ABSTRACT
Prolactin (PRL), a pleiotropic hormone essential for maintenance of corpus luteum (CL) function and pregnancy, transduces its signal through two types of receptors, a short form (PRLR-S) and a long form (PRLR-L). Both types of receptors are expressed in the CL, yet their individual roles are not well defined. We have shown previously that female transgenic mice expressing only PRLR-S display total infertility characterized by defective follicular development and early degeneration of CL, suggesting that expression of PRLR-L is a prerequisite for normal follicular development and maintenance of CL. To determine whether PRLR-L alone is the sole receptor required to maintain normal CL formation, differentiation, and progesterone secretion, we generated two transgenic mice which express only PRLR-L, either ubiquitously (Tg-RL) or in a CL-specific manner (CL-RL). To generate CL-specific expression, we used the HSD17B7 promoter. We found both transgenic mice models cycled normally, displayed no apparent defect in follicular development, and had normal ovulation rates. The STAT5 signaling pathway, considered essential for luteinization and progesterone production, was activated by PRL in both transgenic mice models. However, soon after mating, Tg-RL and CL-RL mice showed early regression of CL, lack of progesterone production, and implantation failure that rendered them totally infertile. Embryo transfer studies demonstrated no embryo abnormalities, and supplementation with progesterone rescued implantation failure in these mice. Close observation revealed lack of luteinization and reduced expression of proteins involved in progesterone biosynthesis despite normal levels of LHCGR (LH-R), ESR1 (ER-alpha), CEBPB (C/EBP-beta) and CDKN1B (p27), proteins essential for luteinization. However, we found VEGFA, a key regulator of angiogenesis and vascularization, to be dramatically reduced in both Tg-RL and CL-RL mice. We also found collagen IV, a marker for the basal lamina of endothelial cells, aberrantly expressed and a discordant organization of endothelial cells in CL. Although luteinization did not occur in vivo, granulosa cells isolated from these mice luteinized in culture. Taken together, these results suggest that a vascularization defect in the CL may be responsible for lack of luteinization, progesterone production, and infertility in mice expressing only PRLR-L. This investigation therefore demonstrates that in contrast to earlier presumptions that PRLR-L alone is able to support normal CL formation and function, both isoforms of the PRL receptor are required in the CL for normal female fertility.
Keywords: corpus luteum, female infertility, ovary, prolactin, prolactin receptor, vascularization
While follicular development and ovulation are unaffected by the sole expression of the long form of the prolactin receptor, luteinization, vascularization, and maintenance of corpus luteum function are impaired.
INTRODUCTION
Embryo implantation and maintenance of pregnancy are dependent upon proper secretion of progesterone by the ovarian corpus luteum (CL), a transient endocrine gland formed from theca and granulosa cells following ovulation. In rodents, one of the primary hormonal stimuli necessary for CL formation and progesterone production is prolactin (PRL), a peptide hormone synthesized and secreted principally by the anterior pituitary [1–7]. Both PRL and PRL receptor (PRLR) null mice are infertile [8, 9]. CL in these mice ceases to grow, undergoes apoptosis, and rapidly regresses, leading to insufficient progesterone production [10–12]. Although several genes important for CL formation, survival, and function have been identified as direct targets of PRL [13–17], the signaling mechanism of PRL-mediated action in the CL is still unclear due in part to the fact that PRL can bind to two types of PRLR, a long (PRLR-L) and short (PRLR-S) form, derived by alternative splicing of the Prlr gene [12, 18]. These two receptors share homology in their extracellular and transmembrane domains and differ in length and composition in the intracellular domains. Although both PRLR-L and PRLR-S are expressed in the ovary, the function of each receptor for PRL-mediated action has not been elucidated [19, 20]. The generation of transgenic mice expressing only PRLR-S [21] allowed us to demonstrate [22–25] that PRL signaling through PRLR-S alone is unable to stimulate the canonical JAK2/STAT pathway in luteal cells or to sustain CL formation or function but causes repression of genes important for maintaining normal follicular development, causing premature ovarian failure. PRLR-S is unable to activate STAT5 upon PRL binding [25, 26], presumably due to the lack of specific tyrosine residues in its intracellular domain [27, 28]. In sharp contrast, the main pathway activated by PRL binding to PRLR-L in the ovary is the JAK2/STAT pathway [14, 17, 19, 29], and functional studies have revealed that activation of STAT5 proteins is essential for CL formation. Double-knockout of the STAT5A and STAT5B proteins led to infertility in female mice, characterized by few or no large CL in the ovary, emphasizing the importance of activation of the JAK2/STAT5 pathway by PRL for CL formation, survival, and function [30]. This led us and other investigators [6, 14, 31, 32] to suggest that PRL signaling through PRLR-L alone and activation of the JAK2/STAT5 pathway may be the sole factor required for CL formation and survival and thus for pregnancy. PRLR-L is ubiquitously expressed and found in the ovary, adrenal gland, kidney, mammary gland, decidua, small intestine, choroid plexus, and pancreas. Because of the broad distribution of PRLR-L and because PRLR-S is often coexpressed in the same tissue, it is difficult to examine the specific role of PRLR-L in the CL by using whole animals. In the present investigation, we generated two PRLR-L transgenic mouse lines on the PRLR null background, one line with ubiquitous expression and the second line with CL-specific expression of PRLR-L, and examined the role of PRL in ovarian development and function in these mice expressing only PRLR-L.
Generation of these transgenic mice did not support the contention that PRLR-L is the only essential receptor necessary for normal CL formation and function. PRL activation of this receptor leads to STAT5 phosphorylation and maintenance of key genes involved in luteinization and corpus luteum formation. However, this PRLR-L-mediated effect appears unable to sustain normal blood vessel formation and vascularization, a crucial event for luteal cell hypertrophy and steroidogenesis. The fact that PRLR-S is the predominant receptor expressed in endothelial cells derived from CL [33] suggests the intriguing possibility that PRLR-L expressed in luteal cells and PRLR-S expressed in the vasculature act in concert to allow normal CL formation and function, ultimately allowing the CL to sustain fertility and pregnancy.
MATERIALS AND METHODS
Generation of Transgenic Mice Expressing PRLR-L, Either Ubiquitously (Tg-RL Line) or in a CL-Specific Manner (CL-RL Line)
To create mice with ubiquitous expression of PRLR-L, a targeting construct was generated similar to the one used previously to generate mice expressing only PRLR-S [21]. The Prlr-L cDNA was generated by PCR and subsequently subcloned into the EF1A-pPolyIII vector (a gift from Dr. Nadine Binart at INSERM, Paris, France). This targeting vector (EF1-RL), used to generate mice with ubiquitous expression of PRLR-L, contains EF1A promoter, SRα enhancer region, Prlr-L cDNA, and a portion of human growth hormone (hGH) cDNA, a tag to identify transgenic mice.
To generate CL-targeted expression of PRLR-L, we cloned and isolated the promoter region of the CL-specific gene, Hsd17b7, by screening mouse 129/SvJ genomic library (Stratagene) with probes for exons 1 and 2. Of the 20 clones selected, the clone containing the 2.8-kb promoter region was subsequently subcloned, resequenced, and confirmed as being upstream of the mouse Hsd17b7 gene by BLAST analysis. Subsequent 5′ truncations of the promoter identified the 1.2-kb region as having the highest promoter activity. Therefore, we replaced the EF1A promoter in the ubiquitous construct with the 1.2-kb HSD17B7 promoter. Additionally, to generate a fusion protein, the Prlr-L cDNA stop sequences were replaced by an enhanced green fluorescent protein (EGFP) sequence, isolated from pEGFP-N1 (Invitrogen).
Both transgenic constructs were tested in culture for their activity levels. Briefly, GG-CL cells, derived from rat CL generated in our laboratory [34], were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Cells were cotransfected with empty vector and either EF1-RL or HSD17B7-RL-EGFP vector with equal amounts of either β-casein or LHRE promoter reporter constructs, respectively. Following transfection, cells were treated either with vehicle or 1 μg/ml ovine PRL (Protein Laboratories Rehovot, Ltd.) for 24 h.
Following confirmation of activity of the transgenic vectors, the constructs were linearized and injected into pronuclei of fertilized oocytes from FVBN mice by the Transgenic Production Services at the University of Illinois at Chicago. Transgenic mice were identified by PCR and Southern blotting using primers/probes to the hGH tag. Transgenic mice were backcrossed with PRLR null (PRLR−/−) mice to generate mice expressing only PRLR-L, either ubiquitously (Tg-RL) or in a CL-specific manner (CL-RL).
Genotyping by PCR and Southern Blot Analysis
Genotyping was performed to identify transgenic mice as previously described [8]. The protocol used for Southern blot analysis has been previously described [35]. For Southern blot analysis, the hGH probe was generated by isolation and purification of hGH cDNA from the transgenic construct. The hGH probe was radiolabled with [32P]deoxycytidine triphosphate by using Rediprime II random primer labeling kit (GE Healthcare). To determine copy number, genomic DNA from wild-type mice was spiked with appropriate amounts of transgenic DNA to generate a standard curve. Blots were visualized by autoradiography using Kodak Biomax MS film (Sigma) with an intensifier screen.
Experimental Animals
Mice were kept under conditions of controlled light (0700–1900 h) and temperature (22°C–24°C) with free access to standard rodent chow and water. All experimental procedures were performed in accordance with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee. Transgene injections, collection of oocytes, and embryo transfer experiments were conducted with the help of Dr. Roberta Franks and Kimberly McLaughlin of the University of Illinois at Chicago Research Resources Center Transgenic Production Service.
To confirm PRL activation of PRLR-L and examine downstream targets, we injected mice s.c. with 200 μg of α-ergocryptine (Sigma) to block endogenous PRL secretion, followed by i.p. injection of 60 μg of ovine PRL (Protein Laboratories Rehovot, Ltd.) 4 h later. Mice were then euthanized at various time points post-PRL injection, and tissues were harvested and analyzed for protein expression.
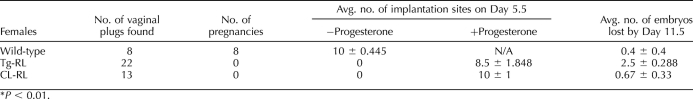
To examine implantation sites, we allowed mice to mate and injected them with vehicle or 3 mg of progesterone daily to maintain pregnancy, beginning on the day the vaginal plug was present (pregnancy Day 0.5). On Day 5.5 of pregnancy, mice were injected with 0.1 ml of a 1% solution of Chicago SkyBlue 6B dye (ACROS Organics) by tail vein injection under isoflurane anesthesia, and the number of implantation sites in the uterus, stained with dye, was recorded. Mice were superovulated by i.p. injection of equine chorionic gonadotropin (eCG) (Sigma), followed 48 h with i.p. injection of human chorionic gonadotropin (hCG; Sigma), and ovaries were collected at various time points thereafter for either histology or protein. An n of 3–5 mice per group was used for all experiments.
RNA Isolation, Semiquantitative PCR, and Quantitative-PCR
RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's protocol. Total RNA was reversed transcribed to cDNA using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) according to the manufacturer's protocol. L19 was used as an internal control for both RT-PCR and quantitative-PCR (Q-PCR). Primers for exon 5 of PRLR were adapted from reference [9]. Q-PCR protocol was adapted from Shehu et al. [35]. Briefly, standard curves were generated by a series of 1:5 to 1:500 dilutions prepared from reverse transcription (RT) products. Five-microliter aliquots of standards or diluted RT products were combined with 1× Fast SYBR Green PCR Master Mix (Applied Biosystems) and 50 nM forward and reverse primers. Reactions were carried out with a Prism 7700 sequence detection system (Applied Biosystems) for 40 cycles (95°C for 15 sec, 60°C for 1 min) after an initial incubation for 10 min at 95°C. Relative expression levels were calculated using the ΔΔCT method, with the Rpl19 mRNA used as an internal control. The following primers were used for Q-PCR: Esr1, 5′-AACCGCCCATGATCTATTCTG-3′ and 5′-AGATTCAAGTCCCCAAAGCC-3′; Cdkn1b, 5′-TGGACCAAATGCCTGACTC-3′ and 5′-GGGAACCGTCTGAAACATTTTC-3′; and Vegfa, 5′-CATCTTCCAGGAGTACCCCGA-3′ and 5′-CACTCCAGGGCTTCATCGTT-3′. Q-PCR primers for Lhcgr (LH-R) and Cebpb (C/EBP/β) were adapted from references [36] and [37], respectively. Primers for Cyp11a1, Hsd3b, and Star were adapted from reference [11].
Immunohistochemistry
Ovaries were collected, processed, paraffin-embedded, and serially sectioned at 5 μm as previously described [22]. Paraffin sections were rehydrated and stained with hematoxylin and eosin by conventional procedures. Immunohistochemistry was performed as previously described [35]. The following antibodies from Santa Cruz Biotechnology were used for immunohistochemistry at the dilutions listed: PRLR M-170 (1:200 dilution), ESR1 (1:150 dilution), VEGF (1:200 dilution), and CDKN1B (1:100 dilution). HSD17B7 antibody was developed in our laboratory [38] and used at 1:250 dilution. The PRLR antibody recognizes the extracellular domain of the receptor. Because our mice express only Prlr-L cDNA, the staining corresponds only to PRLR-L in these mice. TUNEL assay (ApopTag Plus in situ detection kit; Chemicon International) was carried out according to the manufacturer's instructions.
To visualize endogenous PRLR-L-EGFP fusion protein expression in CL-RL mice, we harvested and fixed ovaries for 2 h in a solution of 4% formalin, 7% picric acid, and 10% sucrose. Tissues were placed in Tissue-Tek OCT embedding compound, flash frozen over liquid nitrogen, and sectioned at 5-μm thickness.
Western Blotting
Whole ovarian extracts were prepared by homogenization of tissue in radioimmunoprecipitation assay buffer (Boston BioProducts) containing 1× protease inhibitor cocktail (Calbiochem) and 1 mM sodium orthovanadate. Western blotting was performed as previously described [38]. Antibodies used for immunoblots were PRLR (M-170; Santa Cruz Biotechnology), anti-phospho-STAT5A/B (Millipore), mouse anti-STAT5 (Pan; Invitrogen), and CEBPB (C/EBPβ; Santa Cruz Biotechnology).
Primary Luteinized Granulosa Cells
Immature (21- to 26-day-old) mice were treated with 8 IU of eCG (Sigma Aldrich) for 48 h to stimulate follicular development and then treated with 8 IU of hCG (Sigma) to induce ovulation. Primary luteinized granulosa cells were harvested 12 h post-hCG injection and incubated sequentially in 6 mM ethylene glycol tetraacetic acid (EGTA) in Dulbecco modified Eagle medium (DMEM)/F-12 medium and 0.5 M sucrose in DMEM/F-12. A 30-G needle was used to puncture follicles to release luteinized granulosa cells into medium. Cells were plated at 150 000 cells/well in a 12-well plate precoated with mouse laminin (BD Biosciences) and incubated at 37°C at 5% CO2. Conditioned medium was collected and changed every 24 h for up to 5 days.
Statistical Analysis
Data were examined by t-test, two-way ANOVA, and one-way ANOVA, followed by the Tukey test using Prism software (GraphPad Software Inc.). All error bars represent ±SEM. Values are considered statistically significant at P values of <0.05*, <0.01**, and <0.001*** (asterisks refer to figure legends).
RESULTS
Generation of Ubiquitous (Tg-RL) and CL-Specific (CL-RL) PRLR-L Transgenic Mice
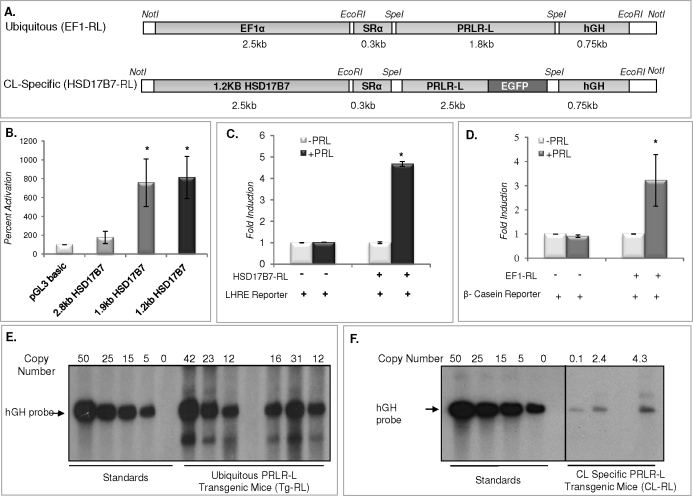
Targeting constructs used to generate mice with either ubiquitous (EF1-RL) or CL-specific (HSD17B7-RL) expression of PRLR-L are shown in Figure 1A. The EF1A promoter, active in all cells, was used to drive ubiquitous expression of PRLR-L, whereas the HSD17B7 promoter was used to drive CL-specific expression of PRLR-L. Both constructs contained a portion of hGH cDNA, which functioned as a tag. The promoter activity of HSD17B7 was tested (Fig. 1B) by transfecting cells with 2.8-kb (full length), 1.9-kb, and 1.2-kb 5′-truncated promoters. The 1.2-kb HSD17B7 promoter had the highest activity and therefore was used to drive CL-specific expression of PRLR-L. To test the activity of the HSD17B7-RL construct (Fig. 1C), GG-CL cells, a luteum-derived cell line generated in our laboratory [34], were cotransfected with the transgenic vector and the LHRE promoter-reporter and subsequently treated with vehicle or PRL. The activity of EF1-RL was examined in cells cotransfected with the β-casein promoter-reporter (Fig. 1D). Both the LHRE and β-casein promoter-reporters are known to be activated by PRL through the PRLR-L form [39–42]. Following PRL treatment, there was a robust stimulation in reporter activity, indicating that both EF1-RL and HSD17B7-RL transgenic receptor constructs are functional.
FIG. 1.
Generation of transgenic mice expressing PRLR-L either ubiquitously (Tg-RL) or in a CL-specific manner (CL-RL). A) Schematic of the targeting construct used to generate mice with ubiquitous (EF1-RL) or CL-specific (HSD17B7-RL) expression of PRLR-L. B) Promoter activity of HSD17B7 was tested by transfecting GG-CL cells with 5′-truncated 2.8-kb (full length), 1.9-kb, and 1.2-kb promoters. To test the activity levels of the transgenic constructs, GG-CL cells were cotransfected with either the HSD17B7-RL (C) or EF1-RL (D) transgenic construct together with either the LHRE or β-casein promoter reporter plasmid, respectively, and treated with or without PRL. Promoter activity experiments are expressed as means ± SEM from three independent experiments performed in triplicate (*P < 0.05). Southern blot analysis was performed with genomic DNA isolated from mouse tail to determine the copy number for both Tg-RL (E) and CL-RL (F) mice, as described in Materials and Methods.
As shown in Figure 1, E and F, several mouse lines carrying either the ubiquitous PRLR-L transgene (Tg-RL) or the CL-specific PRLR-L transgene (CL-RL) were generated. The Tg-RL mouse carrying 31 copies of PRLR-L (Fig. 1E) was chosen to expand the colony, and all Tg-RL mice subsequently used were derived from that mouse. Integration of the CL-RL transgene had a lower copy number (Fig. 1F); therefore, mice with the highest copies were intercrossed. Both strains of transgenic mice were backcrossed to the PRLR null background such that both lines expressed only PRLR-L, either in a ubiquitous or CL-targeted manner.
Expression and Activation of PRLR-L in Tg-RL and CL-RL Female Mice
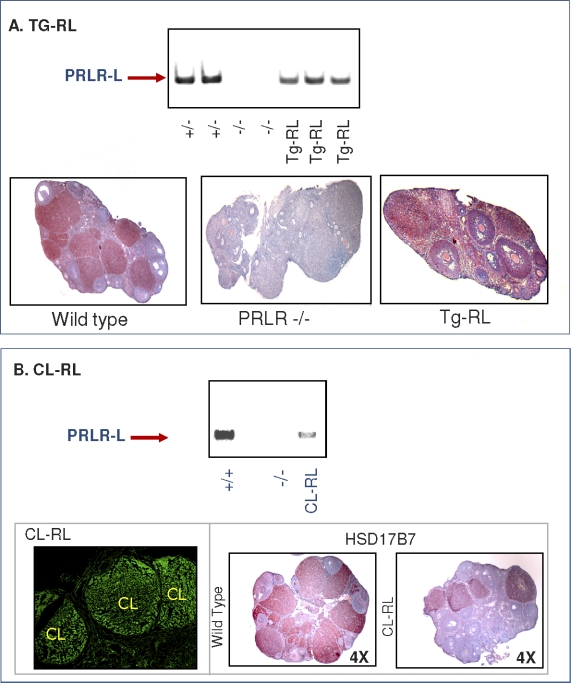
We examined the expression of PRLR-L in the ovaries of transgenic mice by using both RT-PCR and immunochemistry (Fig. 2A). Ovaries were obtained from mice 1.5 days after they mated. As previously reported [43], PRLR-L was detected in both CL and follicles of wild-type mice (Fig. 2A, lower panel). No receptor was found in the ovary of PRLR null mice, whereas it was readily detectable in Tg-RL mice by both RT-PCR (Fig. 2A, upper panel) and immunohistochemistry (Fig. 2A, lower panel). In the Tg-RL mice, the receptor was clearly expressed in both CL and follicles. PRLR-L was also detected by RT-PCR in the ovaries of CL-RL mice expressing the HSD17B7-RL vector (Fig. 2B, upper panel). Green fluorescence, indicative of PRLR-L-EGFP fusion protein expression, was seen only in the CL and not in follicles of CL-RL mice (Fig. 2B, lower left panel), and immunohistochemical analysis confirmed the specific expression of HSD17B7 in the CL (Fig. 2B, lower right panel).
FIG. 2.
Ovarian expression of PRLR-L in Tg-RL and CL-RL mice. Transcript levels of PRLR (exon 5) were examined by RT-PCR in ovaries from wild-type/heterozygote, PRLR null, and Tg-RL mice (A, upper panel) or CL-RL mice (B, upper panel). PRLR-L expression was also detected by immunohistochemistry (shown in red) in ovaries of wild-type, PRLR-null, and Tg-RL mice (A, lower panel). B) Confocal imaging of an ovary from CL-RL mice shows CL-specific expression of the PRLR-L-EGFP fusion protein (lower left panel). Ovaries from wild-type and CL-RL mice were subjected to immunohistochemistry using HSD17B7 polyclonal antibody to confirm CL-specific HSD17B7 expression (B, lower right panels). Reactivity is shown in red, and hematoxylin counterstain is shown in blue.
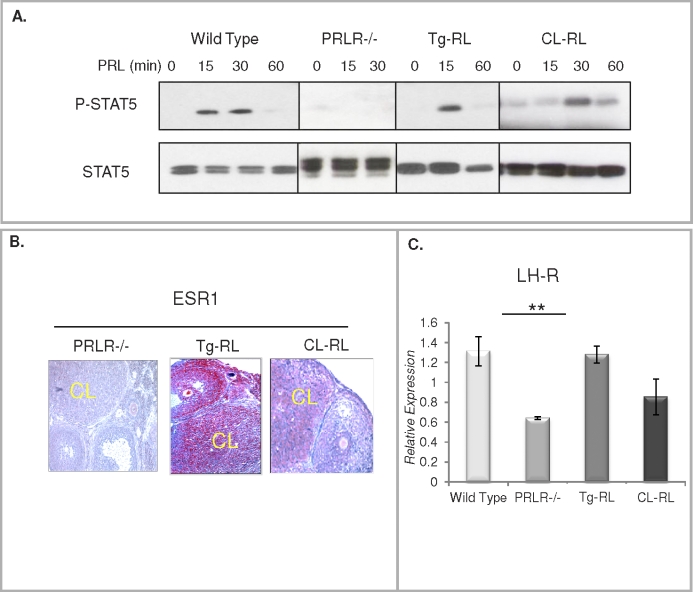
We ascertained that the transgenic PRLR-L is functional in vivo in Tg-RL and CL-RL female mice by examining the ability of PRL to stimulate STAT5 activity. As shown in Figure 3A, a rapid increase in STAT5 phosphorylation was observed in the ovaries of both transgenic mice after in vivo treatment with PRL. Because ESR1, as we have previously shown [20], is normally expressed in the CL, is up regulated by PRL, and is a downstream target of the PRLR-L/STAT5 pathway [29, 44], we examined its ovarian expression in both of the PRLR-L transgenic mice lines. As shown in Figure 3B, ESR1 is nonexistent in PRLR null mice, providing further support to previous reports of PRL-mediated stimulation of ESR1. Interestingly, we found that ubiquitous expression of PRLR-L in Tg-RL mice drives expression of ESR1 in the follicles as well as in the CL (Fig. 3B), whereas the CL-specific transgene induces the expression of ESR1 in CL only. To further establish the activity of these receptors, we used Q-PCR to examine ovarian LHCGR expression, another downstream target of PRL [39, 45]. We found a marked decrease in ovarian LHCGR levels in the PRLR null mice and a significant stimulation in both transgenic mice compared to that in null mice (Fig. 3C). Taken together, these results indicate that the PRLR-L transgenic receptor is functional in vivo in both Tg-RL and CL-RL mice.
FIG. 3.
Activation of PRLR-L in Tg-RL and CL-RL mice. A) Western blot analysis of STAT5 phosphorylation from ovaries of wild-type, PRLR-null, Tg-RL, and CL-RL mice treated with ergocryptine for 4 h, followed by PRL treatment for various time points. B) Immunohistochemical examination of ESR1 expression in ovaries of PRLR null, Tg-RL, and CL-RL mice (shown in red) at Day 1.5 of pregnancy. C) Q-PCR expression of LHCGR in the ovaries of wild-type, PRLR-null, Tg-RL, and CL-RL mice (**P < 0.01).
Luteinization and Progesterone Production Are Defective in Tg-RL and CL-RL Mice
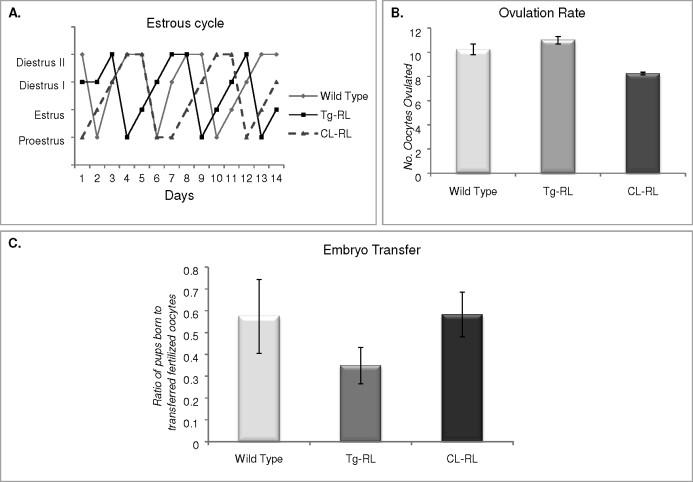
Tg-RL and CL-RL mice have normal estrous cycles (Fig. 4A) and mate with fertile males (as confirmed by a vaginal plug). However, surprisingly, no pregnancy was observed in either transgenic mice line (Table 1). This was not due to a defect in ovulation, because, as shown in Figure 4B, fertilized eggs were found in the oviduct and the ovulation rate in Tg-RL and CL-RL mice appeared to be similar to that of wild-type mice.
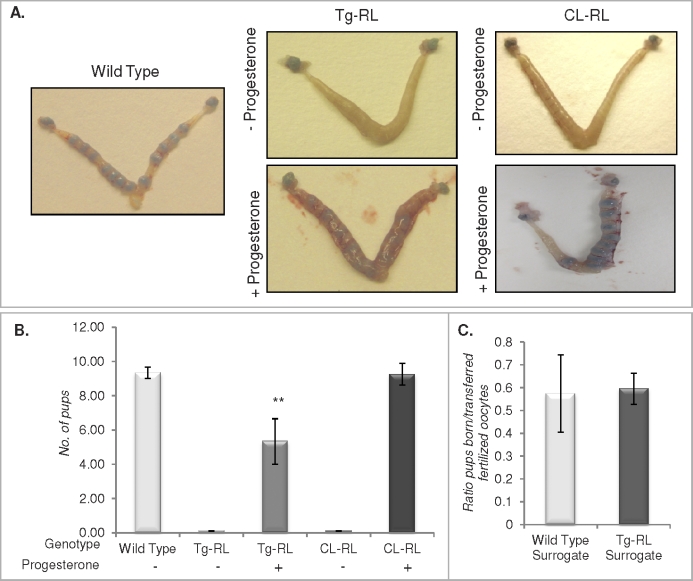
FIG. 4.
Tg-RL and CL-RL mice have normal estrous cycles and ovulation. A) Vaginal smear assays were conducted daily for 14 days to monitor the stage of the estrous cycles of the mice and were graphed to demonstrate the cycling patterns of wild-type, Tg-RL, and CL-RL mice. The graph shows representative cycles monitored from mice (n = 3–5 per group). B) Ovulation rates of wild-type, Tg-RL, and CL-RL mice were determined by collecting and counting the number of oocytes ovulated postmating. C) Fertilized oocytes from wild-type, Tg-RL, or CL-RL mice were transferred to wild-type surrogate mothers. The ratio of the number of pups born to the number of fertilized oocytes implanted per mother was plotted to compare whether the transgenic embryos were able to implant normally compared to wild-type embryos (n = 3–5 mice per group for each experiment).
TABLE 1.
Infertility in Tg-RL and CL-RL mice is rescued with progesterone treatment.*
We examined whether infertility was due to an intrinsic defect in the Tg-RL and CL-RL embryo by transplanting fertilized oocytes from transgenic mice into wild-type surrogate mothers. As shown in Figure 4C, the number of Tg-RL and CL-RL pups born in relation to the number of fertilized oocytes implanted was similar to that in wild-type controls, indicating that Tg-RL and CL-RL fertilized oocytes are indeed able to implant in wild-type mice. However, as shown in Figure 5A, upper panel, no implantation sites were detected in Day 5.5 postmated Tg-RL and CL-RL females. Treatment with progesterone rescued the implantation defect seen in both Tg-RL and CL-RL female mice, indicating that the lack of implantation and pregnancy in Tg-RL and CL-RL female mice was due to an ovarian shortcoming and insufficient progesterone production.
FIG. 5.
Progesterone supplementation cannot completely rescue pregnancy. A) Female mice were mated with fertile males, treated with vehicle or progesterone daily beginning on Day 0.5, and subsequently injected with Chicago Sky Blue dye on Day 5.5 of pregnancy to examine implantation sites. B) At Day 16.5 of pregnancy, following daily administration of vehicle or progesterone, the number of pups present in the uterus was recorded. C) A reverse embryo transfer experiment, where fertilized wild-type oocytes were transferred to wild-type or progesterone-treated Tg-RL surrogate mothers, was conducted.
Interestingly, continued progesterone treatment completely rescued pregnancy in CL-RL mice but not in Tg-RL mice (Fig. 5B) because of significant fetal death by Day 11.5 (Table 1). These findings suggest that ubiquitous expression of PRLR-L may modulate other confounding factors that contribute to infertility in these mice. In addition to the ovary, the uterus was shown to be a target tissue of PRL, where it affects decidual expression of cytokines and enzymes [46]. To determine whether a uterine defect in Tg-RL mice was the reason for such a fetal loss, we conducted a reverse embryo transfer, where fertilized wild-type embryos were transplanted into either a wild-type surrogate mother or a PRLR-L female surrogate treated with progesterone. As shown in Figure 5C, there was no significant embryo loss in PRLR-L surrogates compared to that in wild-type surrogates, indicating that the uterine environment in PRLR-L female mice is capable of maintaining normal pregnancy if supplemented with progesterone. This finding confirmed the fact that the major defect is in the ovaries of both transgenic mice lines.
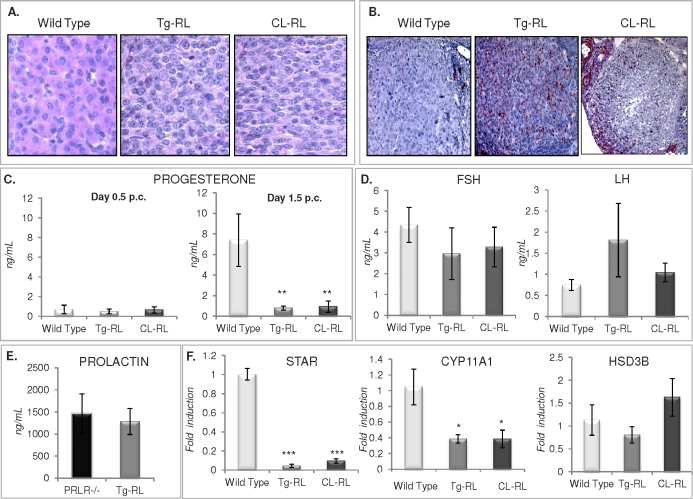
Examination of the ovaries of Tg-RL and CL-RL female mice at 1.5 days after they mated revealed the presence of CL. However, cell hypertrophy, a typical characteristic of luteinization in wild-type mice (Fig. 6A, left panel), was absent in CL of both Tg-RL and CL-RL mice, indicating a defect in the luteinization process in these mice. In addition to defective luteinization, extensive cell death was detected by TUNEL assay in CL of Tg-RL and CL-RL mice but not in wild-type mice (Fig. 6B) by Day 3.5 of pregnancy. Early regression of the CL can lead to a defect in progesterone synthesis, and as expected, serum progesterone levels were low on Day 0.5 postmating in all groups of mice (Fig. 6C, left panel). On Day 1.5 of pregnancy, the characteristic rise in serum progesterone levels seen in wild-type mice was not observed in either Tg-RL or CL-RL mice (Fig. 6C, right panel). No significant difference were observed between FSH and LH levels in wild-type mice and those in transgenic mice (Fig. 6D). PRL levels were elevated in Tg-RL mice (Fig. 6E), similar to levels found in PRLR null and PRLR-S transgenic mice, as previously reported [22]. Not surprisingly, mRNA expression levels of steroidogenic acute regulatory protein (STAR) and P450 side chain cleavage (P450scc [CYP11A1 gene]), two proteins involved in progesterone biosynthesis [41, 42], were significantly reduced in Tg-RL and CL-RL ovaries compared to those in the wild type (Fig. 6F). However, no significant differences in 3-beta-hydroxysteroid dehydrogenase (HSD3B) were observed (Fig. 6F, right panel).
FIG. 6.
Luteinization and impaired progesterone biosynthesis in Tg-RL and CL-RL mice. A) Photomicrographs of CL from hematoxylin-eosin-stained ovaries of wild-type, Tg-RL, and CL-RL mice at Day 1.5 of pregnancy (original magnification ×100). B) TUNEL assay with paraffin-embedded ovarian sections was performed at Day 3.5 of pregnancy, revealing reactivity (seen in red) in the CL of wild-type, Tg-RL, and CL-RL mice (original magnification ×40). C) Serum progesterone levels were measured on Days 0.5 (left panel) and 1.5 (right panel) postmating. D) Serum FSH and LH levels were measured at proestrus phase in wild-type, Tg-RL, and CL-RL mice. E) Serum PRL levels were measured using the NB2 assay from PRLR null and Tg-RL mice. F) Q-PCR analysis of the expression of STAR (left panel), CP11A1 (middle panel), and HSD3B (right panel) in ovaries from wild-type, Tg-RL, and CL-RL mice at Day 1.5 of pregnancy. *P < 0.05; **P < 0.01; ***P < 0.001.
Defective CL Vascularization in Tg-RL and CL-RL Mice
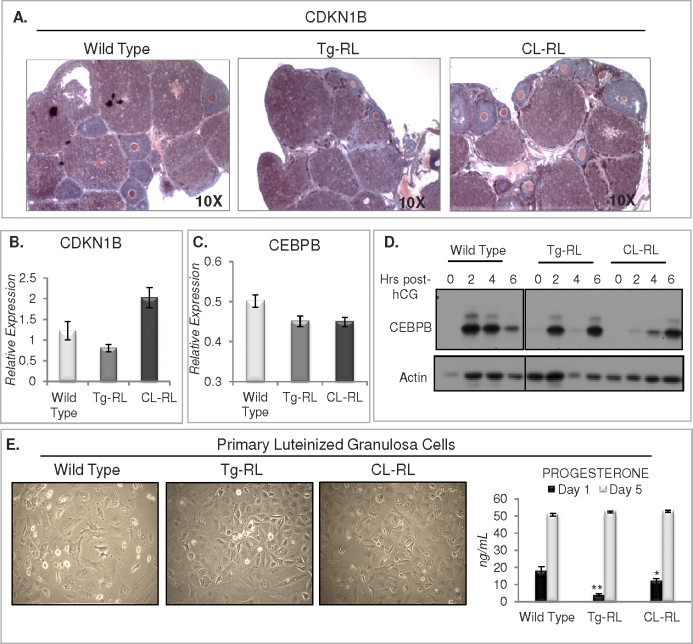
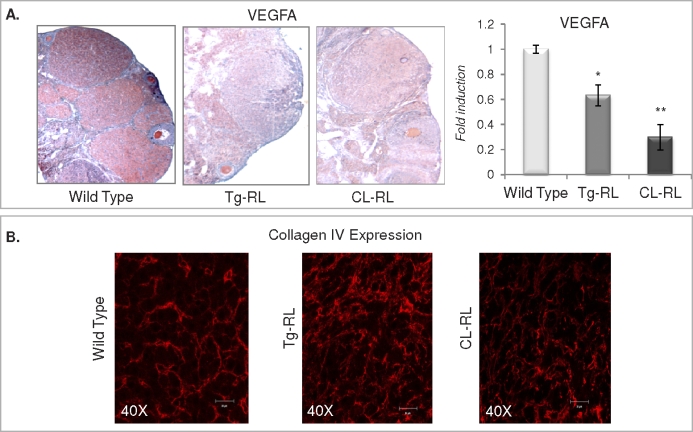
To further investigate the mechanism of defective luteinization in the transgenic mice, we looked at the expression levels of CDKN1B and CCAAT-enhancer binding protein (C/EBP) β, CEBPB, two key proteins that are critical for CL formation. CDKN1B, an inhibitor of cell cycle progression required for cells to exit the cycle and initiate CL formation, was expressed in both transgenic mice lines in response to superovulatory doses of eCG and hCG, similar to that in the wild type as determined by both immunohistochemistry and Q-PCR (Fig. 7, A and B). Transcript levels of CEBPB, a transcription factor induced following the LH surge, was similar in ovaries from wild-type and both Tg-RL and CL-RL transgenic mice (Fig. 7C), and its protein expression was induced following hCG administration (Fig. 7D), indicating that the ovaries of both transgenic mice are responsive to LH. Indeed, we found that after an LH surge, granulosa cells of mice harvested from Tg-RL, and CL-RL luteinize in culture similar to wild-type cells (Fig. 7E), suggesting that these cells have the intrinsic capacity to undergo hypertrophy. We also measured progesterone levels in conditioned medium collected from primary luteinized cells after 1 and 5 days in culture (Fig. 7E, right panel). Luteinized granulosa cells isolated from Tg-RL and CL-RL ovaries produced significantly lower levels of progesterone after 1 day in culture. However, by Day 5, there were no differences in progesterone secretion between cell types, indicating that the defect in CL formation in the ovaries of Tg-RL and CL-RL mice might have been caused by a defect not necessarily intrinsic to luteal cells as they can undergo hypertrophy and produce progesterone in culture. Indeed most of the cells found in the CL were from a vascular origin. The CL has the highest blood flow of any tissue, and neovascularization is essential for CL formation and survival [47]. Vascularization provides a transport network for nutrients and hormones, most importantly progesterone in CL. We found vascular endothelial growth factor A (VEGFA), a key player in CL vascularization [6], was highly expressed in wild-type CL but almost undetectable in CL of both Tg-RL and CL-RL mice (Fig. 8A). Collagen IV is the primary extracellular matrix component of the subendothelial basal lamina [48] and has been shown to modulate angiogenesis [49, 50] and appears as a continuous network in later stages of angiogenesis [51]. Collagen IV staining, a marker for basal lamina of endothelial cells, is aberrantly expressed and reveals discordant organization of endothelial cells in CL of transgenic mice at Day 1.5 of pregnancy, further suggesting that angiogenesis in the CL of these mice is disrupted (Fig. 8B).
FIG. 7.
Examination of markers of luteinization in vivo and of primary luteinized granulosa cell hypertrophy and progesterone production in culture. A) Immunohistochemical expression of CDKN1B (shown in red) in the CL of wild-type, Tg-RL, and CL-RL mice following superovulation. Transcript levels of CDKN1B (B) and CEBPB (C) were detected by Q-PCR from cDNA of ovaries from wild-type, Tg-RL, and CL-RL mice at early pregnancy. D) Western blot analysis of ovarian CEBPB protein induction following hCG injection in wild-type, Tg-RL, and CL-RL mice. E) View of primary luteinized granulosa cells isolated from wild-type, Tg-RL, and CL-RL mice following 5 days in culture (left) and respective progesterone levels in conditioned medium from primary cells at Days 1 and 5 in culture (right).
FIG. 8.
Vascularization is defective in the ovaries of Tg-RL and CL-RL mice. A) Paraffin-embedded ovarian sections from wild-type, Tg-RL, and CL-RL mice at Day 1.5 of pregnancy were probed with VEGFA antibody (left panels). Reactivity is shown in red and hematoxylin counterstain in blue (original magnification ×10). Vegfa mRNA levels were detected by Q-PCR in ovaries from wild-type, Tg-RL, and CL-RL mice (right). B) Immunofluorescence shows collagen IV staining (red) of the basal lamina of endothelial cells in CL of wild-type, Tg-RL, and CL-RL mice at Day 1.5 of pregnancy.
DISCUSSION
While PRL has long been known to be essential for CL formation and function in rodents, the generation of mice lacking either the Prl or Prlr gene made apparent the need for this hormone in the transformation of CL of the cycle to that of pregnancy [8, 9]. In the absence of PRL or PRLR, CL forms after ovulation but secretes very low levels of progesterone and involutes rapidly. Although PRL binds to both PRLR types, it was well accepted but never proven that PRL sustains CL formation and steroidogenic activity acting through the PRLR-L form [29, 44, 52–54]. The generation of transgenic mice expressing only PRLR-S [22], which showed no rescue of the CL, further substantiated this possibility. Similar to PRLR null mice, transgenic mice expressing only PRLR-S have CL that rapidly degenerates and fails to produce progesterone [22]. Moreover, these mice exhibited a severe ovarian pathology characterized by massive follicular recruitment followed by granulosa cell and oocyte death, leading to premature ovarian failure [22]. The findings that CL expresses both PRLR types [19, 20] and that PRLR-S is unable to rescue CL formation or its steroidogenic capacity independently led us to rigorously test the hypothesis that PRLR-L is the sole receptor necessary for CL formation and function.
In the present study, we report that mice expressing PRLR-L, either ubiquitously or in a CL-specific manner, have a severe ovarian impairment because of a defect in normal CL development. Interestingly, the presence of PRLR-L alone in the ovary does indeed allow for PRL stimulation of STAT5 and expression of genes known to be regulated by PRL activation of the PRLR-L, such as ESR1 [29, 44, 55] and LHCGR [10, 39, 40, 45]. Yet, it does not allow for cell survival and CL development, establishing for the first time that PRL activation of STAT5 through PRLR-L is insufficient for the proper formation and maintenance of CL of pregnancy. Unlike transgenic mice, which express only PRLR-S, both Tg-RL and CL-RL mice have normal follicular development and ovulation rates. We have previously shown that both types of PRLR cross-talk in the follicle and that expression of PRLR-S alone leads to severe inhibition in several transcription factors including FOXO3A [22] and SP1 [25] and to downregulation of galactose-1-phosphate uridyl transferase (GALT), an enzyme essential for follicular survival [22]. Coexpression of PRLR-S and PRLR-L in either the follicle or in cultured cells rescues the deleterious suppression by PRL activation of PRLR-S alone on GALT expression and restores normal follicular development [22]. This finding implies that PRLR-L can inhibit PRLR-S-mediated cellular death signaling in the follicle. The mechanism of cross-talk between these two types of receptors remains largely unknown. Recent investigations suggest that both types of PRLR can homo- and heterodimerize in the absence of ligand [56, 57], which may be one means of cross-talk between the receptors.
Another novelty of this investigation is the generation of transgenic mice with CL-specific expression of PRLR-L by using the HSD17B7 promoter. The HSD17B7 enzyme was originally discovered as a 32-kDa protein most abundantly and specifically expressed in the CL [58, 59]. It was not found in any other tissue [58]. During pregnancy, HSD17B7 expression becomes detectable, although at much lower levels, in the decidua and trophoblast [35]. However, because this expression in the conceptus occurs only around Day 9 of pregnancy, long after CL of pregnancy forms and becomes highly active, the HSD17B7 promoter can be used to generate a CL-specific transgene for either early pregnancy study or in nonpregnant mice. To our knowledge, this is the first CL-specific transgenic mouse model ever generated.
Results of the present investigation together with our previous data generated with mice expressing only PRLR-S indicate that neither PRLR subtype alone can transduce the repertoire of signals that prevent cell death and those that are necessary for luteinization and vascularization, two events that occur simultaneously after ovulation that are absolutely necessary for CL formation. With early degeneration of CL as shown by a marked increase in apoptotic cells in CL, it was not surprising that transcript levels of steroidogenic enzymes were low in both Tg-RL and CL-RL mice, contributing to the lack of progesterone production. The facts that CL of PRLR-L-expressing mice express abnormally low levels of VEGFA and have impaired vascularization, as shown by discordant organization of endothelial cells in CL, and that granulosa cells isolated from these mice after ovulation luteinize normally in culture suggest that the apparent reason for defective CL formation is improper vascularization.
Neovascularization is a critical requirement for proper CL formation and progesterone production because it provides an import/export system for nutrients, hormones, cholesterol, and most importantly, progesterone [47]. Staining of collagen IV, the primary extracellular membrane component of the subendothelial basal lamina and a factor involved in angiogenesis (Fig. 8B), revealed a disrupted network of endothelial cells in ovaries of both Tg-RL and CL-RL mice. Of the angiogenic factors present in the ovary, VEGF is the key player in angiogenesis in CL [60]. Among the five different isoforms of VEGF that are expressed in the CL [61], VEGFA is the most important one because disruption of VEGFA signaling [62] or inhibition of VEGFA [60] results in a lack of vascularization of CL and inhibition progesterone production. Our finding that VEGFA expression is markedly reduced in both Tg-RL and CL-RL mice provides the primary explanation for impaired progesterone production in these mice. Indeed, luteal cells respond to PRL activation of PRLR-L with STAT5 activation, and primary luteinized granulosa cells isolated from Tg-RL and CL-RL ovaries can undergo hypertrophy and produce progesterone in culture, suggesting that the lack of nutrient access in vivo due to defective vascularization appears to be causal for defective luteal cell hypertrophy and CL function. The fact that PRLR-S is the predominant receptor expressed in endothelial cells derived from CL, as well as other vasculature sources, and that treatment of anti-PRL antibody leads to a significant reduction of endothelial cell growth [42, 63, 64] implies that the lack of PRLR-S in Tg-RL and CL-RL mice may result in the observed decrease in vascular formation. It remains to be determined whether PRLR-S activation induces VEGFA expression in both luteal and endothelial cells, given that PRLR null [11] and PRLR-L-expressing mice both have a luteal vascularization defect. This suggests the intriguing possibility that PRLR-S expressed in the vasculature and PRLR-L expressed in luteal cells act in concert to permit normal CL formation and function, ultimately allowing CL to sustain fertility and pregnancy.
ACKNOWLEDGMENT
The authors would like to thank Dr. Nadine Binart for providing the EF1A empty construct originally used to generate PRLR-S transgenic mice. We would like to acknowledge The University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core (supported by the Eunice Kennedy Shriver National Institute of Child Health and Development/National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research grant U54-HD28934) for serum analysis. The authors are grateful to Dr. Roberta Franks and Kimberly McLaughlin of the University of Illinois at Chicago Research Resources Center Transgenic Production Service for transgene injections and help with embryo transfer and oocyte collection experiments.
Footnotes
Supported by National Institutes of Health grants HD11119, HD 12356 to G.G., T32 DK07739 to J.A.L., and T32 HL007692 to J.A.L.
REFERENCES
- Morishige WK, Rothchild I. Temporal aspects of the regulation of corpus luteum function by luteinizing hormone, prolactin and placental luteotrophin during the first half of pregnancy in the rat. Endocrinology 1974; 95: 260 274 [DOI] [PubMed] [Google Scholar]
- Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog Horm Res 1981; 37: 183 298 [DOI] [PubMed] [Google Scholar]
- Gibori G, Khan I, Warshaw ML, McLean MP, Puryear TK, Nelson S, Durkee TJ, Azhar S, Steinschneider A, Rao MC. Placental-derived regulators and the complex control of luteal cell function. Recent Prog Horm Res 1988; 44: 377 429 [DOI] [PubMed] [Google Scholar]
- Gibori G. The corpus luteum of pregnancy In: Adashi E, Leung P. (eds.), The Ovary. New York: Raven Press; 1992: 261 317 [Google Scholar]
- Murphy BD, Rajkumar K. Prolactin as a luteotrophin. Can J Physiol Pharmacol 1985; 63: 257 264 [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 2007; 28: 117 149 [DOI] [PubMed] [Google Scholar]
- Kalison B, Warshaw ML, Gibori G. Contrasting effects of prolactin on luteal and follicular steroidogenesis. J Endocrinol 1985; 104: 241 250 [DOI] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 1997; 16: 6926 6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 1997; 11: 167 178 [DOI] [PubMed] [Google Scholar]
- Binart N, Helloco C, Ormandy CJ, Barra J, Clement-Lacroix P, Baran N, Kelly PA. Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology 2000; 141: 2691 2697 [DOI] [PubMed] [Google Scholar]
- Grosdemouge I, Bachelot A, Lucas A, Baran N, Kelly PA, Binart N. Effects of deletion of the prolactin receptor on ovarian gene expression. Reprod Biol Endocrinol 2003; 1: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin V, Binart N, Clement-Lacroix P, Bouchard B, Bole-Feysot C, Edery M, Lucas BK, Touraine P, Pezet A, Maaskant R, Pichard C, Helloco C, et al. From the molecular biology of prolactin and its receptor to the lessons learned from knockout mice models. Genet Anal 1999; 15: 189 201 [DOI] [PubMed] [Google Scholar]
- Stocco C, Callegari E, Gibori G. Opposite effect of prolactin and prostaglandin F(2 alpha) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology 2001; 142: 4158 4161 [DOI] [PubMed] [Google Scholar]
- Dajee M, Kazansky AV, Raught B, Hocke GM, Fey GH, Richards JS. Prolactin induction of the alpha 2-Macroglobulin gene in rat ovarian granulosa cells: stat 5 activation and binding to the interleukin-6 response element. Mol Endocrinol 1996; 10: 171 184 [DOI] [PubMed] [Google Scholar]
- Gaddy-Kurten D, Hickey GJ, Fey GH, Gauldie J, Richards JS. Hormonal regulation and tissue-specific localization of alpha 2-macroglobulin in rat ovarian follicles and corpora lutea. Endocrinology 1989; 125: 2985 2995 [DOI] [PubMed] [Google Scholar]
- Gaddy-Kurten D, Richards JS. Regulation of alpha 2-macroglobulin by luteinizing hormone and prolactin during cell differentiation in the rat ovary. Mol Endocrinol 1991; 5: 1280 1291 [DOI] [PubMed] [Google Scholar]
- Russell DL, Norman RL, Dajee M, Liu X, Hennighausen L, Richards JS. Prolactin-induced activation and binding of stat proteins to the IL-6RE of the alpha 2-macroglobulin (alpha 2M) promoter: relation to the expression of alpha 2M in the rat ovary. Biol Reprod 1996; 55: 1029 1038 [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 1998; 19: 225 268 [DOI] [PubMed] [Google Scholar]
- Russell DL, Richards JS. Differentiation-dependent prolactin responsiveness and stat (signal transducers and activators of transcription) signaling in rat ovarian cells. Mol Endocrinol 1999; 13: 2049 2064 [DOI] [PubMed] [Google Scholar]
- Telleria CM, Parmer TG, Zhong L, Clarke DL, Albarracin CT, Duan WR, Linzer DI, Gibori G. The different forms of the prolactin receptor in the rat corpus luteum: developmental expression and hormonal regulation in pregnancy. Endocrinology 1997; 138: 4812 4820 [DOI] [PubMed] [Google Scholar]
- Binart N, Imbert-Bollore P, Baran N, Viglietta C, Kelly PA. A short form of the prolactin (PRL) receptor is able to rescue mammopoiesis in heterozygous PRL receptor mice. Mol Endocrinol 2003; 17: 1066 1074 [DOI] [PubMed] [Google Scholar]
- Halperin J, Devi SY, Elizur S, Stocco C, Shehu A, Rebourcet D, Unterman TG, Leslie ND, Le J, Binart N, Gibori G. Prolactin signaling through the short form of its receptor represses forkhead transcription factor FOXO3 and its target gene galt causing a severe ovarian defect. Mol Endocrinol 2008; 22: 513 522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi YS, Seibold A, Shehu A, Maizels E, Halperin J, Le J, Binart N, Bao L, Gibori G. Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: involvement of a novel phosphatase. J Biol Chem 2011; 286: 7609 7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi YS, Shehu A, Halperin J, Stocco C, Le J, Seibold AM, Gibori G. Prolactin signaling through the short isoform of the mouse prolactin receptor regulates DNA binding of specific transcription factors, often with opposite effects in different reproductive issues. Reprod Biol Endocrinol 2009; 7: 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi YS, Shehu A, Stocco C, Halperin J, Le J, Seibold AM, Lahav M, Binart N, Gibori G. Regulation of transcription factors and repression of Sp1 by prolactin signaling through the short isoform of its cognate receptor. Endocrinology 2009; 150: 3327 3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J 1994; 13: 4361 4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Clevenger CV. Modulation of growth factor receptor function by isoform heterodimerization. Proc Natl Acad Sci U S A 1996; 93: 5947 4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Ye Y, Clevenger CV. Stoichiometric structure-function analysis of the prolactin receptor signaling domain by receptor chimeras. Mol Cell Biol 1998; 18: 896 905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Barkai U, Zhong L, Fazleabas AT, Gibori G. PRL-induced ERalpha gene expression is mediated by Janus kinase 2 (Jak2) while signal transducer and activator of transcription 5b (Stat5b) phosphorylation involves Jak2 and a second tyrosine kinase. Mol Endocrinol 2001; 15: 1941 1952 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A 1997; 94: 7239 7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol 2005; 68: 49 84 [DOI] [PubMed] [Google Scholar]
- Zhong L, Parmer TG, Robertson MC, Gibori G. Prolactin-mediated inhibition of 20alpha-hydroxysteroid dehydrogenase gene expression and the tyrosine kinase system. Biochem Biophys Res Commun 1997; 235: 587 592 [DOI] [PubMed] [Google Scholar]
- Ricken AM, Traenkner A, Merkwitz C, Hummitzsch K, Grosche J, Spanel-Borowski K. The short prolactin receptor predominates in endothelial cells of micro- and macrovascular origin. J Vasc Res 2007; 44: 19 30 [DOI] [PubMed] [Google Scholar]
- Sugino N, Zilberstein M, Srivastava RK, Telleria CM, Nelson SE, Risk M, Chou JY, Gibori G. Establishment and characterization of a simian virus 40-transformed temperature-sensitive rat luteal cell line. Endocrinology 1998; 139: 1936 1942 [DOI] [PubMed] [Google Scholar]
- Shehu A, Mao J, Gibori GB, Halperin J, Le J, Devi YS, Merrill B, Kiyokawa H, Gibori G. Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol Endocrinol 2008; 22: 2268 2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 1997; 56: 976 984 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009; 324: 938 941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu A, Albarracin C, Devi YS, Luther K, Halperin J, Le J, Mao J, Duan RW, Frasor J, Gibori G. The stimulation of HSD17B7 expression by estradiol provides a powerful feed-forward mechanism for estradiol biosynthesis in breast cancer cells. Mol Endocrinol 2011; 25: 754 766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjurulf E, Selstam G, Olofsson JI. Increased LH receptor mRNA and extended corpus luteum function induced by prolactin and indomethacin treatment in vivo in hysterectomized pseudopregnant rats. J Reprod Fertil 1994; 102: 139 145 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol 1990; 4: 1856 1865 [DOI] [PubMed] [Google Scholar]
- Berlanga JJ, Garcia-Ruiz JP, Perrot-Applanat M, Kelly PA, Edery M. The short form of the prolactin (PRL) receptor silences PRL induction of the beta-casein gene promoter. Mol Endocrinol 1997; 11: 1449 1457 [DOI] [PubMed] [Google Scholar]
- Lesueur L, Edery M, Ali S, Paly J, Kelly PA, Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A 1991; 88: 824 828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DL, Linzer DI. Changes in prolactin receptor expression during pregnancy in the mouse ovary. Endocrinology 1993; 133: 224 232 [DOI] [PubMed] [Google Scholar]
- Frasor J, Park K, Byers M, Telleria C, Kitamura T, Yu-Lee LY, Djiane J, Park-Sarge OK, Gibori G. Differential roles for signal transducers and activators of transcription 5a and 5b in PRL stimulation of ERalpha and ERbeta transcription. Mol Endocrinol 2001; 15: 2172 2181 [DOI] [PubMed] [Google Scholar]
- Gafvels M, Bjurulf E, Selstam G. Prolactin stimulates the expression of luteinizing hormone/chorionic gonadotropin receptor messenger ribonucleic acid in the rat corpus luteum and rescues early pregnancy from bromocriptine-induced abortion. Biol Reprod 1992; 47: 534 540 [DOI] [PubMed] [Google Scholar]
- Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology 2007; 148: 2326 2334 [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol 2003; 1: 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993; 268: 26033 26036 [PubMed] [Google Scholar]
- Wang H, Su Y. Collagen IV contributes to nitric oxide-induced angiogenesis of lung endothelial cells. Am J Physiol Cell Physiol 2011; 300: C979 C988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno E, Iurlaro M, Madri JA, Nicosia RF. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In Vitro Cell Dev Biol Anim 2000; 36: 336 340 [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Madri JA. The microvascular extracellular matrix. Developmental changes during angiogenesis in the aortic ring-plasma clot model. Am J Pathol 1987; 128: 78 90 [PMC free article] [PubMed] [Google Scholar]
- Martel C, Labrie C, Dupont E, Couet J, Trudel C, Rheaume E, Simard J, Luu-The V, Pelletier G, Labrie F. Regulation of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase expression and activity in the hypophysectomized rat ovary: interactions between the stimulatory effect of human chorionic gonadotropin and the luteolytic effect of prolactin. Endocrinology 1990; 127: 2726 2737 [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Periman LM, Simard J, Melner MH. Ovarian 3 beta-hydroxysteroid dehydrogenase and sulfated glycoprotein-2 gene expression are differentially regulated by the induction of ovulation, pseudopregnancy, and luteolysis in the immature rat. Endocrinology 1992; 130: 2192 2200 [DOI] [PubMed] [Google Scholar]
- Risk M, Gibori G. Mechanisms of luteal cell regulation by prolactin In: H. ND (ed.), Prolactin. Boston: Kluwer Academic Publishers; 2001: 265 295 [Google Scholar]
- Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab 2003; 14: 118 123 [DOI] [PubMed] [Google Scholar]
- Gadd SL, Clevenger CV. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol 2006; 20: 2734 2746 [DOI] [PubMed] [Google Scholar]
- Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and hetero-dimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 2006; 20: 1912 1923 [DOI] [PubMed] [Google Scholar]
- McLean MP, Nelson S, Parmer T, Khan I, Steinschneider A, Puryear T, Gibori G. Identification and characterization of an abundant phosphoprotein specific to the large luteal cell. Endocrinology 1990; 126: 1796 1805 [DOI] [PubMed] [Google Scholar]
- Duan WR, Linzer DI, Gibori G. Cloning and characterization of an ovarian-specific protein that associates with the short form of the prolactin receptor. J Biol Chem 1996; 271: 15602 15607 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 1998; 4: 336 340 [DOI] [PubMed] [Google Scholar]
- Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 2006; 63: 601 615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF. Regulation and manipulation of angiogenesis in the primate corpus luteum. Reproduction 2001; 121: 355 362 [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Gualillo O, Pezet A, Vincent V, Edery M, Kelly PA. Dominant negative and cooperative effects of mutant forms of prolactin receptor. Mol Endocrinol 1997; 11: 1020 1032 [DOI] [PubMed] [Google Scholar]
- Saunier E, Dif F, Kelly PA, Edery M. Targeted expression of the dominant-negative prolactin receptor in the mammary gland of transgenic mice results in impaired lactation. Endocrinology 2003; 144: 2669 2675 [DOI] [PubMed] [Google Scholar]