ABSTRACT
Uterine gland development (adenogenesis) in mice begins on Postnatal Day (PND) 5 and is completed in adulthood. Adenogenesis depends on estrogen receptor 1, and progesterone (P4) inhibits mitogenic effects of estrogen on uterine epithelium. This progestin-induced effect has been used to inhibit uterine gland development; progestin treatment of ewes for 8 wk from birth has produced infertile adults lacking uterine glands. The goals of the present study were to determine if a window of susceptibility to P4-mediated inhibition of uterine gland development exists in mice and whether early P4 treatment abolishes adenogenesis and fertility. Mice were injected daily with P4 (40 μg/g) or vehicle during various postnatal windows. Adenogenesis, cell proliferation, and expression of key morphoregulatory transcripts and proteins were examined in uteri at PNDs 10 and 20. Additionally, adenogenesis was assessed in isolated uterine epithelium. Treatment during PNDs 3–9, 5–9, or 3–7 abolished adenogenesis at PND 10, whereas treatments during PNDs 3–5 and 7–9 did not. Critically, mice treated during PNDs 3–9 lacked glands in adulthood, indicating that adenogenesis did not resume after this treatment. However, glands were present by PND 20 and later following treatment during PNDs 5–9 or 3–7, whereas treatment during PNDs 10–16 produced partial inhibition of adenogenesis at PND 20 and later. Epithelial proliferation at PND 10 was low following P4 treatment (PNDs 3–9) but exceeded that in controls at PND 20, indicating a rebound of epithelial proliferation following treatment. Messenger RNA for Wnt, Fzd, and Hox genes was altered by neonatal P4 treatment. All groups cycled during adulthood. Mice treated with P4 during PNDs 3–9, but not during other developmental windows, showed minimal fertility in adulthood. In summary, brief P4 treatment (7 days) during a critical neonatal window (PNDs 3–9) transiently inhibited epithelial proliferation but totally and permanently blocked adenogenesis and adult fertility. This resulted in permanent loss of uterine glands and, essentially, total infertility during adulthood. The narrow window for inhibition of adenogenesis identified here may have implications for development of this methodology as a contraceptive strategy for animals.
Keywords: adenogenesis, ESR1, fertility, uterus
Exposure of neonatal mice to progesterone during a narrowly circumscribed, early postnatal window prevents uterine gland development.
INTRODUCTION
Uterine endometrium consists of luminal epithelium and underlying stroma. In addition to fibroblastic connective tissue, immune cells, and vascular elements, stroma contains endometrial glands that empty into the uterine lumen. Initially, the developing uterus consists of a tubular central epithelium encased in an undifferentiated mesenchymal sheath. Uterine glands originate as invaginations of luminal epithelium and grow into the underlying stroma. In most species, uterine gland development (adenogenesis) begins during the first 2 wk of postnatal life [1, 2]. Uterine adenogenesis is dependent on estrogen receptor 1 (ESR1; also known as estrogen receptor alpha), as shown by Esr1 knockout mice lacking proper gland development [3].
Despite essential roles of ESR1 in adenogenesis and adult uterine epithelial proliferation, neonatal epithelial mitogenesis and adenogenesis do not require steroid hormone support, as indicated by these events occurring normally in neonatal rodents subjected to a combination of hypophysectomy, ovariectomy, and adrenalectomy [4]. Though not established definitively, it has been suggested that growth factors may activate ESR1 signaling neonatally and, thereby, support epithelial proliferation. Neonatal uterine epithelium is not dependent on estrogen for proliferation but is mitogenically responsive to it, as shown by estrogen treatment increasing epithelial proliferation in neonatal mice [5].
Progestins are well-known inhibitors of estrogen actions on uterine epithelium. For example, progesterone (P4) treatment essentially abolishes the robust epithelial proliferation normally induced by estrogen treatment of ovariectomized mice [6]. P4 also inhibits uterine epithelial proliferation in neonatal mice [7]. Thus, P4 inhibits mitogenic signals mediated through activated ESR1, even though ESR1 activation is not dependent on estrogen neonatally.
This ability of postnatal progestin treatment to inhibit epithelial mitogenesis and, thus, uterine gland development was utilized by Bartol and colleagues to produce a sheep model in which uterine gland development was permanently blocked by neonatal progestin treatment [8–11]. These ewes, designated as uterine gland knockout animals [9], were infertile during adulthood despite ovulating fertilizable oocytes, indicating that uterine glands are essential for fertility in sheep [1, 12–14].
Studies with knockout mice have provided insights regarding the development and function of uterine glands. Mice lacking Wnt5a or Wnt7a lack uterine glands and are infertile [15–17], whereas mice containing a stabilized form of β-catenin, which increases Wnt signaling, have increased uterine glands [18]. Foxa2, a transcription factor that is a member of the forkhead transcription factor family, is also essential for normal uterine adenogenesis [19], and Foxa2 knockout mice have impaired fertility. Knockout mice lacking leukemia-inhibitory factor, which normally is produced only in glands of the uterus, are also infertile [20, 21], suggesting that inhibition of uterine gland function is sufficient to induce infertility. Similarly, knockdown of calcitonin, which is only expressed in uterine glandular epithelium, impairs implantation in mice, further emphasizing the critical role of uterine glands in fertility [22, 23].
The ability of neonatal progestin treatment to permanently inhibit uterine gland formation and the necessary role of uterine glands in fertility suggest that neonatal progestin treatment may have promise as a permanent contraceptive methodology in various animal species. Initial studies in sheep involved administration of a progestin over a 2-mo postnatal period [9, 10]. In earlier work, however, progestin treatment for only 2 wk starting at birth produced a temporary inhibition of uterine gland development, but gland development had resumed at Postnatal Day (PND) 26 [8]. If neonatal progestin administration can be developed as a feasible contraceptive strategy, it would be optimal to design a treatment of sufficient length to fully and permanently inhibit uterine gland formation while exposing the animal to progestins for only the minimal time needed.
In mice, uterine gland formation begins at approximately PND 5 [24] and becomes maximal between PNDs 30 and 60 [25]. It is unclear whether progestin treatment would be needed during the entire period of adenogenesis to fully suppress this process or if shorter treatments might suffice. Similarly, it is unclear whether full inhibition of adenogenesis requires progestin treatment beginning at or before gland formation or whether progestin treatment after initiation of adenogenesis might also be effective.
To answer these questions, we treated neonatal mice with P4 for various intervals during postnatal life and examined effects on uterine gland development at the cellular and molecular levels. Results indicate that P4 exposure for as little as 1 wk during a clearly circumscribed neonatal period is sufficient to permanently inhibit uterine adenogenesis in mice. However, this treatment must commence before the onset of uterine adenogenesis to have long-term consequences on uterine glandularity and fertility.
MATERIALS AND METHODS
Animals and Animal Care
Mice (C57BL/6) were housed at 25°C with a 12L:12D photoperiod and given water and a standard rodent diet ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Illinois and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Day of birth was considered as PND 0. Litters were adjusted to 6–8 pups/litter. Only one animal per litter was used for each time point so that the litter could be considered as the experimental unit.
Experimental Groups and P4 Dosing
Previous work suggested that maximal inhibition of uterine epithelial proliferation was obtained at 40 μg/g of P4 [7]. A preliminary experiment was performed in neonatal mice with one injection of this dose of P4 (Sigma-Aldrich), administered in 0.1 ml of corn oil, and this produced almost total inhibition of epithelial proliferation 24 h later. Therefore, this dose was utilized in subsequent experiments.
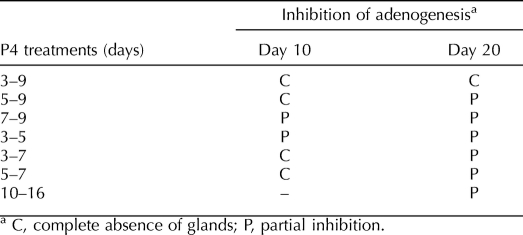
Mice were injected daily with P4 (40 μg/g) during PNDs 3–9, 3–5, 3–7, 5–7, 5–9, 7–9, and 10–16 (n = 12–20 per group) to encompass the period before, during, and after onset of adenogenesis and peak proliferation of nascent glandular epithelium. Control mice were treated with oil vehicle alone during PNDs 3–9. Body weights of pups were recorded during and after P4 treatment.
Uterine Epithelial Separation
To visualize uterine gland development at various ages and following different treatments, uterine epithelium was isolated from stroma using an enzymatic methodology described previously [26]. Briefly, uteri from various groups of 10- and 20-day-old mice (n ≥ 4 per group) were removed and weighed. These uteri, along with adult uteri from control and P4-treated mice (n ≥ 4 per group), were enzymatically dissociated in a solution of 1% trypsin (Difco) in calcium- and magnesium-free Hanks balanced salt solution for 90 min at 4°C, followed by dissection with jeweler's forceps and a Graefe knife to remove intact epithelium from surrounding stroma. The epithelium was then photographed using an Olympus microscope interfaced to an Apple computer system containing ImageJ software from the NIH.
Immunohistochemistry for MKI67 and HAND2
To assess cell proliferation in control and P4-treated uteri, slides were stained for a cell proliferation marker, MKI67, using a mouse anti-human monoclonal immunoglobulin G to human MKI67 (BD Transduction Laboratories). Uteri were fixed by immersion in 10% neutral-buffered formalin, embedded in paraffin using standard techniques, sectioned (thickness, 4 μm), and immunostained for MKI67 following antigen retrieval as described previously [27].
Inhibitory effects of P4 on uterine epithelial proliferation are mediated by the transcription factor HAND2 [28]. Therefore, HAND2 expression was evaluated in control mice at PNDs 1, 5, and 10 to determine if this protein was expressed neonatally. Following antigen retrieval, sections were blocked with 3% rabbit serum and incubated overnight at 4°C with goat polyclonal antibody against HAND2 (sc-9409; Santa Cruz Biotechnology). Secondary antibody staining was performed with the rabbit VECTASTAIN Elite ABC kit (catalog no. PK-6101; Vector Laboratories) and 3,3′-diaminobenzidine substrate kit (Vector Laboratories) according to manufacturer's instructions.
Negative controls for both MKI67 and HAND2 were processed without the corresponding primary antibody. After immunostaining, tissues were counterstained with Mayer hematoxylin (Sigma-Aldrich). Labeling index was determined by analyzing MKI67 staining in approximately 500 uterine epithelial or stromal cells.
In Situ Hybridization
In situ hybridization analyses of mouse uteri were conducted using methods described previously [10]. Briefly, deparaffinized, rehydrated, and deproteinated cross-sections (thickness, 5 μm) of uterine horns were hybridized with radiolabeled sense or antisense cRNA probes generated from linearized plasmid DNA templates using in vitro transcription with [35S-α]uridine triphosphate. After hybridization, washing, and ribonuclease A digestion, slides were dipped in NTB liquid photographic emulsion (Kodak), stored at 4°C for 4–30 days, and developed in Kodak D-19 developer. Slides were counterstained with Gill modified hematoxylin, dehydrated through a graded series of alcohol to xylene, and then coverslipped.
Quantitative Real-Time RT-PCR Analysis
Total RNA was isolated from uteri using the TRIzol Reagent (Invitrogen) according to the manufacturer's recommendations. Quantity and quality of total RNA were determined by spectrometry and denaturing agarose gel electrophoresis, respectively. Complementary DNA was synthesized from total RNA (2 μg) using the iScript Select cDNA Synthesis Kit (Bio-Rad). Real-time PCR analysis of mRNA expression was performed using a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad), with iQ SYBR Green Supermix (Bio-Rad) as the detector, according to manufacturer's recommendations. Primers (Table 1) were designed to amplify cDNAs of approximately 100 bp, and all exhibited similar amplification efficiency (95% ± 3%) as assessed by amplification of cDNA dilution series. PCR cycle parameters were 95°C for 15 sec and 60°C for 1 min for 40 cycles. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (CT) values were determined as the cycle number at which the threshold line crossed the amplification curve. PCRs without template or with total RNA substituted for template were used as negative controls to verify experimental results. After amplification, the specificity of the PCR was determined by both melt-curve analysis and gel electrophoresis to verify that only a single product of the correct size was present. Data were normalized against Gapdh and are shown as the fold-increase (average ± SEM). The fold-changes are equivalent to 2x−y, where x is the CT value of the control and y is the CT value following P4 treatment.
TABLE 1.
Sequences of primers for real-time RT-PCR.
Assessment of Reproductive Cyclicity and Fertility
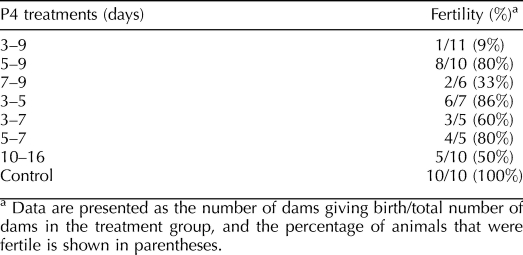
Reproductive cyclicity was assessed by daily vaginal lavage over a period of 15 days in adult animals of all groups. To assess fertility, adult mice (age, ≥60 days; n = 5–11 for each group) were housed with proven male breeders for 14 days. Pregnancies were noted, and litter size was recorded when dams gave birth.
Statistical Analyses
Uterine weight and cell proliferation data were analyzed using one-way ANOVA or Student t-test and are presented as the mean ± SEM. Differences were considered to be significant at P < 0.05. Statistical analysis was carried out using GraphPad Prism 4.0 (GraphPad Software, Inc.).
All quantitative real-time PCR data were subjected to one-way ANOVA, and differences between individual means were compared by a Tukey multiple-range test using GraphPad Prism 4.0. Real-time PCR data were corrected for differences in sample loading using the Gapdh data as a covariate. Tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error. Differences were considered to be significant at P < 0.05. Data are presented as least-square means with SEM.
RESULTS
Effects of P4 Treatment on Uterine Gland Development and Epithelial Proliferation at PNDs 10 and 20
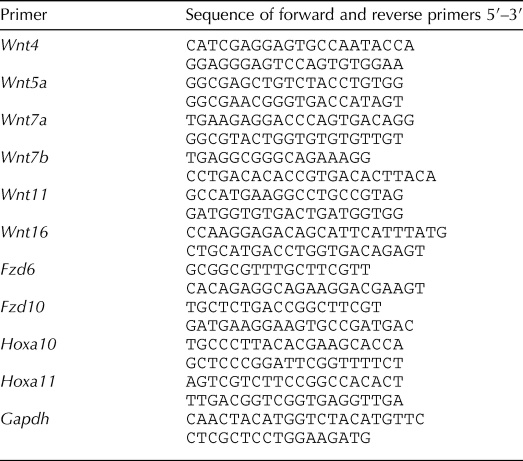
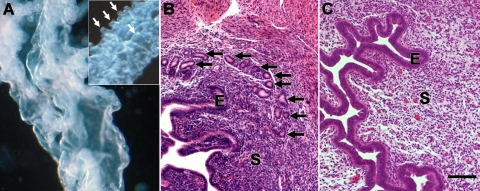
Uterine gland development in the C57BL/6 mice began at PND 5 (Fig. 1A). At PND 10 in control mice, uterine epithelium showed more extensive gland development around the total circumference of the epithelium (Fig. 1B). Treatment during PNDs 3–9, 3–7, 5–7, or 5–9 totally abolished gland development at PND 10; epithelium of these uteri consisted of a cylindrical tube without glands (Fig. 1, C and D, and Table 2). In contrast, in mice treated during PNDs 7–9 or 3–5, some gland development was seen at PND 10 (Table 2).
FIG. 1.
Uterine adenogenesis in C57BL/6 control mice and mice exposed to P4 (40 mg/kg) during PNDs 3–9. A) Adenogenesis begins on PND 5, as shown by the absence (top) and presence (bottom; arrows) of uterine glands in isolated epithelium from a 5- and 6-day-old animal, respectively. B–D) Both gland number and size (arrows) are increased in PND 10 uterine epithelium isolated from control mice (B) compared to PND 6. Treatment with P4 during PNDs 3–7 (C) or 3–9 (D) resulted in total absence of glands in epithelium at PND 10. Bar = 100 μm.
TABLE 2.
Effect of various regimens of neonatal P4 treatment on uterine adenogenesis at 10 and 20 days postnatal.
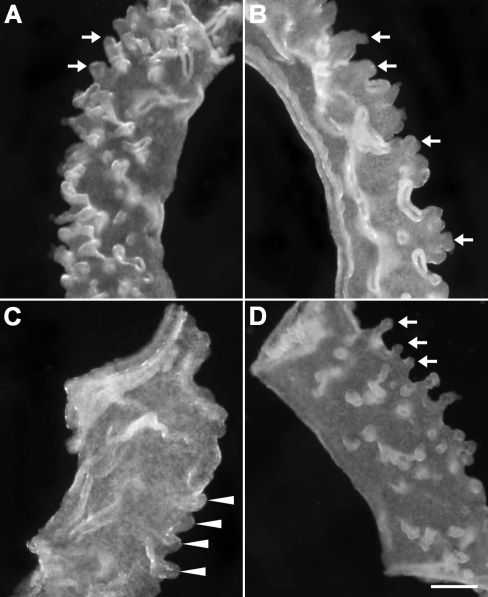
By PND 20, glands in control mice were more extensive, both in size and in number (Fig. 2A). Mice treated with P4 during PNDs 3–7 (Fig. 2B), 5–7, or 5–9 showed distinct gland formation by PND 20, despite absence of glands in these groups at PND 10 (Table 2). In contrast, the absence of glands at PND 10 in mice treated during PNDs 3–9 persisted at PND 20 (Fig. 2C and Table 2), indicating that glandular morphogenesis did not resume after the last P4 treatment at PND 9. In mice treated with P4 during PNDs 10–16, gland development at PND 20 was sharply reduced compared to that in controls (Fig. 2D).
FIG. 2.
Adenogenesis in 20-day-old control mice and mice exposed to P4 postnatally. Adenogenesis in controls (arrows) was more extensive at PND 20 (A) than at PND 10 (compare with Fig. 1B). By PND 20, mice treated with P4 during PNDs 3–7 (B) showed gland development (arrows) despite lacking glands at PND 10 (compare with Fig. 1D). In contrast, at PND 20, uterine epithelium from mice treated with P4 during PNDs 3–9 still lacked any gland development (C). Note that the uterine epithelial folds indicated by arrowheads in C are not glands. When mice were treated with P4 during PNDs 10–16, by PND 20 glands were apparent (D), although less extensive than in controls (A), and gland number appeared reduced compared to controls at PND 10. Bar = 100 μm.
Uterine weights from control and P4-treated (PNDs 3–9) mice were not different at PND 10. In contrast, by PND 20, uterine weights in the P4-treated group were approximately one-third less (P < 0.05) than those in the controls (data not shown).
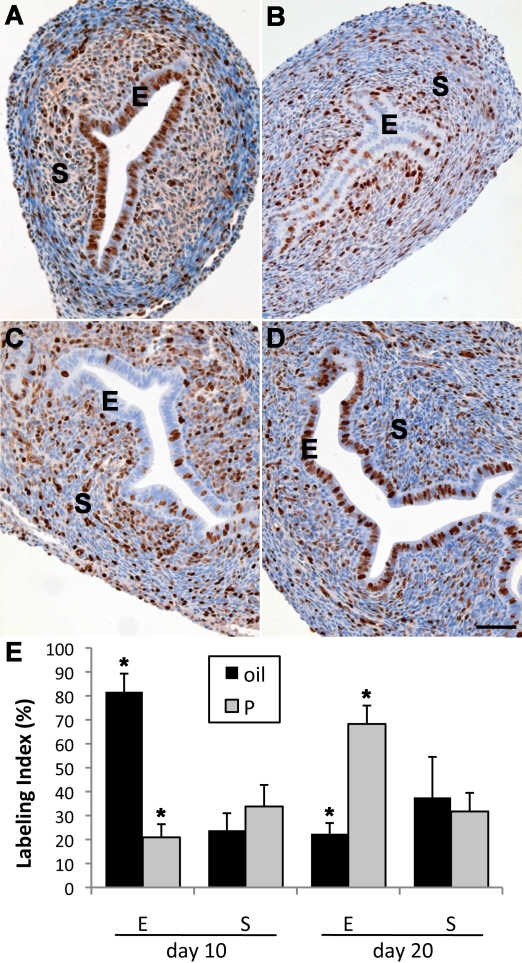
Analyses of MKI67 staining indicated that uterine stromal and epithelial proliferation at PND 10 was robust in control mice (Fig. 3, A and E). Conversely, epithelial proliferation at PND 10 was low following P4 treatment during PNDs 3–9 (Fig. 3, B and E), and similar results were seen at this age with other P4 treatments (e.g., PNDs 3–7 and 5–9; data not shown). In contrast to the sharply inhibited epithelial proliferation, stromal proliferation showed a trend toward an increase in mice treated with P4 during PNDs 3–9, although this did not reach significance (Fig. 3, B and E). Epithelial proliferation in control mice was much lower at PND 20 than at PND 10 (Fig. 3, C and E). In contrast, epithelial proliferation at PND 20 in mice treated with P4 during PNDs 3–9 greatly exceeded that in controls (Fig. 3, D and E), indicating a large and extended rebound of epithelial proliferation after cessation of P4 treatment at PND 9. Significantly, despite robust epithelial proliferation at PND 20 in mice treated with P4 during PNDs 3–9, gland development was absent (Fig. 3D). These data agreed with results observed with isolated epithelium.
FIG. 3.
Effects of neonatal P4 treatment on uterine cell proliferation as measured by immunohistochemical staining of MKI67. At PND 10, extensive proliferation was seen in both epithelium (E) and stroma (S) of control uteri (A). In contrast, uterine epithelial proliferation in 10-day-old mice treated with P4 during PNDs 3–9 was dramatically reduced (B), although stromal (S) proliferation remained robust. By PND 20 in control uteri, both epithelial and stromal proliferation (C) was reduced compared to PND 10. In contrast, 20-day-old uterine epithelium in mice that received P4 during PNDs 3–9 showed robust epithelial proliferation (D) exceeding that of controls. Bars = 100 μm. Quantitation of uterine epithelial and stromal labeling index in the groups mentioned above indicates that epithelial proliferation in mice treated during PNDs 3–9 with P4 is sharply reduced compared to controls at PND 10 but significantly exceeds controls by PND 20 (E). Stromal labeling index was not significantly different than control in P4 (P)-treated mice at PNDs 10 or PND 20, despite a trend toward an increase in the former. *P < 0.05 vs. control. Data are shown as mean ± SEM.
HAND2 Is Expressed in the Neonatal Uterus
Expression of HAND2, a key mediator of antiproliferative effects of P4 on uterine epithelium [28], was high in stroma of PND 5 uterus (Fig. 4). Conversely, differentiating myometrial and epithelial layers did not show significant HAND2 staining. Similar results were seen at PNDs 1 and 10 (data not shown).
FIG. 4.
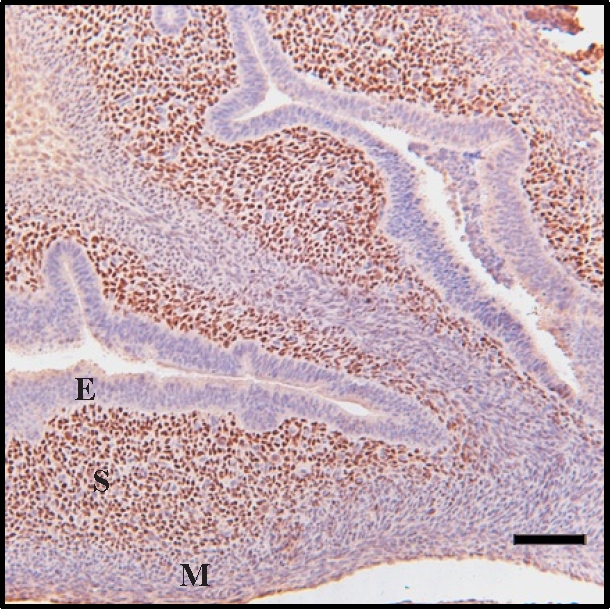
Immunohistochemical staining of HAND2 in the 5-day-old neonatal mouse uterus. Strong staining was seen in stroma (S) cells, whereas both epithelium (E) and myometrium (M) were unstained. Bar = 100 μm.
P4 Regulates Wnt, Fzd, and Hox Gene Expression in Neonatal Uterus
Wnt and Hox genes are conserved, critical regulators of development in female reproductive tract organs, including the uterus [15, 16, 29]. To determine the potential role of Wnts, their Fzd receptors, and Hox genes in the P4-induced disruption of gland formation, we examined the spatial localization and quantitative expression of Wnt, Fzd, and Hox genes by in situ hybridization and real-time RT-PCR, respectively (Figs. 5–7). Uterine gene expression was compared in uteri from control mice and mice treated with P4 during PNDs 3–9, because this was the regimen that permanently inhibited uterine gland development and adult fertility. Mice were killed either on PND 10, a day after the conclusion of P4 treatment, or on PND 20 to examine these key genes involved in uterine adenogenesis.
FIG. 5.
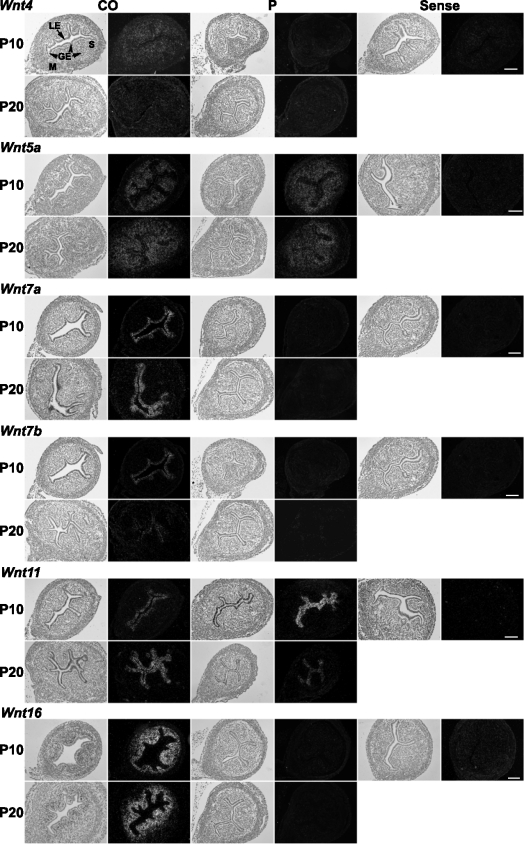
Spatial localization of Wnt gene expression was examined by in situ hybridization analysis in uteri from control mice (CO) and mice that received P4 (P) injections daily during PNDs 3–9 (n = 5 per treatment per day). In each panel portion, representative photomicrographs of in situ hybridization results are shown with light-field and dark-field illumination, respectively. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; S, stroma. Bars = 100 μm.
FIG. 7.
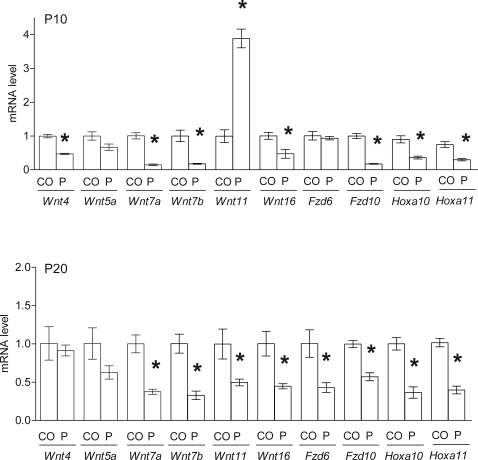
Wnt, Fzd, and Hox gene expression was quantitated by real-time RT-PCR analysis in uteri from control (CO) mice and mice that received P4 (P) injections daily during PNDs 3–9. *P < 0.05 vs. CO.
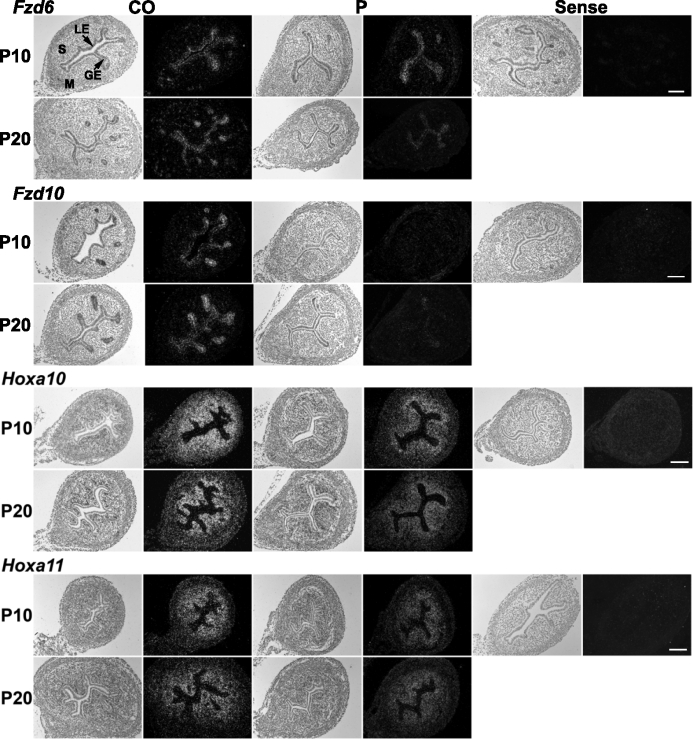
Epithelial Wnt7a and Wnt7b expression (Figs. 5 and 7) and Fzd10 expression (Figs. 6 and 7) were suppressed at PND 10 and PND 20 in mice exposed to P4 during PNDs 3–9 (P < 0.05). Fzd6 was inhibited in the epithelium on PND 20 but not affected on PND 10 (Figs. 6 and 7). Exposure to P4 during PNDs 3–9 transiently increased Wnt11 in uterine epithelium on PND 10 but decreased it below control level by PND 20 (P < 0.05) (Figs. 5 and 7). Stromal Wnt16, Hoxa10, and Hoxa11 were inhibited on PNDs 10 and 20 after P4 exposure during PNDs 3–9 (P < 0.05) (Figs. 5–7). Wnt4 was decreased in stroma on PND 10 (P < 0.05) but unaffected on PND 20. P4 exposure did not alter Wnt5a in neonatal uterus.
FIG. 6.
Spatial localization of Fzd and Hox gene expression was examined by in situ hybridization analysis in uteri from control mice (CO) and mice that received P4 (P) injections daily during PNDs 3–9 (n = 5 per treatment per day). In each panel portion, representative photomicrographs of in situ hybridization results are shown with light-field and dark-field illumination, respectively. GE, glandular epithelium; LE, luminal epithelium; M, myometrium; S, stroma. Bars = 100 μm.
Cyclicity, Fertility, and Adult Uterine Histology
Females in all treated and control groups displayed normal estrous cycles. Thus, P4 treatment did not impair reproductive cyclicity.
All dams in the control group (10/10) gave birth to viable litters, with an average litter size of 7.2 ± 1.1. Mice treated with P4 showed variable reductions in fertility that were related, to some extent, with duration of P4 treatment. For example, mice treated with P4 during PNDs 3–5 or 3–7 showed modest decreases in fertility, with 86% and 60% of the dams, respectively, becoming pregnant and delivering litters (Table 3). Litter sizes in several groups (PNDs 7–9, 5–9, 3–5, and 10–16) were not different from those of controls, but in the groups receiving P4 treatment during PNDs 5–7 and 3–7, litter size averaged from four to five pups. The one mouse that became pregnant after P4 treatment during PNDs 3–9 delivered only three pups.
TABLE 3.
Fertility in adult mice given various regimens.
Adult uteri from mice treated with P4 during PNDs 3–9 were almost devoid of uterine glands (Fig. 8, A and C), in contrast to the extensive uterine glands typically present in control uteri (Fig. 8, A and B). Interestingly, the uterus from the one mouse that became pregnant in this group had 15 identifiable glands in the uterine cross-section examined (data not shown).
FIG. 8.
Adult uteri lack glands following neonatal P4 treatment during PNDs 3–9. A) Isolated uterine epithelium from an adult mouse that was treated during PNDs 3–9 with P4. This epithelium showed an absence of glands, although folds of uterine epithelium were still present. In contrast, adult uterine epithelium normally showed extensive glands that covered the epithelial surface (inset). B and C) Histological sections of adult control uteri (B) showed extensive glands (arrows), whereas uteri exposed to P4 during PNDs 3–9 (C) lacked glands in adulthood, corroborating observations in isolated epithelial whole mounts (A). E, epithelium; S, stroma. Bar = 200 μm (A) and 100 μm (B and C).
The duration of and age at initiation of P4 treatment had major effects on ultimate gland number and fertility in adulthood. In sharp contrast to mice treated with P4 during PNDs 3–9, mice treated with P4 during PNDs 3–5, 3–7, or 5–9 showed gland development similar to that of controls during adulthood (data not shown), and the majority of these animals were fertile (Table 3). Treatment of mice for 1 wk starting at PND 10 also did not abolish fertility during adulthood (Table 3), and extensive adenogenesis was seen during adulthood in this group (data not shown).
DISCUSSION
The present results indicate that uterine adenogenesis in the C57BL6 mice occurs at approximately the same time as reported previously for BALB/c mice [25]. Uterine adenogenesis is not completed until sometime between PND 30 and PND 60 [25]. The most critical finding of the present study may be that total and irrevocable inhibition of adenogenesis does not require P4 treatment for the entire period of adenogenesis. Instead, treatment for a short portion of this period permanently prevents this process. This critical window for inhibiting adenogenesis is narrow and sharply defined, as discussed below.
The degree and permanency of inhibition of adenogenesis induced by P4 depends on both treatment duration and age at initiation. Exposure to P4 during several treatment periods (PNDs 3–9, 5–9, and 3–7) abolished gland development at PND 10. However, a shorter, 3-day regimen of P4 just as adenogenesis begins (PNDs 3–5) produced only partial inhibition of adenogenesis at PND 10. Thus, 3 days of P4 at the onset of adenogenesis produced only temporary inhibition of gland development that was reversed shortly after cessation of P4 treatment. A longer treatment of 5–7 days was required to induce full suppression of adenogenesis at PND 10.
Two treatments (P4 on either PNDs 5–9 or PNDs 3–7) totally inhibited adenogenesis on PND 10, but gland development was evident by PND 20. In striking contrast, P4 treatment during PNDs 3–9 produced an aglandular phenotype at both PND 10 and PND 20. Thus, permanent abolition of murine endometrial gland development requires P4-induced inhibition of adenogenesis beginning on PND 3 and maintenance of this antiadenogenic condition for 7 days. Results suggest that P4-induced inhibition of endometrial gland development during this critical, 7-day window of neonatal life (i.e., PNDs 3–9) establishes organizational conditions inhibitory to adenogenesis and from which the endometrium cannot recover upon cessation of P4 treatment.
Previous work with an ovine model that used neonatal progestin treatment to inhibit uterine adenogenesis produced a similar phenomenon of initial gland suppression followed by resumption of adenogenesis after cessation of progestin treatment. Bartol et al. [8] reported that treatment of ewes during PNDs 0–13 with a progestin inhibited adenogenesis at PND 13; however, gland development was apparent by PND 26 (i.e., 13 days after withdrawal of progestin). Uterine adenogenesis in ewes begins between birth and PND 7 [1, 14, 30]. Thus, uterine tissues, including nascent glands, would have been exposed to progestin for between 7 and 13 days with this treatment regimen. Based on the present results, the progestin treatment of Bartol et al. [8] may not have encompassed the entire critical window for progestin-sensitive development of uterine glands. Therefore, after this progestin treatment, the ovine uterus initiated adenogenesis in a manner analogous to that described here for mice following short P4 treatments.
Progestin treatment following initiation of adenogenesis can inhibit development of uterine glands temporarily. However, adenogenesis resumes when P4 is withdrawn and can proceed to essentially normal uterine glandularity (and adult fertility). This is illustrated by the treatments during PNDs 7–9 and 10–16. Treatment starting at PND 10, when gland development was well underway, and continued until PND 16 produced inhibition of adenogenesis at PND 20 compared to controls. Indeed, adenogenesis in this group at PND 20 was similar to that in controls at PND 10, indicating that P4 treatment during this period almost completely halted adenogenesis. In adulthood, however, these mice had extensive gland development and were fertile. This was similar to P4 treatment during PNDs 7–9, which also began after gland development had commenced. It likely is impossible to inhibit adenogenesis irreversibly with P4 after this process is initiated. A temporary stasis of adenogenesis can be induced, but these treatments simply inhibit adenogenesis during P4 exposure. Following P4 administration, epithelial proliferation resumes and gland development is strongly reinitiated, and gland development in adults is comparable to controls.
The P4 regimen used effectively inhibited epithelial proliferation, as shown by low epithelial MKI67 labeling at PND 10 following P4 treatment during PNDs 3–9 or 5–9 compared to controls. Uterine epithelial cells still labeling with MKI67 at PND 10 may reflect a subset of these cells for which proliferation is not inhibited by P4 and/or proliferation of uterine epithelial stem cells [31] that are not mitotically inhibited by P4.
Stromal proliferation was significant in controls at PND 10, and P4 treatment during PNDs 3–9 produced a trend toward a further increase. This is consistent with previous work that P4 stimulates proliferation of neonatal stromal cells [5], the converse of inhibitory effects on epithelial proliferation.
In control mice, epithelial proliferation was lower at PND 20 than PND 10, consistent with literature indicating steadily decreasing epithelial proliferation postnatally [4]. In contrast, epithelial proliferation at PND 20 in mice treated with P4 during PND 3–9 exceeded both that in controls at PND 20 and that on PND 10 in mice treated during PNDs 3–9. Clearly, the inhibited epithelial proliferation produced by neonatal P4 treatment is temporary and reversible. More importantly, a striking, extended rebound of epithelial proliferation occurs following neonatal P4 treatment such that even 10 days after P4 treatment, epithelial proliferation in treated mice exceeds that in controls. Similar patterns of increased uterine epithelial cell proliferation, induced as a specific consequence of progestin withdrawal, have been described for neonatal lambs [8].
Increased epithelial proliferation following cessation of P4 could result from direct effects of P4 treatment and consequent inhibition of epithelial proliferation holding the uterus in a less mature state. Following P4 treatment, uteri may exhibit the increased proliferation characteristic of younger, less mature organs. Another possibility is that cessation of P4 treatment may result in something analogous to catch-up growth, in which a parameter rebounds to greater than control levels as it recovers from inhibition.
HAND2 is a protein produced in uterine stromal cells that mediates the inhibitory effects of P4 on estradiol-induced uterine epithelial proliferation in adult mice [28]. HAND2 expression is minimal in ovariectomized adult mice and is strongly stimulated by P4. Our present results indicate that HAND2 is detectable immediately after birth and during the neonatal period when P4 is being administered. The strong expression of HAND2 in neonatal uterine stroma when endogenous P4 concentrations are minimal suggests that HAND2 expression is not dependent on P4 during early postnatal life. This is consistent with our observations that HAND2 expression is similar in neonatal control and P4 receptor knockout mice (Davila et al., unpublished results). Thus, despite the obligatory role of P4 in inducing HAND2 expression in adult uteri, expression of this protein is not dependent on the progesterone/progesterone receptor signaling pathway in neonatal uteri. The critical role of HAND2 in mediating adult inhibition of epithelial proliferation by P4, combined with the neonatal expression of HAND2, suggests that HAND2 could be involved in or essential for inhibitory P4 effects on neonatal epithelial proliferation, although this remains to be definitively established.
Neonatal P4 treatment altered expression of a number of Wnt genes and their Fzd receptors at both PND 10 and PND 20. To our knowledge, this is the first report that neonatal P4 effects on adenogenesis are accompanied by altered expression of the Wnt, Fzd, and Hoxa genes. Recent work with neonatal administration of the synthetic estrogen diethylstilbestrol (DES), which also inhibits adenogenesis [29], revealed changes in Wnt4, Wnt7a, and Wnt11 at PNDs 5 and/or 15 following DES administration from birth to PND 5. These genes showed similar patterns of change in expression following neonatal P4 administration, suggesting that genes altered similarly in both models may be involved in inhibition of adenogenesis induced by either hormone. In contrast, some changes in gene expression were seen with one treatment but not the other. For example, P4 suppressed Fzd6 expression whereas DES did not, and DES decreased Wnt5a expression whereas P4 did not. Effects seen with one hormone but not the other may reflect unique uterine effects of estrogen or progestins. Expression of Hoxa10 and Hoxa11 was decreased at both 10 and 20 days following P4 treatment during PNDs 3–9. Wnt7a maintains uterine Hoxa10 and Hoxa11 expression [2, 15, 16, 32]. Thus, reduced Hoxa10 and Hoxa11 expression may reflect downstream events resulting from decreased Wnt7a.
Mice given various P4 treatments cycled during adulthood. Thus, neonatal P4 did not affect development or function of the hypothalamo-pituitary-ovarian axis or related mechanisms required to support adult cyclicity and ovulation. This result is consistent with earlier data indicating that adult ewes treated with progestin for 8 wk postnatally underwent puberty and cycled normally in adulthood [12].
Fertility studies confirmed the essential role of uterine glands in successful reproduction established with the sheep model [1, 12–14]. Despite the striking and, in some cases, extended effects of various P4 treatments, glands were present at PND 20, and in adulthood, the group showed some fertility. However, both litter size and percentage fertility tended to decrease with extended P4 treatments. Conversely, all but 1 of 11 mice treated during PNDs 3–9 were infertile. Histological examination revealed two to three rudimentary glands, at most, per uterine cross-section. In contrast, the one mouse treated with P4 during PNDs 3–9 that proved to be fertile in adulthood had 15 glands in the uterine cross-section examined. An analogous condition was reported for adult ewes treated neonatally with a progestin to induce the aglandular uterine phenotype [14]. Whereas the neonatal progestin exposure induced a glandless phenotype in the majority of ewes, this phenotype was not completely penetrant. Degrees of endometrial glandularity were observed in some progestin-exposed ewes. Moreover, the capacity of neonatally progestin-exposed adults to support conceptus development was related positively to the extent of endometrial glandularity. In the present study, it was unclear whether the adenogenesis and fertility in the one mouse treated with P4 during PNDs 3–9 reflected individual variation in the response to this treatment or an error during dosing. Regardless, both the presence of glands in only one mouse and the observed fertility of this mouse emphasize the strong link between uterine gland development and fertility.
In summary, we identified a narrow developmental window in neonatal mice during which inhibition of uterine adenogenesis permanently inhibits adenogenesis and adult infertility. This 1-wk window shows that it is unnecessary to suppress gland development during the entire period of adenogenesis to totally and permanently block this process. The ability of early P4 treatment to inhibit adenogenesis and adult fertility suggests that this methodology could be effective for sterilization of companion animals and other species. However, this approach would not be feasible if P4 had to be given for an extended period during neonatal/juvenile life. The present results suggest strongly that this would not be the case and that the necessary critical window for treatment would be only a fraction of the entire period of gland development. Observations emphasize the potential value of this approach for sterilizing various species if the time of initiation of uterine adenogenesis, the pattern of gland development, and the critical window of progestin susceptibility can be determined.
ACKNOWLEDGMENT
The authors thank Terry Medrano for her proofreading of the figures and manuscript.
Footnotes
Supported by the Billie A. Field Endowment, University of Illinois (P.S.C.), and funding from the University of Florida. The work at the University of Illinois was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06 RR16515 from the National Center for Research Resources, National Institutes of Health. J.D. was supported by National Institute of Environmental Health Sciences training grant ES07326 during conduct of this research.
REFERENCES
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68: 85 122 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 1993; 90: 11162 11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y, Okamoto S, Kitamura Y, Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology 1983; 113: 582 587 [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Cunha GR. Estrogen stimulation of deoxyribonucleic acid synthesis in uterine epithelial cells which lack estrogen receptors. Endocrinology 1986; 119: 390 396 [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 1998; 139: 4708 4713 [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Cunha GR. Effects of progestins and glucocorticoids on deoxyribonucleic acid synthesis in the uterus of the neonatal mouse. Endocrinology 1985; 117: 2520 2526 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Coleman DA, Wolfe DF, Riddell MG. Ovine uterine morphogenesis: effects of age and progestin administration and withdrawal on neonatal endometrial development and DNA synthesis. J Anim Sci 1988; 66: 3000 3009 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287 302 [PubMed] [Google Scholar]
- Spencer TE, Stagg AG, Joyce MM, Jenster G, Wood CG, Bazer FW, Wiley AA, Bartol FF. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 1999; 140: 4070 4080 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Gray CA. Sheep uterine gland knockout (UGKO) model. Methods Mol Med 2006; 121: 85 94 [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL, Bazer FW, Spencer TE. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod 2000; 62: 448 456 [DOI] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289 300 [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 2001; 64: 1608 1613 [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 2004; 131: 2061 2072 [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201 3211 [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Filant J, Hayashi K, Rucker EB, III, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 2011; 85: 386 896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. Beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 2010; 83: 396 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A 1991; 88: 11408 11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature 1992; 359: 76 79 [DOI] [PubMed] [Google Scholar]
- Ding YQ, Zhu LJ, Bagchi MK, Bagchi IC. Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology 1994; 135: 2265 2274 [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Bagchi MK, Bagchi IC. Attenuation of calcitonin gene expression in pregnant rat uterus leads to a block in embryonic implantation. Endocrinology 1998; 139: 330 339 [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Li AX, Luo K, Cunha GR. Strain differences in the ontogeny of estrogen receptors in murine uterine epithelium. Endocrinology 1990; 126: 2592 2596 [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat 1989; 186: 1 20 [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Cooke PS, Cunha GR. A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol Endocrinol Metab 1986; 251: E630 E636 [DOI] [PubMed] [Google Scholar]
- Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult Sertoli cells following conditional knock out of the gap junctional protein GJA1 (connexin 43). Biol Reprod 2007; 76: 804 812 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011; 331: 912 916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, III, Spencer TE, Demayo FJ, Lydon JP, Maclean JA., II WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011; 84: 308 319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley AA, Bartol FF, Barron DH. Histogenesis of the ovine uterus. J Anim Sci 1987; 64: 1262 1269 [DOI] [PubMed] [Google Scholar]
- Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells 2006; 24: 1529 1538 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Bagnell CA. Uterine development and endometrial programming. Soc Reprod Fertil Suppl 2006; 62: 113 130 [PubMed] [Google Scholar]