ABSTRACT
The matrix metalloproteinases (MMPs) are postulated to facilitate follicular rupture. In the present study, expression of the stromelysins (MMP3, MMP10, MMP11) was analyzed in the periovulatory human and rat ovary. Human granulosa and theca cells were collected from the dominant follicle at various times after human chorionic gonadotropin (hCG). Intact rat ovaries, granulosa cells, and residual tissue (tissue remaining after granulosa cell collection) were isolated from equine CG (eCG)-hCG-primed animals. Mmp10 mRNA was highly induced in human granulosa and theca cells and intact rat ovaries, granulosa cells, and residual tissue. Localization of MMP10 to granulosa and theca cells in both human and rat ovarian follicles was confirmed by immunohistochemistry. Mmp3 mRNA was unchanged in human cells and rat granulosa cells, but increased in intact rat ovaries and residual tissue. Mmp11 mRNA decreased following hCG treatment in human granulosa and theca cells as well as rat granulosa cells. Regulation of Mmp10 in cultured rat granulosa cells revealed that the EGF inhibitor AG1478 and the progesterone receptor antagonist RU486 suppressed the induction of Mmp10 mRNA, whereas the prostaglandin inhibitor NS398 had no effect. Studies on the Mmp10 promoter demonstrated that forskolin plus PMA stimulated promoter activity, which was dependent upon a proximal AP1 site. In conclusion, there are divergent patterns of stromelysin expression associated with ovulation, with a marked induction of Mmp10 mRNA and a decrease in Mmp11 mRNA, yet a species-dependent pattern on Mmp3 mRNA expression. The induction of Mmp10 expression suggests an important role for this MMP in the follicular changes associated with ovulation and subsequent luteinization.
Keywords: extracellular matrix, granulosa cells, matrix metalloproteinase, ovulation, ovulatory cycle, proteinases, theca cells
Expression of the metalloproteinase Mmp10 mRNA is stimulated by hCG prior to follicular rupture in both the human and the rat ovary, indicating involvement in ovulation and subsequent luteinization.
INTRODUCTION
During the ovulatory process, there is an orchestrated breakdown of the apical wall of the ovulatory follicle to allow oocyte release. This remodeling of the ovarian follicular extracellular matrix (ECM), as well as the matrix of the cumulus oocyte complex, is postulated to occur through the actions of a broad array of proteinases, including metallo-, serine, and thiol proteinases [1]. The expression and activity of these proteinases are set in motion by the mid-cycle surge of luteinizing hormone (LH) in numerous species [1–7]. However, little is known about the expression of these proteinases across the human periovulatory period, due to the complexities of analyzing gene expression in the human ovary. During a preliminary screen of the matrix metalloproteinases (MMPs) in human periovulatory follicles, we observed an increase in the mRNA expression of stromelysin-2 (MMP10), which has yet to be characterized throughout the periovulatory period in either the human or the rat.
The stromelysins are a class of proteinases within the MMP family that were originally named based upon their stromal-derived origin and capacity to degrade structural proteins of the ECM. Stromelysin-1 (MMP3) was identified while exploring proteinase activity in rabbit synovial fibroblasts [8]. Shortly thereafter, screening of a human tumor cDNA library resulted in the identification of stromelysin-2 (MMP10), which exhibited high sequence homology with stromelysin-1 [9]. Stromelysin-3 (MMP11) was later identified by differential screening of a human breast cancer cDNA library as a gene that was elevated in invasive carcinomas [10]. Although the stromelysins share similar structural characteristics and substrate specificity, the most significant differences among these proteinases are their differential pattern of expression and regulation [11, 12].
The pattern of expression for the stromelysins has been explored in the ovary of various species. To date, Mmp11 has been the most extensively characterized member of the stromelysin family in the ovary. Mmp11 mRNA is highly abundant in the ovary of the rat [13], mouse [14, 15], and fish [16]. While surveying normal rat tissues for expression of Mmp11 mRNA, Okada et al. observed the highest levels of Mmp11 mRNA in the ovary and uterus [13]. In the mouse, the expression of Mmp11 mRNA does not change during the periovulatory period, and was associated with atretic follicles [14, 15]. In contrast, Mmp11 mRNA was elevated prior to ovulation in the fish (Oryzias latipes), and was associated primarily with the oocyte [16]. Less is known about the other members of the stromelysin family in the ovary. Mmp3 has been reported in the porcine [17] and human ovary [18], but was undetectable in the mouse ovary [14]. There is limited to no information on Mmp10 in the ovary. In the present study, we examined the expression patterns of the stromelysins throughout the periovulatory period in human preovulatory follicles and rat ovaries.
MATERIALS AND METHODS
Materials and Reagents
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Molecular biological enzymes, molecular size markers, oligonucleotide primers, pCRII-TOPO Vector, culture media, and Trizol were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA).
Human Granulosa and Theca Cell Collection
Human granulosa and theca cells from periovulatory follicles were collected as previously described [19]. Briefly, follicular tissue was collected from patients undergoing laparoscopic tubal sterilization at four different periovulatory periods (preovulatory, early ovulatory, late ovulatory, and postovulatory groups) to distinguish expression levels across the ovulatory period. To obtain high-quality patient material, only women with proven fertility, regular menstrual cycles, and without hormonal medications were included. Women were monitored with repeated transvaginal ultrasound for an average of two cycles to insure normal cyclicity. For follicle collection, follicular growth was monitored by transvaginal ultrasound to enable performing surgery at a stage when the dominant follicle was preovulatory (≥14 mm and ≤17.5 mm). Since detection of the preovulatory rise in LH levels requires repeated measurements of serum LH, we chose to administer exogenous human chorionic gonadotropin (hCG) to mimic the LH surge and enable retrieval of follicles at defined stages. Immediately prior to surgery, serum levels of LH, progesterone, and estradiol were analyzed to confirm the ovulatory phase category and that the LH surge had not been initiated. For samples collected at the preovulatory phase, surgery was performed prior to the LH surge when the dominant follicle was ≥14 mm and ≤17.5 mm. The remaining patients received an injection of hCG (s.c., 250 μg rhCG, Ovitrelle; Organon, Oss, Netherlands) to mimic the endogenous LH surge. Subsequently, patients underwent surgery at varying times following hCG injection: early ovulatory phase (12 to ≤18 h), late ovulatory phase (>18 to ≤34 h), and postovulatory phase (>44 to ≤70 h). Frequent transvaginal ultrasound examinations have determined that rupture occurs approximately 36–38 h after hCG [19, 20].
The whole dominant follicle was excised and either 1) fixed and embedded in tissue blocks for immunohistochemistry, or 2) the granulosa and theca cells were isolated and frozen for subsequent mRNA expression analysis by real-time reverse transcriptase PCR. For cell isolation, granulosa cells were collected by gently bisecting the follicle to release the loosely attached cells. The mural granulosa cells were then gently scraped from the follicle wall and pooled with the loosely attached granulosa cells. Theca cells were harvested mechanically from the remnant of the follicle. Theca cells were obtained from all four periovulatory phases, but granulosa cells could not be collected from the postovulatory group. This study was approved by the human ethics committee at the University of Gothenburg, and informed written consent was obtained from all patients before surgery.
Rat Ovarian Tissue Collection
Immature 15-day old female Sprague-Dawley rats (Harlan Sprague-Dawley Inc., Indianapolis, IN) were maintained at room temperature with food and water provided ad libitum. Rats were injected s.c. with 10 IU of equine CG (eCG) at 22–23 days of age to stimulate ovarian follicular development. At 48 h after eCG injection, the rats were injected with 10 IU of hCG to induce ovulation. Rats were killed at varying time points after hCG treatment (0, 4, 8, 12, and 24 h). Whole ovaries, granulosa cells (in vivo), and remaining residual tissue (in vivo) were collected, snap frozen, and stored at −70°C for later analysis of mRNA expression by real-time PCR. Intact ovaries were also collected and embedded in paraffin for immunohistochemical analysis. The residual tissue represents the tissue remaining after granulosa cell collection and is a heterogeneous tissue comprised of theca, interstitial, endothelial, and stromal cells, as well as remaining granulosa cells. All animal procedures for these experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Rat Granulosa and Theca-Interstitial Cell Culture
Ovaries were collected from rats 48 h after eCG administration and granulosa cells were isolated, as described previously [21]. Briefly, granulosa cells were isolated by follicular puncture, pooled, filtered, pelleted by centrifugation, and resuspended in Opti-MEM I medium supplemented with 0.05 mg/ml of gentamycin and 1× ITS. The cells (5 × 105 cells/ml) were cultured in the absence or presence of hCG with various reagents (RU486, NS398, or AG1478, discussed in detail below) for varying lengths of time. For the regulation studies (RU486, NS398, or AG1478), reagents were added and cells preincubated for 30 min before treatment with hCG. Cells were then cultured for an additional 8 h before collection and analysis of mRNA expression.
Following granulosa cell isolation, the remaining tissue was then digested to obtain theca-interstitial cells, as described previously [22]. Briefly, the remaining tissue was incubated for 90 min in medium containing collagenase (2.5 mg/ml) and deoxyribonuclease (10 μg/ml), centrifuged, washed, and the dispersed cells subjected to 5-min gravity purification. Theca-interstitial cells represent a more purified population of theca cells than found in the residual tissue. Theca-interstitial cells were cultured in the absence (control) or presence of forskolin (FSK) plus phorbol 12-myristate-13-acetate (PMA; Sigma Aldrich) to mimic the actions of LH/hCG for varying lengths of time (0, 6, 12, and 24 h). After the appropriate length of culture, granulosa and theca-interstitial cells were collected, snap frozen, and stored at −70°C for later analysis of mRNA expression by real-time PCR.
RNA Isolation and Reverse Transcription
Total RNA was isolated from human and rat ovarian tissues using Trizol reagent according to the manufacturer's protocol (Thermo Scientific, Wilmington, DE). Reverse transcription of 1 μg of total RNA was used to synthesize first-strand cDNA through the use of SuperScript III with Oligo (dT)18 primers for use with real-time PCR according to the manufacturer's protocol (Invitrogen), as previously described [19].
Real-Time PCR of Human mRNA
TaqMan 20× Gene Expression Assay solutions containing the oligonucleotide primers for human MMP3 (TaqMan primer set Hs00968308_m1), human MMP10 (TaqMan primer set Hs00233987_m1), human MMP11 (TaqMan primer set Hs00968295_m1), and human GAPDH (VIC/MGB probe 4326317E), an internal endogenous control gene, were purchased from Applied Biosystems (Foster City, CA). The thermal cycling steps were programmed to include an initial setup of 2 min at 50°C, an initial denaturation step for 10 min at 95°C, and then 15 sec at 95°C and 1 min at 60°C for 45 cycles, followed by 1 min at 95°C, 30 sec at 58°C, and 30 sec at 95°C for ramp dissociation. The performance of the assay mix and endogenous control was tested through analysis of the amplification plots and plate sample values generated in the MxPro real-time PCR analysis program (Stratagene) following each real-time PCR reaction. The relative amount of stromelysin mRNA in each sample was calculated following the 2−ΔΔCT method, and normalized to the endogenous reference gene, GAPDH.
Real-Time PCR of Rat mRNA
Oligonucleotide primers for rat Mmp3 (forward 5′-GCCAGATTTGGTTCTTCCAA-3′, reverse 5′-AGTAGGCATAGCCCTCAGCA-3′), Mmp10 (forward 5′-ACCCCACTCACATTCTCC-3′, reverse 5′-CCATTTCTCATCATCATCG-3′), Mmp11 (forward 5′-ACCCAAATGGAGGAAAAACC-3′, reverse 5′-CTGGAGAATGTGAGTGGGGT-3′), and Rpl32 (forward 5′-GAAGCCCAAGATCGTCAAAA-3′, reverse 5′-AGGATCTGGCCCTGGCCCTTGAATCT-3′), an internal endogenous control gene, were designed based on the previously reported sequences of rat Mmp3 (GenBank, NM_133523.2), Mmp10 (GenBank, NM_133514), Mmp11 (GenBank, NM_012980.1), and Rpl32 (GenBank, NM_013226) using PRIMER3 software (SDSC Biology Workbench, San Diego, CA). The thermal cycling steps were designed to include 2 min at 50°C, an initial denaturation step for 10 min at 95°C, and then 15 sec at 95°C, 30 sec at 58°C, and 45 sec at 72°C for 45 cycles, followed by 1 min at 95°C, 30 sec at 58°C, and 30 sec at 95°C for ramp dissociation. Each of the three MMPs was sequentially analyzed in the same sample (i.e., ovary, granulosa cell, etc.) from three individual experiments over a period of a few months. The relative amount of mRNA in each sample was calculated following the 2−ΔΔCT method and normalized to Rpl32.
Generation of Mmp10 Luciferase Reporter Vector
Genomic DNA was isolated from tail samples collected from rats using an easy-DNA kit (Invitrogen). Primers corresponding to varying fragment lengths of the upstream promoter region of the Mmp10 gene were designed with restriction enzyme sites incorporated (SacI and NheI). Amplified fragments were of the following sizes: 1083 bp (−1047/+36), 237 bp (−201/+36), 128 bp (−92/+36), and 68 bp (−32/+36). The fragments corresponding to the Mmp10 gene promoter were cloned into the pCRII-TOPO vector (Invitrogen) and digested with the SacI and NheI restriction enzymes, as described previously [23]. These fragments were then subcloned into a multiple cloning site of the pGL3 basic vector (Promega, Madison, WI).
Site-directed point mutations of the Mmp10 promoter were generated using a QuikChange II site-directed mutagenesis kit (Stratagene), according to the manufacturer's protocol. The following sequence for the Mmp10 promoter oligonucleotide primers were generated, with the site-directed point mutation specifically designed within the AP1 binding site (shown in lowercase), 5′-CGATTAAAATTAGCACCCACAtgcaacaTGCTTAGTGACCTAAACACCC-3′, and GATA2 binding site (shown in lowercase), 5′-GTAGGTTGAGAGATTCACTGATGTAAttggTCCTCTTCATCCTATGAATGCACTT-3′. Rat granulosa cells were isolated 48 h after eCG injection, as described above. These granulosa cells were then transfected with firefly luciferase reporter plasmids (pGL3-Mmp10 promoter constructs) and the renilla luciferase vector (pRLTK vector) using a Lipofectamine 2000 reagent (Invitrogen), and allowed to incubate at 37°C for 24 h. Following incubation, granulosa cells were treated in the absence (control) or presence of FSK (10 μM) plus PMA (20 nM) for 6 h. FSK + PMA was utilized to mimic the action of LH/hCG due to the potential down-regulation of LH receptors [24]. Firefly and renilla luciferase activity was then measured using a dual-luciferase reporter assay system (Promega) on a Tecan Infinite 200 microplate reader (Tecan U.S., Durham, NC). Luminescence was monitored for a period of 10 sec for each reaction, with firefly luciferase activity then normalized to renilla activity.
Immunohistochemistry of MMP10
Human follicles and rat ovaries were sectioned at 8 um, deparaffinized, and washed in ethanol, followed by PBS prior to antigen retrieval using DakoCytomation Target Retrieval solution (Dako Inc., Carpinteria, CA) for 20 min at 100°C. After blocking with normal goat serum for 20 min, sections were incubated with MMP10 antibody (human, 5 μg/ml; rat, 0.05 μg/ml; Novus Biologicals, Littleton, CO) for 2 h at room temperature, then washed in PBS and incubated for 30 min with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). Staining was visualized using Vectastain Universal Elite ABC kit, as per manufacturer's instructions (Vector Laboratories). Diaminobenzidine enzyme was used for visualization, producing a brown precipitate. Sections were then counterstained with hematoxylin. No primary antibody was incubated with sections serving as negative controls. Images were captured using Nikon Elements imaging software (Nikon, Melville, NY).
Immunoassay of Androstenedione and Testosterone
Concentrations of androstenedione and testosterone in conditioned culture media following theca-interstitial cell culture were assayed using an Immulite kit on an Immulite 1000 (Siemens Healthcare Diagnostics, Los Angeles, CA). Assay sensitivity was 0.3 ng/ml for androstenedione and 15 ng/dL for testosterone. The intra-assay and interassay coefficients of variation were 9.9% and 9.1%, respectively, for androstenedione and 6.5% and 12.2%, respectively, for testosterone.
Statistical Analyses
All data are presented as means ± SEM. A two-way ANOVA was used to test for differences in mRNA expression at various times following hCG administration in rat whole ovary, granulosa and theca-interstitial cell samples, and human granulosa and theca cell samples. If ANOVA revealed significant effects of treatment, the means were compared by Duncan or Tukey test, with P < 0.05 considered significant. Statistical analyses were performed using SPSS 18.0 computational software (SPSS Inc., IBM Company, Chicago, IL).
RESULTS
Expression of Stromelysin mRNA in the Rat Ovary
The expression of mRNA for Mmp3 in intact rat ovaries transiently increased at 8 and 12 h following hCG administration with a maximal induction at 12 h, compared to the 0 h time point (Fig. 1A). This increase in expression occurred prior to the expected time of ovulation in this model (14–16 h post-hCG [25]). Levels of Mmp3 mRNA then declined at 24 h after hCG and were not different from the 0-h control (data not shown).
FIG. 1.
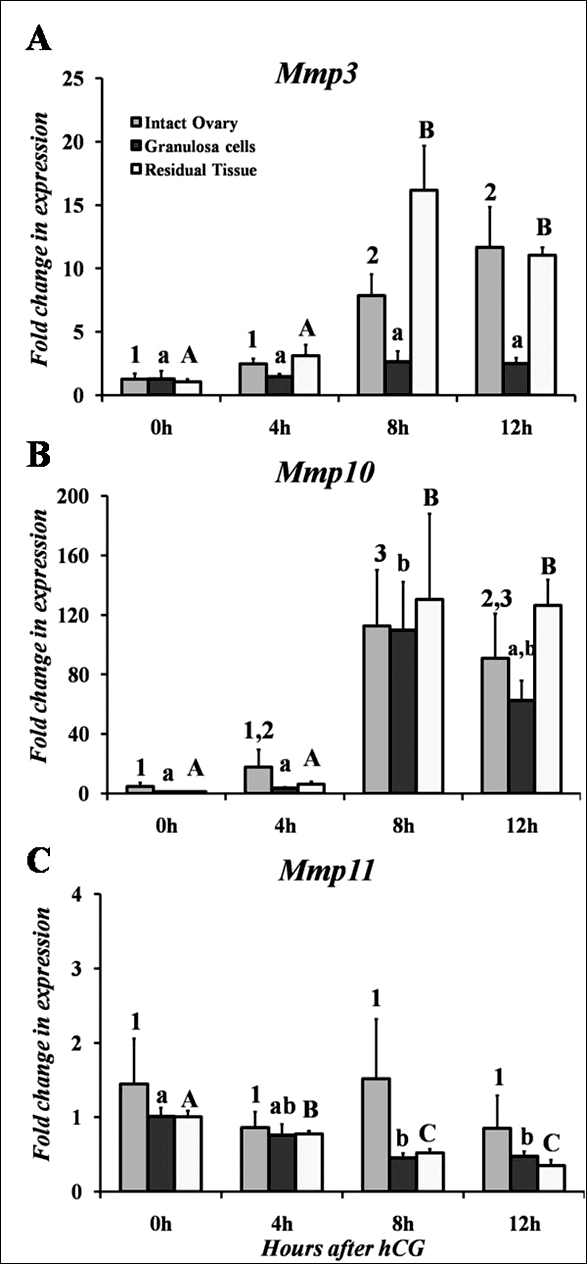
A–C) Real-time PCR analysis of the mRNA profiles for the stromelysin class of MMPs in intact rat ovaries, granulosa cells collected in vivo, and residual tissue collected in vivo. Relative levels of mRNA for each stromelysin member were normalized to Rpl32 for each sample. Values represent the mean ± SEM (n = 5 animals/time point for intact ovary, n = 3–5 animals/time point for granulosa cells in vivo, and n = 4 animals/time point for residual tissue in vivo). Bars with no common superscripts (number, intact ovary; lowercase letter, granulosa cells; uppercase letter, residual tissue) among the respective MMP member in each panel are significantly different (P < 0.05).
The expression of Mmp10 mRNA in the intact rat ovary demonstrated a similar expression profile to that of Mmp3 with an induction at 8 and 12 h after hCG (Fig. 1B). The accumulation of Mmp10 mRNA peaked at 8 h following hCG treatment before decreasing at the 24-h time point to 0-h control levels (data not shown). Examination of Mmp11 mRNA levels revealed no significant changes throughout the periovulatory period and expression levels were low, as reflected by the CT values at all time points (Fig. 1C).
Expression of Stromelysin mRNA in Rat Granulosa Cells and Residual Tissue In Vivo
In granulosa cells collected after hCG in vivo, there was no change in Mmp3 mRNA expression (Fig. 1A). In contrast, in the remaining residual tissue after granulosa cell collection the mRNA levels for Mmp3 increased by 8 h after hCG administration and remained elevated at 12 h (Fig. 1A). Thus, the induction of Mmp3 by hCG in the intact ovary is not mimicked by the expression in granulosa cells collected in vivo, but corresponds to expression in the residual tissue.
Similar to the expression pattern in the intact rat ovary, Mmp10 mRNA increased in granulosa cells collected in vivo at 8 h after hCG stimulation (Fig. 1B). Likewise, the levels of Mmp10 mRNA in the residual tissue mimicked the pattern of expression in intact ovaries with an induction by hCG at 8 h, which remained elevated at 12 h (Fig. 1B).
The expression of Mmp11 mRNA displayed an interesting pattern in that the levels of mRNA decreased in the presence of hCG. In granulosa cells collected in vivo, expression of Mmp11 mRNA was highest in the absence of hCG stimulation (e.g., 0 h) and decreased throughout the periovulatory period (Fig. 1C). A similar pattern was observed in residual tissue collected after hCG stimulation (Fig. 1C). The differences in expression pattern between the whole ovary and the isolated compartments may reflect a loss of tissue during isolation and filtration of granulosa cells and residual tissue.
Expression of Stromelysin mRNA in the Human Ovary
In human granulosa and theca cells collected in vivo prior to hCG stimulation (preovulatory phase), MMP10 mRNA expression was low (Fig. 2A). After hCG administration, there was a striking increase in the levels of MMP10 mRNA between the preovulatory and early ovulatory periods, which remained elevated at the late ovulatory phase (Fig. 2A). In theca cells, MMP10 mRNA expression returned to preovulatory levels by the postovulatory period. The postovulatory phase is not presented due to difficulties in collecting granulosa cells following ovulation. This finding of a marked stimulation of MMP10 mRNA expression in human granulosa and theca cells prior to ovulation correlates with the induction observed in rat granulosa cells and rat residual tissue collected in vivo (Fig. 1B). These findings must be interpreted with the caveat that some of the theca cell expression may represent contamination by granulosa cells.
FIG. 2.
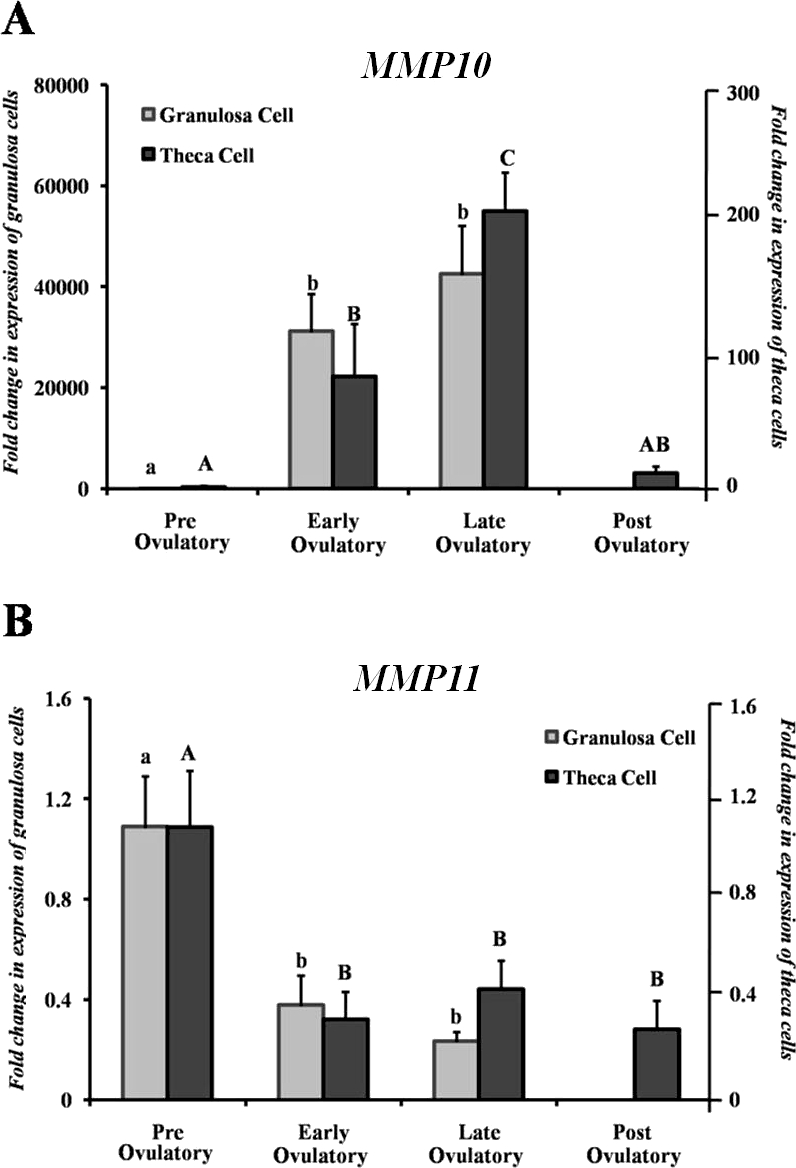
Analysis of MMP10 (A) and MMP11 (B) mRNA in human granulosa and theca cells isolated from the dominant follicle in women undergoing elective surgery throughout the periovulatory period after hCG treatment. Relative levels of MMP10 and MMP11 mRNA were normalized to GAPDH for each sample. Values represent the mean ± SEM (n = 5 patients/time point). Bars with no common superscripts in each panel are significantly different (P < 0.05).
The levels of MMP11 mRNA decreased after hCG administration in both human granulosa and thecal cells. In granulosa cells, expression of MMP11 mRNA decreased approximately 65% after hCG and remained low through the late ovulatory period (Fig. 2B). In the theca cells, hCG administration resulted in a 70% decline in MMP11 mRNA levels that remained low throughout the early and late ovulatory period (Fig. 2B).
For MMP3, mRNA expression was extremely low and did not change throughout the periovulatory period in either granulosa or theca cells (data not shown).
Expression of Stromelysin mRNA in Cultured Rat Granulosa and Theca Interstitial Cells
To explore the regulation of stromelysin expression, studies were undertaken to determine whether the expression patterns for the stromelysins observed in vivo could be mimicked in vitro. In granulosa cells collected in vivo after hCG, there was no change in Mmp3 mRNA expression (Fig. 1A). In contrast, when granulosa cells were isolated and cultured in the presence of hCG, the mRNA levels for Mmp3 rapidly and transiently increased by 4 h after gonadotropin stimulation, before declining to control levels by 12 h (Fig. 3A). Thus, the induction of Mmp3 by hCG in granulosa cells collected from intact ovaries is not mimicked by the expression in cultured granulosa cells. A similar lack of association was discerned with cultured theca-interstitial cells compared to residual tissue. Whereas expression of Mmp3 mRNA levels in residual tissue was stimulated by hCG (Fig. 1A), there was no change in mRNA levels for Mmp3 after FSK + PMA, which was used to mimic an hCG stimulus, in theca-interstitial cells (Fig. 4A). This difference in expression patterns of Mmp3 mRNA levels may reflect the contribution of other cell types present in the residual tissue or the loss of responsiveness during isolation of the theca-interstitial cells. However, responsiveness of the theca-interstitial cells to a FSK + PMA stimulus was confirmed by an approximate 4- to 5-fold induction of testosterone (6-h control = 69 ng/dl; 6 h FSK + PMA = 329 ng/dl) and androstenedione (6-h control = 0.8 ng/ml; 6 h FSK + PMA = 2.9 ng/ml) along with an increase in Mmp10 mRNA (discussed below).
FIG. 3.
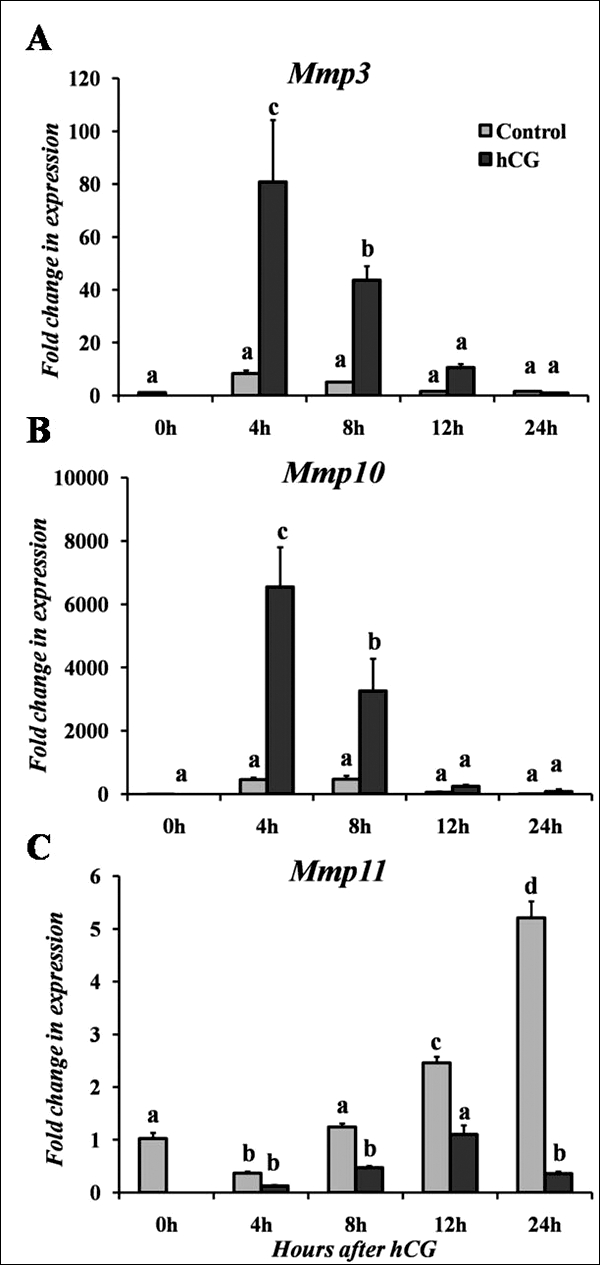
Real-time PCR analysis of stromelysin mRNA expression in rat granulosa cells cultured in vitro for Mmp3 (A), Mmp10 (B), and Mmp11 (C). Cells were cultured in the absence (Control) or presence of hCG for varying lengths of time. Relative levels of mRNA for each stromelysin member were normalized to Rpl32 for each sample. Values represent the mean ± SEM (n = 3–4 animals/time point). Bars with no common superscripts in each panel are significantly different (P < 0.05).
FIG. 4.
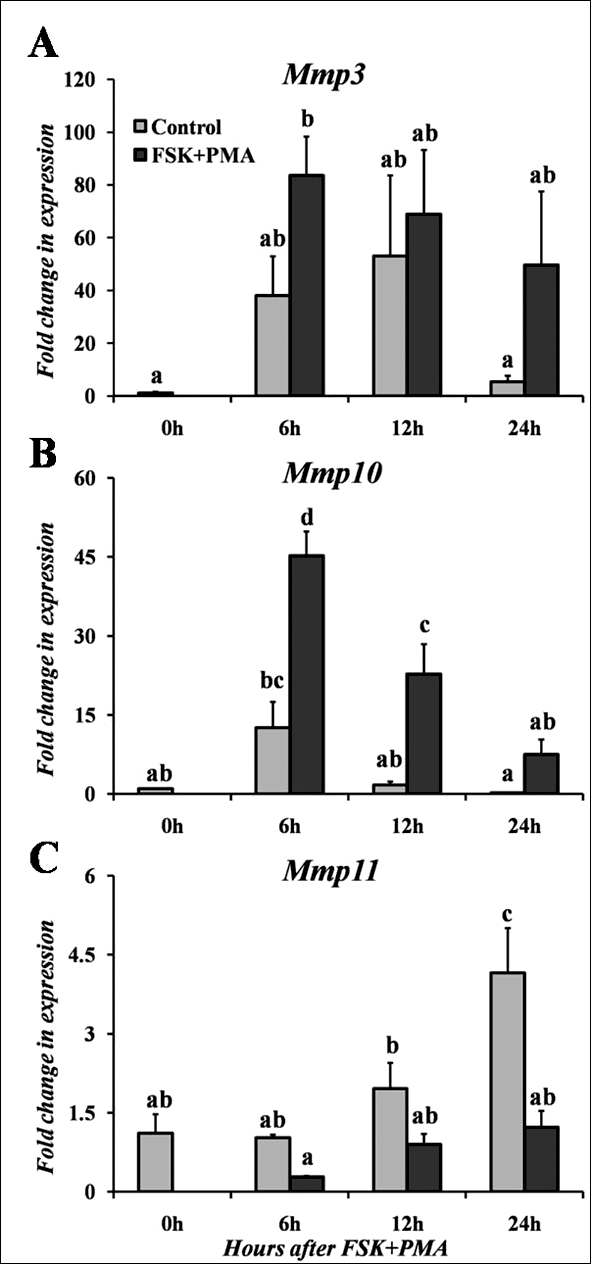
Real-time PCR analysis of stromelysin mRNA expression in rat theca-interstitial cells cultured in vitro for Mmp3 (A), Mmp10 (B), and Mmp11 (C). Cells were collected from eCG-primed immature rats and cultured in the absence (Control) or presence of FSK + PMA for varying lengths of time. Relative levels of mRNA for each stromelysin member were normalized to Rpl32 for each sample. Values represent the mean ± SEM (n = 3–4 animals/time point). Bars with no common superscripts in each panel are significantly different (P < 0.05).
For Mmp10, hCG induced a rapid and transient induction in cultured granulosa cells at 4 h after treatment (Fig. 3B). This induction of Mmp10 mRNA by hCG remained elevated at 8 h before decreasing to control levels by 12 h (Fig. 3B). Likewise in theca-interstitial cells, there was an induction in Mmp10 mRNA expression following treatment with FSK + PMA, which peaked at 6–12 h of treatment before returning to the basal control level by 24 h of treatment (Fig. 4B). This induction of Mmp10 in cultured granulosa and theca-interstitial cells corresponds with the induction occurring at the early periovulatory period in the intact rat ovary as well as granulosa cells and residual tissue collected in vivo, although the hormonal stimulation occurs slightly earlier in cultured cells.
Mmp11 mRNA levels in cultured cells displayed an unusual pattern of expression, where cells without treatment exhibited higher amounts of Mmp11 mRNA than hormonally stimulated cells. When granulosa cells were cultured in vitro, expression of Mmp11 mRNA increased in untreated cells (absence of hCG), with the highest induction at 24 h (Fig. 3C). In contrast, hCG treatment actually decreased the expression of Mmp11 mRNA in cells at 8, 12, and 24 h. A similar trend was detected in cultured theca-interstitial cells, where Mmp11 mRNA increased at 24 h in control cells (absence of FSK + PMA, Fig. 4C). Treatment with FSK + PMA resulted in no change in expression of Mmp11 mRNA over time. These findings in cultured cells support the in vivo observations in granulosa cells and residual tissue that an hCG stimulus actually diminishes the expression of Mmp11 mRNA.
Regulation of Mmp10 mRNA in the Rat Ovary
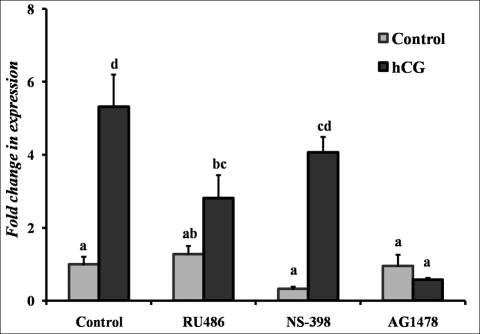
To begin to understand the regulation of Mmp10 mRNA expression, studies were conducted to explore the LH/hCG-mediated signaling pathways. Rat granulosa cells were cultured in the absence or presence of hCG (1 IU) with the addition of various inhibitors—progesterone receptor antagonist RU486 (1 μM), prostaglandin-endoperoxide synthase 2 inhibitor NS-398 (1 μM), and EGF receptor (EGFR) tyrosine kinase selective inhibitor AG1478 (1 μM)—for 6 h. The inhibition of hCG-induced expression of Mmp10 mRNA by RU486 and AG1478 suggests a dependence on the progesterone and EGFR signaling pathways, respectively (Fig. 5). Treatment with NS-398 was without effect.
FIG. 5.
Hormonal regulation of the hCG-induced Mmp10 mRNA expression in rat granulosa cells following 6 h of culture. Cells were pretreated in media alone (Control), with the progesterone receptor antagonist RU486 (1 μM), the prostaglandin-endoperoxide synthase 2 inhibitor NS-398 (1 μM), or the EGFR tyrosine kinase selective inhibitor AG1478 (1 μM) for 30 min. Cells were then cultured in the absence (Control) or presence of hCG (1 IU) plus each of the inhibitors of various hormonal signaling pathways for 6 h. Relative levels of mRNA for Mmp10 were normalized to the Rpl32 gene in each sample following real-time PCR and expressed as a fold change relative to the untreated 0-h control (mean ± SEM; n = 3–6 independent culture experiments). Values with no common superscripts in each panel are significantly different (P < 0.05).
Inhibition of Mmp10 Expression by AP1 Site-Directed Mutagenesis in Rat Granulosa Cells
We next examined the regulation of the Mmp10 promoter. Four rat Mmp10 promoter constructs (−1047/+36, −201/+36, −92/+36, and −32/+36) were transfected into granulosa cells collected from ovaries after 48 h of eCG priming. After 24 h of preincubation, treatment of granulosa cells with FSK + PMA for 6 h had no effect on the activity of the −32/+36 promoter construct, but stimulated expression in the other three constructs compared with the activity of cells cultured without FSK + PMA (Fig. 6). When activity was normalized to the corresponding control, the −92/+36 construct displayed an approximately 6-fold induction by FSK + PMA, whereas the −201/+36 and −1047/+36 constructs resulted in a nearly 11-fold induction (Fig. 6, inset).
FIG. 6.
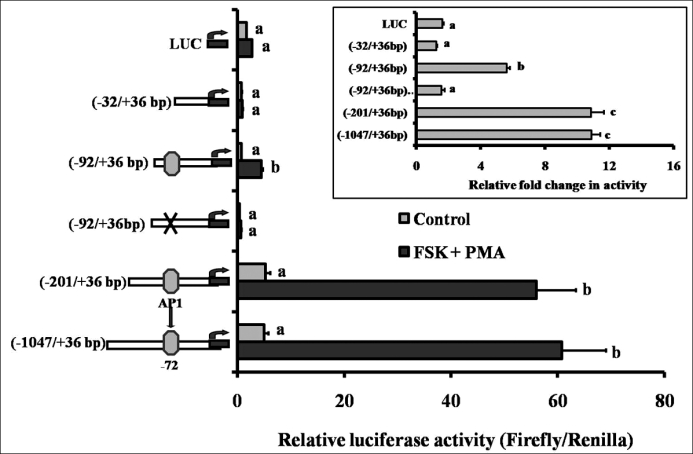
Regulation of the Mmp10 promoter by FSK + PMA in rat granulosa cells. Granulosa cells were isolated from gonadotropin-primed immature rats (48 h post-eCG), transfected with various rat Mmp10 promoter constructs for 24 h, and subsequently cultured with FSK + PMA for 6 h. Relative levels of the promoter activity where normalized to the luciferase:renilla ratio, and data are presented as the relative luciferase activity. Bars with no common superscripts within a promoter construct are significantly different from their respective control (P < 0.05). Inset: data are presented as the fold change in activity relative to the corresponding control. Bars with no common superscripts are significantly different (P < 0.05). Values represent the mean ± SEM (n = 3–4).
To determine the importance of the consensus AP1 binding site at −72 bp (TGAGTCA) of the Mmp10 promoter, site-directed mutants were generated and transfected into cultured preovulatory granulosa cells. Mutation of the proximal AP1 binding sequence abolished the induction of Mmp10 promoter luciferase activity by FSK + PMA in the −92/+36 construct (Fig. 6, A and B). These findings suggest that AP1 is a critical factor for the up-regulation of Mmp10 by LH.
To explore the induction of Mmp10 activity detected in larger promoter constructs (−201/+36, −1047/+36), additional potential transcription factor binding sites upstream of the proximal AP1 site were investigated. Site-directed mutants of the GATA2 binding site at −132 bp of the Mmp10 promoter were generated, but revealed no inhibition of FSK + PMA-stimulated Mmp10 promoter luciferase activity in the −201/+36 construct (data not shown). These findings suggest that the GATA2 site does not regulate Mmp10 promoter activity.
Localization of MMP10 in Human Follicles and Rat Ovaries
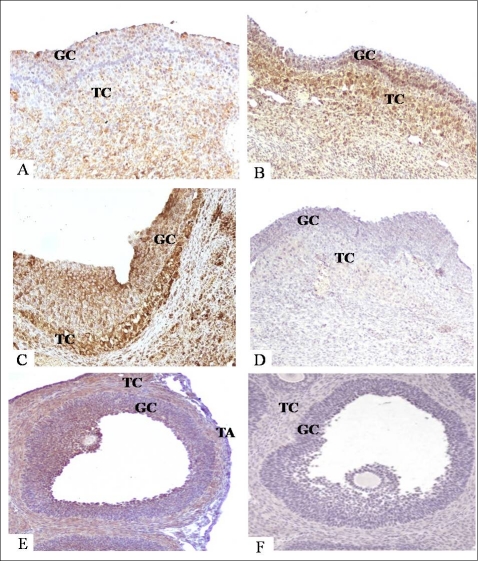
To determine the correlation between stromelysin mRNA and protein levels, MMP10 protein was localized in both human follicles and rat ovaries. MMP10 was scattered throughout the granulosa and theca layers in the human preovulatory follicle (Fig. 7A). Intense staining was observed in the granulosa and theca cells in early and late ovulatory follicles (Fig. 7, B and C, respectively). In the rat, MMP10 immunostaining was concentrated in the thecal cell layers and interestingly, in antral granulosa cells of the follicle prior to ovulation (Fig. 7E). Staining was not observed in the tunica albuginea at the follicular apex. These findings correlate with the mRNA observations in the human and rat, in which MMP10 is highly abundant throughout the follicular cell layers prior to ovulation.
FIG. 7.
Protein localization of MMP10 by immunohistochemical staining in human preovulatory (A), early ovulatory (B), and late ovulatory (C) dominant follicles, and rat ovary (E) at 8 h after hCG stimulation. Representative images of human follicles (A–D) and rat ovaries (E and F) were collected at defined times after hCG stimulation and processed for immunohistochemistry. MMP10 reaction product was visualized with diaminobenzidine producing a brown precipitate. Sections were then counterstained with hematoxylin. Negative control sections, with no primary antibody, are illustrated for a human follicle collected at the late ovulatory period (D) and a rat ovary collected at 8 h after hCG (F). GC, granulosa cells; TA, tunica albuginea; TC, theca cells. Original magnification ×20 (A–C, F), ×30 (D), ×15 (E).
DISCUSSION
Coordinated spatiotemporal expression patterns of the MMPs have been postulated to regulate ECM turnover associated with breakdown of the follicular wall [2]. The present study is the first investigation to compare the spatiotemporal expression for members of the stromelysin family of MMPs during the periovulatory period in the human and rat ovary. A major strength of the present study is that it was performed on human ovarian tissue obtained from well-defined stages of the ovulatory process. Interestingly, our results illustrate a similarity of expression among certain stromelysins that are involved in the ovulatory process of both humans and rats, while simultaneously highlighting differences between these species.
The stromelysin that was coordinately up-regulated in both the human and rat was MMP10, which raises the question as to the significance of the rapid induction of this stromelysin in the ovary of both species. The stromelysins were originally named based upon their stromal origin and ability to cleave the ECM of the stroma. Their actions on components of the ECM, such as collagen, fibronectin, laminin, aggrecan, elastin, and fibrinogen, have been well documented [26, 27]. Thus, one potential function of ovarian MMP10 is to act on the ECM during the follicular remodeling process. Additionally, the stromelysins have been shown to activate other proteinases, such as the collagenases [27, 28]. Of interest is the finding that MMP10 can act synergistically with plasmin to activate the latent form of collagenase (MMP1), thus creating a “superactive enzyme” that is approximately 10-fold more active than when activated with plasmin alone [29, 30]. Therefore, MMP10 may also function in the activation of other proteinases involved with the ovulatory process.
An alternative action of MMP10 may be to regulate the vascularity of the follicle as it transforms to the CL. Following the LH surge, there is intense angiogenesis and increased permeability of blood vessels during formation of the CL [31]. During early embryogenesis, repression of MMP10 maintains the integrity of the vascular endothelium [32]. When MMP10 is induced by disruption of histone deacetylase 7, there is a failure in endothelial cell-cell adhesion, with consequent dilatation and rupture of blood vessels [32]. Likewise, overexpression of MMP10 has been reported to compromise vascular integrity [33]. Thus, it is tempting to speculate that the induction of MMP10 by hCG in the theca and granulosa cells disrupts the vascular bed in the theca, thereby allowing angiogenesis as the granulosa cells become vascularized.
Although the function of MMP10 in the ovary remains enigmatic, experiments were conducted to begin to understand the regulation of Mmp10. Previous studies have reported that the Mmp10 promoter was regulated by inflammatory stimuli [33]. This observation is important, as the process of ovulation has been proposed to be an inflammatory reaction [34, 35], suggesting that ovarian Mmp10 may be regulated by LH/hCG-induced inflammatory mediators. To begin to understand the regulation of ovarian Mmp10, promoter assays were performed. In the present study, FSK + PMA stimulated Mmp10 promoter activity in the −92/+36 construct compared to the −32/+36 construct. Analysis of the promoter structure between −92 and −32 revealed the presence of an AP1 site. The AP1 site is highly conserved between human and rats, and functions as a transcription factor binding site for the proto-oncogenes JUN and FOS [36, 37]. In endothelial cells, an AP1 site in the proximal region of the Mmp10 promoter was critical for the induction of Mmp10 transcriptional activity by thrombin [38]. Experiments to determine the role of the proximal AP1 site in ovarian expression of Mmp10 by site-directed mutagenesis revealed that this site was critical for induction by FSK + PMA, confirming a common regulatory pathway between murine endothelial cells [38] and rat granulosa cells.
The induction of Mmp10 mRNA by hCG in granulosa cells appears to be mediated, in part, by the progesterone and the EGFR signaling pathways. Similar findings on the regulation of Mmp10 have been reported in other model systems. In human endometrium, progesterone up-regulates the expression of MMP10 mRNA [39, 40]. In colon cancer cells, the EGFR signaling pathway regulates MMP10 expression [41], with induction being blocked by both AG1478 and EGFR antibodies. In the ovary, LH/hCG produces a transient up-regulation of the progesterone receptor as well as the EGF-like growth factors ampiregulin and epiregulin [42–44], which are crucial for ovulation, steroidogenesis, oocyte maturation, and cumulus expansion [45]. The possibility exists, therefore, that the actions of progesterone and the EGF peptides on these aspects of ovulation may be partially mediated by MMP10.
The current findings support a similarity between the stromelysins involved in the ovulatory process in the human and rat; however, at the same time, our findings underscore differences with previous studies in other species and experimental paradigms. These differences indicate that expression of the ovarian stromelysins appears to vary depending upon the specific family member, stage of the ovarian cycle, and species. For example, there is a similarity in the periovulatory induction for MMP10 mRNA in both the human and the rat in the present study. Levels of MMP10 mRNA were markedly up-regulated in human and rat granulosa cells collected in vivo after an hCG stimulus, as well as in human theca cells and residual rat ovarian tissue. Similar findings of a periovulatory increase in MMP10 mRNA were reported in macaque dominant follicles [46]. In the mouse, however, Mmp10 mRNA in intact ovaries after hCG was undetectable by Northern blot analysis [14]. These differences may be species related or due to differences in the sensitivity of the assay methodology.
Likewise, there are species differences in the expression of Mmp3. For instance, Mmp3 mRNA was undetectable in murine ovaries after hCG by Northern blot analysis [14], whereas, in the present study, expression of Mmp3 mRNA was induced by hCG in the rat ovary, but was unchanged in the human granulosa or theca cell compartments. In the human, MMP3 protein has also been reported to be unchanged in the apical wall of atretic follicles, the tunica albuginea, and corpus luteum of normal ovaries [18]. These disparate expression patterns of Mmp3 mRNA highlight the differences in the responsiveness to LH/hCG between species.
Somewhat surprising was the paradoxical responsiveness in the expression of Mmp3 mRNA in rat granulosa cells to exogenous hCG in vivo compared to in vitro. Whereas levels of Mmp3 mRNA were unchanged after hCG in vivo, hCG stimulated an increase in Mmp3 mRNA expression within 4 h in cultured cells. This responsiveness in vitro may be due to a number of factors, including: 1) removal of an endogenous ovarian inhibitory influence, 2) disruption of cell-cell communication, or 3) an induction by an artificial culture system. However, mRNA for Mmp3 has been shown to be induced in porcine granulosa cells by bradykinin [17]. Thus, in both rat and porcine granulosa cells, Mmp3 mRNA is induced by signals associated with ovulation.
There are numerous reports of Mmp11 in the ovary. The original description of Mmp11 mRNA from a survey of normal rat tissues reported the highest levels in the ovary and uterus [13]. In the present study, the expression of Mmp11 mRNA after hCG stimulation did not change across the periovulatory period in intact rat ovaries. These observations are similar to those in the mouse, where Mmp11 mRNA is highly abundant, but does not change after hCG [14]. In contrast are reports in the fish (O. latipes), where Mmp11 mRNA expression was approximately four times greater in ovaries collected at ovulation compared to levels in ovaries obtained 12 h before ovulation [16]. Interestingly, after hCG administration in both the human and the rat, the levels of MMP11 mRNA actually declined in granulosa cells, theca cells, and residual tissue (rat) collected in vivo. This observation was confirmed in the rat, where addition of hCG to cultured granulosa and theca-interstitial cells resulted in levels of Mmp11 mRNA that were lower than in untreated control cells. To date, this is the first report of an inhibition of any of the MMPs by hCG in the ovary. The significance of this observation deserves further investigation.
In summary, the present findings are the first documentation of parallel changes in the expression of mRNA for MMP10 and MMP11 during the periovulatory period in the human dominant follicle and the rat ovary. The rapid and marked induction of MMP10 expression after hCG administration suggests an important role for this MMP in the many changes associated with ovulation and luteinization. The decline in mRNA levels for MMP11 after hCG treatment is a unique observation among the ovulatory MMPs, and may reflect a switch from a proliferative granulosa cell to a differentiated luteal cell phenotype as the follicle transforms into the corpus luteum.
Footnotes
Supported by National Institutes of Health grants P20 RR15592 and HD057446 to T.E.C., and by Swedish Research Council grant 11607 to M.B.
REFERENCES
- Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med 2006; 24: 228 241 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428 465 [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta 2005; 1751: 95 109 [DOI] [PubMed] [Google Scholar]
- Goldman S, Shalev E. MMPs and TIMPS in ovarian physiology and pathophysiology. Front Biosci 2004; 9: 2474 2483 [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res 2003; 44: 50 57 [PubMed] [Google Scholar]
- Ny T, Wahlberg P, Brandstrom IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol 2002; 187: 29 38 [DOI] [PubMed] [Google Scholar]
- Smith MF, Ricke WA, Bakke LJ, Dow MPD, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol 2002; 191: 45 56 [DOI] [PubMed] [Google Scholar]
- Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem 1985; 260: 12367 12376 [PubMed] [Google Scholar]
- Muller D, Quantin B, Gesnel MC, Million-Collard R, Abecassis J, Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J 1988; 253: 187 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990; 348: 699 704 [DOI] [PubMed] [Google Scholar]
- Van TC, Potworowski EF, St-Pierre Y. Stromelysin-1 (MMP-3) is inducible in T lymphoma cells and accelerates the growth of lymphoid tumors in vivo. Biochem Biophys Res Commun 2004; 315: 884 891 [DOI] [PubMed] [Google Scholar]
- Van TC, Alain T, Kossakowska AE, Urbanski S, Potworowski EF, St-Pierre Y. Stromelysin-2 (matrix metalloproteinase 10) is inducible in lymphoma cells and accelerates the growth of lymphoid tumors in vivo. J Immunol 2004; 173: 3605 3611 [DOI] [PubMed] [Google Scholar]
- Okada A, Saez S, Misumi Y, Basset P. Rat stromelysin 3: cDNA cloning from healing skin wound, activation by furin and expression in rat tissues. Gene 1997; 185: 187 193 [DOI] [PubMed] [Google Scholar]
- Hagglund AC, Ny A, Leonardsson G, Ny T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology 1999; 140: 4351 4358 [DOI] [PubMed] [Google Scholar]
- Hagglund AC, Basset P, Ny T. Stromelysin-3 is induced in mouse ovarian follicles undergoing hormonally controlled apoptosis, but this metalloproteinase is not required for follicular atresia. Biol Reprod 2001; 64: 457 463 [DOI] [PubMed] [Google Scholar]
- Ogiwara K, Matsui H, Kimura A, Takahashi T. Molecular cloning and partial characterization of medaka fish stromelysin-3 and its restricted expression in the oocytes of small growing follicles of the ovary. Mol Reprod Dev 2002; 61: 21 31 [DOI] [PubMed] [Google Scholar]
- Kimura A, Kihara T, Ohkura R, Ogiwara K, Takahashi T. Localization of bradykinin B(2) receptor in the follicles of porcine ovary and increased expression of matrix metalloproteinase-3 and -20 in cultured granulosa cells by bradykinin treatment. Biol Reprod 2001; 65: 1462 1470 [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M, Rechberger T, Jakimiuk AJ, Skorupski P, Jakowicki JA. Evaluation of matrix metalloproteinases-1 and -3 concentrations in the tunic albuginea, the apical wall of atretic follicles and the corpus luteum of normal human ovaries. Gynecol Endocrinol 2000; 14: 25 31 [DOI] [PubMed] [Google Scholar]
- Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt K, Brannstrom M. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod 2006; 12: 725 736 [DOI] [PubMed] [Google Scholar]
- Andersen AG, Als-Nielsen B, Hornnes PJ. Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Hum Reprod 1995; 10: 3202 3205 [DOI] [PubMed] [Google Scholar]
- Mann JS, Kindy MS, Edwards DR, Curry TE., Jr Hormonal regulation of matrix metalloproteinase inhibitors in rat granulosa cells and ovaries. Endocrinology 1991; 128: 1825 1832 [DOI] [PubMed] [Google Scholar]
- Wu Q, Sucheta S, Azhar S, Menon KM. Lipoprotein enhancement of ovarian theca-interstitial cell steroidogenesis: relative contribution of scavenger receptor class B (type I) and adenosine 5′-triphosphate-binding cassette (type A1) transporter in high-density lipoprotein-cholesterol transport and androgen synthesis. Endocrinology 2003; 144: 2437 2445 [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE, Jr, Ko C. Development and application of a rat ovarian gene expression database (rOGED). Endocrinology 2004; 145: 5384 5396 [DOI] [PubMed] [Google Scholar]
- Liu J, Park ES, Curry TE, Jr, Jo M. Periovulatory expression of hyaluronan and proteoglycan link protein 1 (Hapln1) in the rat ovary: hormonal regulation and potential function. Mol Endocrinol 2010; 24: 1203 1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridaran R, Rodriguez-Sierra JF, Blake CA. Ovarian involvement in the timing mechanism that controls ovulation in rats. Biol Reprod 1979; 21: 505 509 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17: 463 516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991; 5: 2145 2154 [PubMed] [Google Scholar]
- Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, Lakey R, Middleton J, Cawston TE, Richards CD, Rowan AD. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum 2006; 54: 3244 3253 [DOI] [PubMed] [Google Scholar]
- He C, Wilhelm SM, Pentland AP, Marmer BL, Grant GA, Eisen AZ, Goldberg GI. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A 1989; 86: 2632 2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauper V, Wilhelm SM, Seperack PK, DeClerck YA, Langley KE, Osthues A, Tschesche H. Direct activation of human neutrophil procollagenase by recombinant stromelysin. Biochem J 1993; 295 (pt 2): 581 586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol 2006; 4: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 2006; 126: 321 334 [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Orbe J. Martinez de LS, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, Paramo JA. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci 2008; 13: 2916 2921 [DOI] [PubMed] [Google Scholar]
- Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod 1980; 22: 73 106 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 2002; 64: 69 92 [DOI] [PubMed] [Google Scholar]
- Sharma SC, Richards JS. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 2000; 275: 33718 33728 [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. AP1- and NF-kappaB-binding sites conserved among mammalian WNT10B orthologs elucidate the TNFalpha-WNT10B signaling loop implicated in carcinogenesis and adipogenesis. Int J Mol Med 2007; 19: 699 703 [PubMed] [Google Scholar]
- Orbe J, Rodriguez JA, Calvayrac O, Rodriguez-Calvo R, Rodriguez C, Roncal C. Martinez de LS, Barrenetxe J, Reverter JC, Martinez-Gonzalez J, Paramo JA. Matrix metalloproteinase-10 is upregulated by thrombin in endothelial cells and increased in patients with enhanced thrombin generation. Arterioscler Thromb Vasc Biol 2009; 29: 2109 2116 [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007; 76: 102 117 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Garcia J, Kolp L, Cheadle C, Rodriguez A, Vlahos NF. The impact of luteal phase support on gene expression of extracellular matrix protein and adhesion molecules in the human endometrium during the window of implantation following controlled ovarian stimulation with a GnRH antagonist protocol. Fertil Steril 2010; 94: 2264 2271 [DOI] [PubMed] [Google Scholar]
- Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol 2009; 296: G755 G763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Sarge OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 1991; 5: 967 978 [DOI] [PubMed] [Google Scholar]
- Natraj U, Richards JS. Hormonal regulation, localization, functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 1993; 133: 761 769 [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol 2004; 33: 281 291 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 2009; 27: 52 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo MC, Murphy MJ, Talcott BS, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology 2011; 152: 3963 3974 [DOI] [PMC free article] [PubMed] [Google Scholar]