media flow, and embryo development demonstrated a detrimental influence of fluid flow [65], which may be explained by shear stress and/or continual and complete removal of autocrine growth-promoting factors. Obviously the flow rate is important, can be regulated, and needs to be fully tested to determine the ranges for no effect, beneficial impact, or detrimental outcomes. Yet, in a controlled dynamic culture system, other factors such as the flow pattern, media volumes, complete refresh or recirculation, and friction/impact of cell-surface interactions are usually not independent and can influence both the overall fluid dynamics that the cell experiences as well as the biology of the cell. Some of these interactive forces and their influence on embryo development have been discussed previously [66].
The concept of dynamic media flow in embryo culture is not new. In the 1990s, numerous reports were generated using perfusion systems to generate fluid flow for dynamic embryo culture but yielded little or no benefit [67–69]. These types of studies utilized macroscale designs, modifications of soluble media composition, and/or somatic cell cocultures that in some instances interfered with possible interpretations. Advances in microfluidics and somatic cell culture in the 1990s and early 2000s paved the way for applications to gamete, embryo, and embryonic stem cell studies. These microfluidic platforms or systems allow automated approaches to achieve precise media movement and may prove to be more amenable for widespread use. These microfluidic systems can be envisioned as microscale perfusion systems that have the ability to operate over long periods with very little or no manual manipulation required. In adherent cell systems, including culture of human embryonic stem cells [70], perfusion devices have been operated successfully for over 1 wk [70–72]. Microfluidic systems that have been used to generate controlled dynamic embryo culture will be analyzed. It should be recognized that additional means of generating fluid flow at the microscale are available [73, 74] yet remain to be tested in relation to gamete and embryo culture.
Integrated gravity-driven flow in a microfluidic platform was first described for the isolation of motile sperm from human seminal plasma [75, 76]. A similar approach has been reported for microinsemination of mouse [77–79] and porcine oocytes [80]. Conceptually, these designs could also be used for embryo culture; however, they lack precise control and are not currently compatible with long-term (days to weeks) dynamic culture.
The use of syringe-based pumping for generating controlled dynamic embryo culture was first described in the laboratories of Drs. Wheeler and Beebe [81]. While the syringe-based pumps are far from microscale, they can be integrated with microfluidic platforms. Typically, the syringe-based pump is used to perfuse media into a reservoir. Microchannels originate from the bottom of the reservoir. Adherent cells, such as embryonic stem cells, can be cultured in the microchannels without flow to facilitate their attachment [70] prior to the syringe-based pump activation and generation of fluid flow in the microchannels. Nonadherent cells such as embryos are usually pipetted into the bottom of the reservoir closely located to the opening of the microchannel. Embryos then passively enter the microchannel and roll down the microchannel to an area where the microchannel is semioccluded; this thus forms a barrier and serves as a stopping point for the embryos in the microchannel. Syringe-based pumps can then be used to generate controlled fluid flow over the stationary embryos in the microchannel. Hickman and coworkers [65] used such a system to investigate changing fluid flow in microchannels on mouse embryo development. The flow rates that were examined did not enhance development compared to a static culture, and some of the evaluated flow rates actually compromised embryo development. This study begins to emphasize the interactive importance of flow rate, manner of flow delivery, and fluid dynamic surrounding the embryo, all of which are stresses that can be generated in controlled dynamic culture.
Attempts to actually design a microscale system using microfluidics specifically for dynamic culture of embryos began in our laboratories as a collaboration between embryo biologists and biomedical engineering experts in the early 2000s. A microfluidic system was designed for testing the influence of controlled fluid flow on embryo development. Concerted efforts were extended in making the design practical for clinical embryology. There was a strong desire not to place the embryos into the microchannels, where they may be unobtainable in the future, but instead to place the embryos into a microfunnel with microchannels acting as conduits for delivering and removing media from the bottom of the microfunnel. PDMS was used in the initial studies because of its optical transparency, biocompatibility, gas permeability, and ease of prototyping. However, early in the device's development, we recognized one of the primary challenges of working with PDMS: evaporation through the PDMS is significant and could result in marked shifts in media osmolality. This drawback of PDMS was circumvented by the design and use of a sandwich membrane that remained flexible, yet protected against evaporation [82]. The last major component was a mechanism to generate fluid flow in a controllable and long-term way. For this we adapted piezoelectric, movable pins on a commercially available Braille display that would typically be used to assist blind individuals in reading computer messages. These Braille pins are cone shaped, about the size of microfluidic microchannels, and can be programmed to move up, down, and in sequence spatially and temporally [83]. The use of the Braille pin actuators, where pins in sequence pressed against the flexible sandwich membrane, caused deformation of the PDMS microchannels, and like stepping on a garden hose, generated controlled, precise fluid flow. Importantly, we were able to produce an automated dynamic culture system for embryos that was not reliant on interconnections, tubings, or external pumps, which are historically known to make microfluidic systems complicated and inconvenient for practical use. Further details of the system can be obtained in the original manuscript [66].
Once the system appeared to work, mouse embryos were placed into the microfunnel reservoirs and allowed to settle to the bottom of the microfunnel; the cartridge was then placed with the Braille pins aligned under the microchannels. The rise and fall of the programmed Braille pins in a controlled fashion produced a pulsatile media flow through the bottom of the funnel (Fig. 5). A culture of 1-cell mouse zygotes for 96 h in the microfluidic-controlled dynamic system resulted in significantly greater advanced blastocyst development in comparison to controls and significantly higher blastocyst cell numbers compared to static culture controls; importantly, the system closely recapitulated results obtained from in vivo-derived blastocysts [66].
FIG. 5.
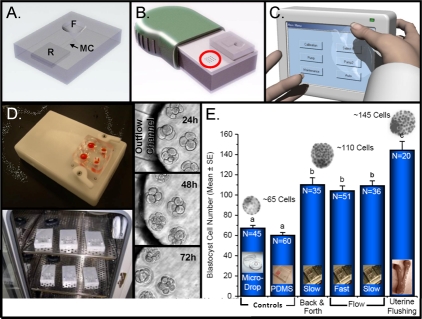
Composite schematics, photographs, micrographs, and graph representing concepts, prototypes, and data used to produce and test a microfluidic culture platform that allows a continual, consistent, and controllable dynamic embryo culture. A–C) Schematics of conceptual components of a microfluidic embryo culture system. A) A conceptual microfluidic cartridge made of the transparent and gas-permeable elastomer PDMS. This cartridge has a funnel (F) for media overlaid with oil and contains the embryos, a media reservoir to house the culture media (R), and microchannels (MCs) that connect R to the bottom of F. Each MC is 150 μm by 30 μm , and embryos do not enter the MCs. B) A conceptual mechanical platform for placement of the cartridge and actuation of fluid movement. This platform is approximately the size of a hand-held device and has two sets of Braille pins (one set encircled in red). The cartridge is placed on the platform with the Braille pins aligned under the MCs. Movement of the Braille pins upward displaces fluid in the MCs, and numerous pins in the moving sequence cause a pumping of the fluid through the MCs, resulting in automated pumping of fluid into and out of the bottom of F. C) A conceptual motherboard that contains the computer program to drive the movement of the Braille pins. This computerized motherboard sits outside the tissue-culture incubator and is connected to the Braille-actuation platform by a small cable, allowing complete closure of the incubator and separation of the two components. D) Prototype Braille-actuation platform (white) and microfluidic cartridge (clear with orange fluid to visualize F and R) are shown at top. Such prototypes have been used to evaluate the influence of dynamic microfluidic culture on mouse, bovine, and human embryos. A photograph of six Braille-actuation platforms in a tissue-culture incubator is shown below. Micrographs on the side represent human embryos development over time (24–72 h) with prototypes of dynamic microfluidic culture. E) Graph of mouse embryo development from the 1-cell stage following 96 h of culture with various conditions indicated on the x-axis. Values are total blastocyst cell count (mean ± SEM). Columns with different letters are significantly different (P < 0.01). The devices shown are the property of Incept Biosystems.
In an attempt to elucidate a biological mechanism to explain the level of advancement of mouse embryo development after 96 h of controlled microfluidic dynamic culture, experiments were performed with 24-h intervals of dynamic culture at the beginning or end of the culture period. It was found that a minimum of 48 h of dynamic culture was needed to see a beneficial impact on blastocyst development, and this effect did not depend on the stage of embryo development [66, 84]. Additionally, it was found that the developmental benefit was proportional to the duration of dynamic culture. Subsequent experiments were performed to determine if the improved blastocyst development observed with microfluidic dynamic culture translated into improved implantation or pregnancy rates. Indeed, it was observed that mouse embryos grown under microfluidic dynamic culture conditions had increased implantation rates, lower rates of absorption, and higher ongoing pregnancy rates [66]. Most recently, experiments have begun to address the influence of static versus dynamic culture conditions for preimplantation mouse embryos and placental imprinted gene expression. The outcomes from these studies could be extremely important considering the long-term health of offsprings derived by assisted reproductive technologies.
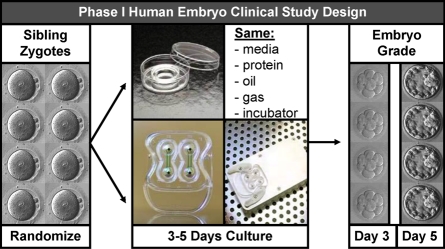
As mentioned earlier, the dependence and interaction of the flow rate, pattern, refresh rate, removal of embryo-secreted factors that may be beneficial, removal of embryo-secreted factors or by-products that may be waste, and shear stress all must be considered in a controllable dynamic culture system. Using a Fluent simulation system to compare factors influencing embryo culture in a static microdrop, in a microchannel with flow, and in a microfunnel with flow, we were able to speculate as to the chemical and mechanical reasons for the superior embryo development in the microfunnel system with flow [66]. Interestingly, this modeling system suggests that the dynamic microfunnel system benefits simultaneously fluid mechanical stimulation to the embryos and retention of secreted autocrine factors. Despite the fact that the fluid flowed into, within, and out of the designed microfunnel, it was found that the fluid, while dynamic, showed a pattern of circulating within the bottom of the microfunnel. While this was not necessarily achieved through design, this flow pattern appears to be beneficial for embryo development. A similar microfluidic dynamic culture system has been shown to be safe for the culture of human pronuclear zygotes to the blastocyst stage (Alegretti and Smith, unpublished data), and currently institutional review board-approved informed consent studies are underway in the United States and Brazil using sibling zygotes to ascertain if this microfluidic dynamic culture system is suboptimal, equivalent to, or beneficial for human embryo development in comparison to contemporary static culture approaches (Fig. 6).
FIG. 6.
Schematic of experimental design of an ongoing phase I clinical trial to assess the influence of microfluidic dynamic embryo culture on human embryo development. The study is being performed in the United States and Brazil under institutional board review and approval. Patients with equal to or greater than eight pronuclear zygotes are asked to volunteer and are provided informed consent documents prior to participation. Once consent is obtained, sibling zygotes from a consenting couple are randomly assigned to standard static group culture and dynamic microfluidic culture. The culture media, protein source, oil overlay, gaseous conditions, and temperature all remain constant. The outcome measures are embryo development at Day 3 (cell number and fragmentation) or Day 5 blastocyst grading of expansion and organization of inner cell mass and trophectoderm. The device shown is the property of Incept Biosystems.
Finally, Mizuno and coworkers [85, 86] have adopted a “womb-on-a-chip” design that incorporates endometrial cell coculture in a separated chamber with embryo growth in a different area that is bathed with conditioned media from the endometrial cells. This elegant system has been suggested to improve mouse embryo development; however, one has to question the practical and clinical utility of this coculture system when significant efforts over the last decades have been made to define culture components and their impact on development, genetics, and epigenetics.
CONCLUDING COMMENTS
It is apparent that the platform used for culture can influence preimplantation embryo development. Modifications of traditional static culture approaches, such as the microwell, have yielded some promising results, likely by concentrating tropic autocrine/paracrine factors. Dynamic culture systems are a relatively new and exciting area of study that offers a means of exploring the physical requirements of developing embryos as well as a method for manipulating the surrounding microenvironment in an attempt to improve development in vitro. The emerging data suggests that both simple forms of agitation and more advanced means for directing fluid movement may be beneficial for developing human embryos; therefore, more well-designed studies are required to determine the causative factors for the observed benefits. It is unclear whether the benefits of dynamic approaches are due to disruption of local gradients, causing a removal/dispersion of harmful by-products, replenishing of tropic nutrients, stimulation of mechano-receptors and activation of trophic signaling pathways, or a combination of these factors.
In addition to benefitting embryo development, perhaps more intriguing is the potential of various dynamic culture systems, primarily perfusion systems, to integrate various laboratory procedures onto a single device. Demonstration of this potential was demonstrated recently by combining IVF and subsequent embryo culture successfully on the same microfluidic device using mouse embyros [78, 79, 85]. Important to this approach, perfusion culture platforms offer a means to seamlessly vary various media over the developing embryos, thereby reducing associated stresses from repeated handling of the embryos as they are moved from dish to dish as well as reducing stress from abrupt changes of differing media. Using this approach, embryos would no longer be limited to exposure to solely single or sequential culture media with one or two replenishments during the culture period. This idea of exposing gametes and embryos to gradually changing solutions may be especially useful for approaches such as cryopreservation by either slow-rate freezing or vitrification. In both methods of cryopreservation, manual stepwise changes in the cryopreservation solution exposures cause cell shrinkage and reexpansion that can be detrimental to subsequent gamete/embryo cryosurvival and/or function. The ability to automate gradual cryosolution exchange over the cell, while maintaining the cell in a stationary position, could have numerous biological and technical advantages.
While the potential of these platforms is readily apparent, it is also clear that widespread adoption of these technologies has been slow. This stems, in part, to the inherent cautious nature of those involved in embryology and IVF. Often, deviation from established methods and protocols is difficult. However, other factors influencing implementation of novel culture platforms also exist. While the lack of mass production and commercial availability has likely stifled the adoption of more user-friendly static approaches like WOWs or variations of microwell approaches, failure to widely accept dynamic platforms is due, at least in part, to variability in operation and the fact that the approach is such an extreme departure from current practices. Furthermore, while simplified dynamic approaches like a tilting system can be implemented relatively easily with current culture dishes, more complex microfluidic approaches still face design and/or operation pitfalls that can make them more labor intensive to utilize. The cost of any new technology is also a valid concern that influences its ability to be utilized on a large scale. This cost may not only be higher for the platform/dishes themselves, but may include additional/new incubators or other equipment. Certainly elucidation of the causative factors of the observed benefits from novel culture platforms may persuade some to accept change and implement these new devices, but until a clear and significant commercial and/or clinical benefit can be demonstrated to offset the cost and perceived additional effort, widespread adoption of these approaches will remain difficult.
ACKNOWLEDGMENT
Professors Smith and Takayama are founders and stockholders of Incept Biosystems, a microfluidic company. All experiments described in this manuscript were performed independent of Incept Biosystems. No financials or materials were received by Incept Biosystems. Professors Smith and Takayama possess patents pertaining to microfluidics and embryo culture. Professors Smith and Takayama participate in Conflict of Interest Management at the University of Michigan.
Footnotes
Research in the authors' laboratories (G.D.S. and S.T.) on culture platforms have been supported by the National Institutes of Health (NIH; HD 049607), the United States Department of Agriculture (USDA), the Michigan Economic Development Corporation (MEDC; GR 696), the U.S. Army Research Laboratory and the U.S. Army Research Office under contract/grant number DAAD19-03-1-0168, and the Coulter foundation.
REFERENCES
- Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol 2007; 21: 83 100 [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod 2005; 20: 3376 3384 [DOI] [PubMed] [Google Scholar]
- Westrom L, Mardh PA, Mecklenburg CV, Hakansson CH. Studies on ciliated epithelia of the human genital tract. II. The mucociliary wave pattern of fallopian tube epithelium. Fertil Steril 1977; 28: 955 961 [DOI] [PubMed] [Google Scholar]
- Paltieli Y, Weichselbaum A, Hoffman N, Eibschitz I, Kam Z. Laser scattering instrument for real time in-vivo measurement of ciliary activity in human fallopian tubes. Hum Reprod 1995; 10: 1638 1641 [DOI] [PubMed] [Google Scholar]
- Fauci L, Dillon R. Biofluidmechanics of reproduction. Annu Rev Fluid Mech 2006; 38: 371 394 [Google Scholar]
- Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update 2011; 17: 541 557 [DOI] [PubMed] [Google Scholar]
- Smith GD, Swain JE, Bormann CL. Microfluidics for gametes, embryos, and embryonic stem cells. Semin Reprod Med 2011; 29: 5 14 [DOI] [PubMed] [Google Scholar]
- Reed ML, Woodward BJ, Swain JE. Single or group culture of mammalian embryos: the verdict of the literature. J Reprod Stem Cell Biol 2011; 2: 77 87 [Google Scholar]
- Bormann C, Swain J, Ni Q, Kennedy R, Smith G. Preimplantation embryo secretome identification. Fertil Steril 2006; 86: s116 [Google Scholar]
- Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril 2006; 86: 678 685 [DOI] [PubMed] [Google Scholar]
- Cortezzi SS, Garcia JS, Ferreira CR, Braga DP, Figueira RC, Iaconelli A, Jr, Souza GH, Borges E, Jr, Eberlin MN. Secretome of the preimplantation human embryo by bottom-up label-free proteomics. Anal Bioanal Chem 2011; 401: 1331 1339 [DOI] [PubMed] [Google Scholar]
- Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol 2008; 20: 292 304 [DOI] [PubMed] [Google Scholar]
- Thouas GA, Jones GM, Trounson AO. The “GO” system—a novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction 2003; 126: 161 169 [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod 1992; 7: 558 562 [DOI] [PubMed] [Google Scholar]
- Ali J. Continuous ultra micro drop culture yields higher pregnacy and implantation rates than either large-drop culture or fresh-medium replacement. Clin Embryol (Online) 2004; 7: 1 23 [Google Scholar]
- Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, Callesen H. New method for culture of zona-included or zona-free embryos: the well of the well (WOW) system. Mol Reprod Dev 2000; 55: 256 264 [DOI] [PubMed] [Google Scholar]
- Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo “cross talk” in vitro. Dev Biol 2005; 284: 62 71 [DOI] [PubMed] [Google Scholar]
- Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006; 131: 269 277 [DOI] [PubMed] [Google Scholar]
- Somfai T, Inaba Y, Aikawa Y, Ohtake M, Kobayashi S, Akai T, Hattori H, Konishi K, Imai K. Culture of bovine embryos in polyester mesh sections: the effect of pore size and oxygen tension on in vitro development. Reprod Domest Anim 2010; 45: 1104 1109 [DOI] [PubMed] [Google Scholar]
- Canseco RS, Sparks AE, Pearson RE, Gwazdauskas FC. Embryo density and medium volume effects on early murine embryo development. J Assist Reprod Genet 1992; 9: 454 457 [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Spitzer A, Batt PA. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol Reprod 1994; 50: 390 400 [DOI] [PubMed] [Google Scholar]
- Donnay I, Van Langendonckt A, Auquier P, Grisart B, Vansteenbrugge A, Massip A, Dessy F. Effects of co-culture and embryo number on the in vitro development of bovine embryos. Theriogenology 1997; 47: 1549 1561 [DOI] [PubMed] [Google Scholar]
- O'Doherty EM, Wade MG, Hill JL, Boland MP. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology 1997; 48: 161 169 [DOI] [PubMed] [Google Scholar]
- Goovaerts IG, Leroy JL, Van Soom A, De Clercq JB, Andries S, Bols PE. Effect of cumulus cell coculture and oxygen tension on the in vitro developmental competence of bovine zygotes cultured singly. Theriogenology 2009; 71: 729 738 [DOI] [PubMed] [Google Scholar]
- Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000; 54: 741 756 [DOI] [PubMed] [Google Scholar]
- Fujita T, Umeki H, Shimura H, Kugumiya K, Shiga K. Effect of group culture and embryo-culture conditioned medium on development of bovine embryos. J Reprod Dev 2006; 52: 137 142 [DOI] [PubMed] [Google Scholar]
- Spindler RE, Crichton EG, Agca Y, Loskutoff N, Critser J, Gardner DK, Wildt DE. Improved felid embryo development by group culture is maintained with heterospecific companions. Theriogenology 2006; 66: 82 92 [DOI] [PubMed] [Google Scholar]
- Spindler RE, Wildt DE. Quality and age of companion felid embryos modulate enhanced development by group culture. Biol Reprod 2002; 66: 167 173 [DOI] [PubMed] [Google Scholar]
- Moessner J, Dodson WC. The quality of human embryo growth is improved when embryos are cultured in groups rather than separately. Fertil Steril 1995; 64: 1034 1035 [DOI] [PubMed] [Google Scholar]
- Almagor M, Bejar C, Kafka I, Yaffe H. Pregnancy rates after communal growth of preimplantation human embryos in vitro. Fertil Steril 1996; 66: 394 397 [DOI] [PubMed] [Google Scholar]
- Rieger D, Schimmel T, Cohen J, Cecchi M. Comparison of GPS and standard dishes for embryo culture: set-up and observation times, and embryo development. Proceedings of the 14th World Congress on In Vitro Fertilization 2007; 141 [Google Scholar]
- Ali J, Shahata MA, Al-Natsha SD. Formulation of a protein-free medium for human assisted reproduction. Hum Reprod 2000; 15: 145 156 [DOI] [PubMed] [Google Scholar]
- Melin J, Lee A, Foygel K, Leong DE, Quake SR, Yao MW. In vitro embryo culture in defined, sub-microliter volumes. Dev Dyn 2009; 238: 950 955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajta G, Korosi T, Du Y, Nakata K, Ieda S, Kuwayama M, Nagy ZP. The well-of-the-well system: an efficient approach to improve embryo development. Reprod Biomed Online 2008; 17: 73 81 [DOI] [PubMed] [Google Scholar]
- Taka M, Iwayama H, Fukui Y. Effect of the well of the well (WOW) system on in vitro culture for porcine embryos after intracytoplasmic sperm injection. J Reprod Dev 2005; 51: 533 537 [DOI] [PubMed] [Google Scholar]
- Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 2009; 137: 415 425 [DOI] [PubMed] [Google Scholar]
- Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M, Hattori H, Kobayashi S, Hashiyada Y, Konishi K, Imai K. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod 2010; 83: 970 978 [DOI] [PubMed] [Google Scholar]
- Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev 2010; 22: 32 39 [DOI] [PubMed] [Google Scholar]
- Villa-Diaz L, Nandivada H, Ding J, Nogueira-de-Souza N, Krebsbach P, O'Shea K, Lahann J, Smith G. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nature Biotechnology 2010; 28: 581 583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth PJ, Watson TJ, Leese HJ. Prediction of porcine blastocyst formation using morphological, kinetic, and amino acid depletion and appearance criteria determined during the early cleavage of in vitro-produced embryos. Biol Reprod 2007; 77: 765 779 [DOI] [PubMed] [Google Scholar]
- Raty S, Walters EM, Davis J, Zeringue H, Beebe DJ, Rodriguez-Zas SL, Wheeler MB. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip 2004; 4: 186 190 [DOI] [PubMed] [Google Scholar]
- Raty S, Davis J, Beebe D, Rodriguez-Zas S, Wheeler M. Culture in microchannels enhances in vitro embryonic development of preimplantation mouse embryos. Theriogenology 2001; 55: 241 [Google Scholar]
- Beebe D, Wheeler M, Zeringue H, Walters E, Raty S. Microfluidic technology for assisted reproduction. Theriogenology 2002; 57: 125 135 [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Liu L, Smith PJ, Keefe DL. Noninvasive measurement of potassium efflux as an early indicator of cell death in mouse embryos. Biol Reprod 2000; 63: 851 857 [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod 2000; 62: 1866 1874 [DOI] [PubMed] [Google Scholar]
- Lane M, Hooper K, Gardner DK. Effect of essential amino acids on mouse embryo viability and ammonium production. J Assist Reprod Genet 2001; 18: 519 525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod 1993; 48: 377 385 [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Increase in postimplantation development of cultured mouse embryos by amino acids and induction of fetal retardation and exencephaly by ammonium ions. J Reprod Fertil 1994; 102: 305 312 [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod 2003; 69: 1109 1117 [DOI] [PubMed] [Google Scholar]
- Zander DL, Thompson JG, Lane M. Perturbations in mouse embryo development and viability caused by ammonium are more severe after exposure at the cleavage stages. Biol Reprod 2006; 74: 288 294 [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Summers MC. Discrepancies between the effects of glutamine in cultures of preimplantation mouse embryos. Reprod Biomed Online 2004; 9: 70 73 [DOI] [PubMed] [Google Scholar]
- Fukui Y, Lee ES, Araki N. Effect of medium renewal during culture in two different culture systems on development to blastocysts from in vitro produced early bovine embryos. J Anim Sci 1996; 74: 2752 2758 [DOI] [PubMed] [Google Scholar]
- Hoppe PC, Pitts S. Fertilization in vitro and development of mouse ova. Biol Reprod 1973; 8: 420 426 [DOI] [PubMed] [Google Scholar]
- Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, Braun F, Sadek F, van der Ven H. Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reprod Biomed Online 2006; 13: 228 234 [DOI] [PubMed] [Google Scholar]
- Cohen J, Ooms MP, Vreeburg JT. Reduction of fertilizing capacity of epididymal spermatozoa by 5 alpha-steroid reductase inhibitors. Experientia 1981; 37: 1031 1032 [DOI] [PubMed] [Google Scholar]
- Zeilmaker GH. Fusion of rat and mouse morulae and formation of chimaeric blastocysts. Nature 1973; 242: 115 116 [DOI] [PubMed] [Google Scholar]
- Matsuura K, Hayashi N, Kuroda Y, Takiue C, Hirata R, Takenami M, Aoi Y, Yoshioka N, Habara T, Mukaida T, Naruse K. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod Biomed Online 2010; 20: 358 364 [DOI] [PubMed] [Google Scholar]
- Koike T, Matsuura K, Naruse K, Funahashi H. In-vitro culture with a tilting device in chemically defined media during meiotic maturation and early development improves the quality of blastocysts derived from in-vitro matured and fertilized porcine oocytes. J Reprod Dev 2010; 56: 552 557 [DOI] [PubMed] [Google Scholar]
- Kim MS, Bae CY, Wee G, Han YM, Park JK. A microfluidic in vitro cultivation system for mechanical stimulation of bovine embryos. Electrophoresis 2009; 30: 3276 3282 [DOI] [PubMed] [Google Scholar]
- Mizobe Y, Yoshida M, Miyoshi K. Enhancement of cytoplasmic maturation of in vitro-matured pig oocytes by mechanical vibration. J Reprod Dev 2010; 56: 285 290 [DOI] [PubMed] [Google Scholar]
- Isachenko E, Maettner R, Isachenko V, Roth S, Kreienberg R, Sterzik K. Mechanical agitation during the in vitro culture of human pre-implantation embryos drastically increases the pregnancy rate. Clin Lab 2010; 56: 569 576 [PubMed] [Google Scholar]
- Isachenko V, Maettner R, Sterzik K, Strehler E, Kreinberg R, Hancke K, Roth S, Isachenko E. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod Biomed Online 2011; 22: 536 544 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod 2006; 75: 45 55 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev 2007; 74: 1287 1294 [DOI] [PubMed] [Google Scholar]
- Hickman D, Beebe D, Rodriguez-Zas S, Wheeler M. Comparison of static and dynamic medium environments for culturing of pre-implantation mouse embryos. Comp Med 2002; 52: 122 126 [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod 2010; 25: 613 622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JM, Reggio BC, Godke RA, Hansel W. Perfusion culture system for bovine embryos: improvement of embryo development by use of bovine oviduct epithelial cells, an antioxidant and polyvinyl alcohol. Reprod Fertil Dev 1997; 9: 411 418 [DOI] [PubMed] [Google Scholar]
- Goverde HJ, Peeters RH, Willems PH. The development of a superfusion system for studying intracellular and secretory processes in embryos. In Vitro Cell Dev Biol Anim 1994; 30A: 819 821 [DOI] [PubMed] [Google Scholar]
- Pruitt JA, Forrest DW, Burghardt RC, Evans JW, Kraemer DC. Viability and ultrastructure of equine embryos following culture in a static or dynamic system. J Reprod Fertil Suppl 1991; 44: 405 410 [PubMed] [Google Scholar]
- Villa-Diaz LG, Torisawa YS, Uchida T, Ding J, Nogueira-de-Souza NC, O'Shea KS, Takayama S, Smith GD. Microfluidic culture of single human embryonic stem cell colonies. Lab Chip 2009; 9: 1749 1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005; 5: 401 406 [DOI] [PubMed] [Google Scholar]
- Hung PJ, Lee PJ, Sabounchi P, Aghdam N, Lin R, Lee LP. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip 2005; 5: 44 48 [DOI] [PubMed] [Google Scholar]
- Zhang C, Xing D, Li Y. Micropumps, microvalves, and micromixers within PCR microfluidic chips: advances and trends. Biotechnol Adv 2007; 25: 483 514 [DOI] [PubMed] [Google Scholar]
- Pennathur S. Flow control in microfluidics: are the workhorse flows adequate? Lab Chip 2008; 8: 383 387 [DOI] [PubMed] [Google Scholar]
- Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem 2003; 75: 1671 1675 [DOI] [PubMed] [Google Scholar]
- Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online 2003; 7: 75 81 [DOI] [PubMed] [Google Scholar]
- Suh RS, Zhu X, Phadke N, Ohl DA, Takayama S, Smith GD. IVF within microfluidic channels requires lower total numbers and lower concentrations of sperm. Hum Reprod 2006; 21: 477 483 [DOI] [PubMed] [Google Scholar]
- Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L, Mitchelson K, Wang J, Huang G, Qiao J, Cheng J. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip 2010; 10: 2848 2854 [DOI] [PubMed] [Google Scholar]
- Ma R, Xie L, Han C, Su K, Qiu T, Wang L, Huang G, Xing W, Qiao J, Wang J, Cheng J. In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development. Anal Chem 2011; 83: 2964 2970 [DOI] [PubMed] [Google Scholar]
- Clark SG, Haubert K, Beebe DJ, Ferguson CE, Wheeler MB. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab Chip 2005; 5: 1229 1232 [DOI] [PubMed] [Google Scholar]
- Davis J, Raty S, Eddington D, Wheeler M, Glasgow I, Beebe D. Development of microfluidic systems for the culture of mammalian embryos. First International IEEE EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Lyon, France, October 12-14, 2000 [Google Scholar]
- Heo YS, Cabrera LM, Song JW, Futai N, Tung YC, Smith GD, Takayama S. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem 2007; 79: 1126 1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Zhu X, Futai N, Cho BS, Takayama S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc Natl Acad Sci U S A 2004; 101: 15861 15866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann C, Cabrera L, Heo Y, Takayama S, Smith G. Dynamic microfluidic embryo dynamic microfluidic embryo culture enhances blastocyst development of murine and bovine embryos. Proceedings from the 14th World Congress on in Vitro Fertilization 2007; 84 [Google Scholar]
- Mizuno J, Ostrovidov S, Nakamura H, Akaishi K, Inui H, Sakai Y, Fujii T, Anzai K, Watanabe A. Human ART on chip: development of microfluidic device for IVF and IVC. Hum Reprod 2007; 22: P432 [Google Scholar]
- Mizuno J, Ostrovidov S, Sakai Y, Fujii T, Nakamura H, Inui H. Human ART on chip: improved human blastocyst development and quality with IVF-chip. Fertil Steril 2007; 88: S101 [Google Scholar]