ABSTRACT
The expressions of genes involved in cholesterol efflux increase, whereas those involved in extracellular cholesterol uptake decrease, during spontaneous functional regression of the primate corpus luteum (CL). This may result from liver x receptor (LXR) alpha (official symbol NR1H3) and/or beta (official symbol NR1H2) control of luteal gene transcription, because these nuclear receptor superfamily members are key regulators of cellular cholesterol homeostasis. Therefore, studies were conducted to assess endogenous LXR ligands in the primate CL through the luteal phase, and to determine the effect of synthetic or natural LXR ligands on cholesterol efflux and uptake in functional primate luteal cells. Using high-performance liquid chromatography tandem mass spectrometry, three LXR ligands were identified and quantified in the rhesus macaque CL, including 22R-hydroxycholesterol (22ROH), 27-hydroxycholesterol (27OH), and desmosterol. Levels of 22ROH paralleled serum progesterone concentrations, whereas mean levels of 27OH tended to be higher following the loss of progesterone synthesis. Desmosterol was present throughout the luteal phase. Functional macaque luteal cells treated with the synthetic LXR agonist T0901317 or physiologically relevant concentrations of the endogenous luteal ligands 22ROH, 27OH, and desmosterol had increased expression of various known LXR target genes and greater cholesterol efflux. Additionally, T0901317 reduced low-density lipoprotein receptor protein and extracellular low-density lipoprotein uptake, whereas 27OH decreased low-density lipoprotein receptor protein, most likely via a posttranslational mechanism. Collectively, these data support the hypothesis that LXR activation causes increased cholesterol efflux and decreased extracellular cholesterol uptake. In theory, these effects could deplete the primate CL of cholesterol needed for steroidogenesis, ultimately contributing to functional regression.
Keywords: corpus luteum, functional regression, liver x receptor, luteolysis, reverse cholesterol transport, rhesus macaque
Liver x receptor activation in primate luteal cells increases cholesterol efflux and reduces extracellular cholesterol uptake from low-density lipoprotein, contributing to luteal regression by limiting cholesterol availability.
INTRODUCTION
The corpus luteum (CL) is a highly steroidogenic gland that relies primarily on blood-borne cholesterol to support its steroid production because de novo synthesis of cholesterol is only important under severely lipid-deprived states [1–3]. During nonfertile menstrual cycles, the primate CL ceases progesterone (P4) production approximately 2 wk after ovulation in a process known as functional regression. The drop in P4 levels results in remodeling and apoptosis of luteal tissue (structural regression) and the initiation of the next ovarian cycle. Although functional regression is the key event in this process, the factors controlling the loss in P4-synthesizing capability in the primate CL are not known. We recently identified liver x receptor (LXR)-mediated reverse cholesterol transport (RCT) as a potential initiator of functional regression in the primate CL [4, 5]. Both LXR subtypes (α and β) were expressed in the CL, with LXRα (NR1H3) mRNA and protein levels increasing just prior to the drop in serum P4 levels. In parallel with increased LXRα (NR1H3) expression, mRNA levels of known LXR target genes involved in cholesterol efflux, including the ATP-binding cassette subfamilies A1 (ABCA1) and G1 (ABCG1), increased during spontaneous functional regression of natural menstrual cycles [4, 5]. Additionally, the LXRs and RCT components were coexpressed within the large steroidogenic luteal cells, which have numerous intracellular lipid droplets during peak P4 synthesis that become localized to extracellular regions in regressing luteal tissue [4]. This indicates that the LXRs and RCT may actively deplete intracellular cholesterol stores in the primate CL such that the P4-producing potential of the CL is diminished.
In addition to promoting cholesterol efflux, the LXRs may also inhibit extracellular cholesterol uptake from circulating lipoproteins. Low-density lipoprotein receptor (LDLR) and scavenger receptor B1 (SCARB1) mRNA and protein levels decrease during spontaneous functional regression of the primate CL [4, 5]. In human adrenal cells, synthetic LXR agonists decreased mRNA levels of both SCARB1 and LDLR [6]. Furthermore, in human liver cells LXR agonists directly induce transcription of the E3 ubiquitin ligase-inducible degrader of the LDLR (IDOL [official symbol MYLIP]), which targets the LDLR for ubiquitin-mediated degradation [7]. Thus, the LXRs may have transcriptional and posttranslational effects to reduce lipoprotein receptor expression, and consequently inhibit extracellular cholesterol uptake.
The LXRs were originally identified as orphan nuclear receptors belonging to the steroid hormone receptor superfamily [8]. They are now known to bind various precursors or derivatives of cholesterol, thereby serving as “cholesterol sensors” that will stimulate RCT in cases of cholesterol excess [9]. Several endogenous LXR agonists have been identified, with the most notable being hydroxylated cholesterol variants (“oxysterols”), or biosynthetic precursors of cholesterol [9], whereas cholesterol itself or steroids produced by the CL are not LXR agonists [10]. Agonists potentially present in the CL include byproducts of steroidogenesis (22R-hydoxycholesterol [22ROH]); cholesterol hydroxylation via specific cytochrome P450 enzymes (24S-hydroxycholesterol [24SOH], 25-hydroxycholesterol [25OH], and 27-hydroxycholesterol [27OH]); a shunt in the cholesterol biosynthetic pathway (24,25-epoxy cholesterol [24,25-epoxy]); and cholesterol biosynthesis (desmosterol, the immediate precursor to cholesterol) [9]. Although there is an increase in LXR target gene expression coinciding with functional regression of the primate CL [4, 5], the presence of endogenous LXR ligands and the functional consequence of luteal LXR activation are unknown.
To further test our hypothesis that LXR-mediated RCT and inhibition of cholesterol uptake cause functional regression of the primate CL, the aims of this study were to: 1) identify and quantify endogenous LXR ligands present in the primate CL throughout the natural luteal phase, and 2) determine whether LXR activation by synthetic or endogenous ligands results in increased cholesterol efflux and reduced extracellular lipoprotein uptake in fully functional primate luteal cells.
MATERIALS AND METHODS
CL Collection
All animal protocols were approved by the Oregon National Primate Research Center's Animal Care and Use Committee. For identification of endogenous LXR ligands, CL (n = 7 per stage) were collected between Days 3 and 4 (early, developing CL), Days 7 and 8 (mid, fully functional CL), Days 10 and 12 (mid-late, late functional CL), Days 14 and 16 (late, period of functional regression), or Days 18 and 19 (very late, menses) after the midcycle luteinizing hormone (LH) surge, as described elsewhere [11]. Late-stage CL were further divided into two groups, with those having serum P4 levels >1.5 ng/ml at the time of lutectomy considered functional CL, and those with P4 <0.5 ng/ml considered to be regressed CL [5]. For the luteal cell cultures, CL (n = 6–9 per experiment) were collected during the mid luteal phase (Days 7–8 post-LH surge).
Sterol Extraction
Frozen (−80°C) CL pieces (approximately 15–40 mg) were weighed and homogenized in 2 ml of PBS. Stable isotope-labeled homologues for each sterol of interest were added to serve as internal standards, and the final volume of the homogenate was brought to 3 ml with PBS. Lipids were extracted by addition of 6.6 ml of a chloroform/methanol mixture (1:1 [v:v]) and vortexing for 2 min. Extracts were centrifuged for 5 min at 1400 × g, and the lower organic layer was collected and dried under nitrogen at 37°C. The lipid extracts were dissolved in 2 ml of toluene and added to a 100-mg silica solid-phase extraction column (Biotage AB, Charlottesville, VA) preconditioned with hexane. The column was washed with 1 ml of hexane. Desmosterol and 24,25-epoxy were eluted with 8 ml of 0.5% isopropanol in hexane, and the oxysterols (22ROH, 24SOH, 25OH, and 27OH) were eluted with 8 ml of 30% isopropanol in hexane [12]. Each fraction was dried under nitrogen, dissolved in 80 μl of methanol, and transferred to an autosampler vial.
High-Performance Liquid Chromatography Tandem Mass Spectrometry Analyses
Extracts were subjected to high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) using a 250 × 2.1 mm (inner diameter), 5-μm BetaBasic C18 high-performance LC (HPLC) column (ThermoHypersil, Waltham, MA) and a Thermo TSQ Quantum Triple Quadrupole mass spectrometer equipped with an atmospheric pressure chemical ionization source (San Jose, CA). The ionization interface was operated in the positive mode with settings as previously described [13]. The LC-MS/MS system was composed of an inline Thermo Surveyor autosampler and HPLC pump. The HPLC column temperature was 10°C. The isocratic mobile phase consisted of methanol:acetonitrile:water (14:0.6:1 by volume) delivered at a flow rate of 0.4 ml/min [13]. A 15-min column wash was included. A total of 25 μl of each calibrant or unknown was injected onto the HPLC column. The mass spectrometer was operated using selected reaction monitoring for sterol identification and quantification. Authentic standards were used for optimization of reaction conditions, including HPLC retention times, collision energies, and selected reaction monitoring precursor to product ion selection.
Quantification of Oxysterols
Calibration curves for quantification were created using authentic standards with deuterium-labeled homologues (Avanti Polar Lipids Inc., Alabaster, AL) used as internal standards (0.5–3 ng/μl). Calibrator concentrations ranged from 0.04 to 3 ng/μl for each oxysterol. Calibration curves were generated by performing a least-squares linear regression for calibrant peak area ratios obtained (oxysterol:oxysterol-deuterium) vs. specified concentration in nanograms per microliter (ng/μl). Calculated quantities of each sterol in CL samples were normalized to tissue weight. To assess ion suppression (the effect of matrix), endogenous sterols and/or isotopically labeled standards from 2-fold increasing quantities of rhesus macaque liver and purified sterols spiked into PBS were extracted in parallel with individual CL samples. Liver tissue was used because it was presumed to be rich in oxysterols and is more readily available than luteal tissue.
Luteal Cell Cultures
Dispersed luteal cell cultures were prepared by collagenase digestion of freshly collected mid luteal phase CL as described elsewhere [14]. Luteal cells (3 × 104 to 4 × 104) were plated in 96-well fibronectin-coated plates in serum-free media (Dulbecco modified Eagle medium/Ham F-12 containing 10 mM HEPES buffer, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mg/ml gentamicin, 3 mg/ml bovine serum albumin, 20 mg/ml insulin, 5 μg/ml transferrin, 25 nM selenium, and 10 μg/ml aprotinin) and maintained at 37°C with 5% CO2 in air. This study had two treatment arms: 1) synthetic LXR agonist and 2) endogenous LXR agonists. Luteal cells were incubated with their respective treatments for 24 h and then harvested for mRNA and protein isolation, or subjected to cholesterol efflux and lipoprotein uptake assays. The first set of experiments employed a 2 × 2 factorial design involving 1 μM synthetic LXR agonist T0901317 (T09; Cayman Chemical Co., Ann Arbor, MI) or 0.1% dimethyl sulfoxide (DMSO) vehicle control, in the presence or absence of 0.5% (w:v) 2-hydroxypropyl-β-cyclodextrin (β-CDX; Sigma-Aldrich Inc., St. Louis, MO). The second set of treatments involved 1 and 5 μM doses to approximate the physiologic range of the endogenous LXR agonists identified and quantified by LC-MS/MS—22ROH, 27OH, and desmosterol, with an equal dose of cholesterol serving as the negative control. All treatments in the endogenous LXR agonist arm involved coadministration of β-CDX. A concentration of 0.5% β-CDX was selected because it was the lowest concentration recommended by the manufacturer. The use of β-CDX can cause enrichment or depletion of cellular sterols depending on its concentration relative to sterols in the media [15]. Cotreatment of luteal cells with β-CDX was always used in the endogenous LXR agonist treatments to facilitate cellular uptake of hydrophobic sterols. Because of potential differences in sterol enrichment between various doses of extracellular sterols (i.e., 1 vs. 5 μM concentrations), comparisons were only made between treatments involving an equal concentration of extracellular sterol. In the T09 treatment arm that lacked extracellular sterols in the treatment medium, β-CDX was also employed to determine the effects on basal LXR target gene expression in a sterol-depleted state.
Quantitative Real-Time PCR and Western Blot Analyses
After the media were aspirated, cells were harvested in Trizol reagent (100 μl per well; Invitrogen Inc., Carlsbad, CA) and stored at −80°C. Extraction of mRNA and protein was performed according to the manufacturer's recommendations. Quantitative real-time PCRs (Q-PCRs) for the ATP-binding cassette subfamilies A1 (ABCA1) and G1 (ABCG1), LXRα (NR1H3), LDLR, and SCARB1 were performed as described previously [4, 5]. The Q-PCR for IDOL (MYLIP) was performed using forward and reverse primers 5′-ACTCGCCTCTGAAGTCCTCAGA-3′ and 5′-TCAGCTTGCGCAGCTTCTC-3′, respectively, along with the following TaqMan probe: 5′-6FAM-TGAGGGCCTCAGCTGCCAGCA-MGBNFQ-3′. Western blot analyses for ABCA1, ABCG1, LXRα (NR1H3), LDLR, SCARB1, and β-tubulin (TUBB), as a loading control, were performed as described previously [4]. Because of the limited number of cells used for each treatment, no protein assay was performed, and the entire amount of protein recovered from each replicate was loaded into a single well for Western blot analysis. Proteins were detected by sequential probing of the membrane, with the membrane being stripped of previously bound antibody prior to addition of a new antibody using a Western blot stripping buffer according to the manufacturer's recommendations (Thermo Scientific, Rockford, IL). The loading control TUBB was previously determined to be invariant throughout the luteal phase [11]. Densitometry analysis was performed using Quantity One version 4.3.1 software (Bio-Rad, Hercules, CA), and the ratio of target protein:TUBB was calculated to account for differences in protein content per lane.
Cholesterol Efflux Assay
Following a 24-h treatment period, cells incubated in clear-bottomed, black-sided 96-well plates (Greiner Bio-One Inc., Monroe, NC) were loaded with 6 μg/ml BODIPY-cholesterol (Avanti Polar Lipids) for 1 h in media containing 0.5% β-CDX to promote absorption of BODIPY-cholesterol. The fluorescent BODIPY-cholesterol has been previously shown to closely mimic the characteristics of cholesterol and to be a suitable substitute for tritiated cholesterol in efflux assays [16]. Following BODIPY-cholesterol loading, the cells were washed once with PBS, and serum/phenol red-free media containing 10 μg/ml apolipoprotein A1 (APOA1) was incubated with the cells for 2 h at 37°C. The media were collected and centrifuged at 2000 × g for 10 min to pellet detached cells, and the supernatant was transferred to empty wells within the original 96-well plate. BODIPY fluorescence was determined in cells and media using a SpectraMax M5 Microplate reader (Molecular Devices Inc., Sunnyvale, CA) with an excitation/emission of 485/515 nm. Background fluorescence from control cells not receiving BOIDPY-cholesterol was subtracted from all groups, and cholesterol efflux was calculated as the percentage of fluorescence in the media relative to the total fluorescence (cells + media), which automatically corrects for differences in cell number and total BODIPY-cholesterol loaded.
Lipoprotein Uptake Assays
Following a 24-h treatment period, cells were incubated for 2 h in serum-free media containing 10 μg/ml fluorescently labeled low-density lipoprotein (Dil-LDL; Invitrogen) or 25 μg/ml fluorescently labeled high-density lipoprotein (Dil-HDL; Intracel Resources LLC, Frederick, MD), plus 5 μg/ml Hoechst 33342 (Invitrogen). After aspirating the media, cells were washed once with PBS and then immersed in PBS. Fluorescence was determined as described earlier using excitation/emission of 544/571 nm for Dil-labeled lipoproteins, and 355/460 nm for Hoechst 33342. Background fluorescence from cells not receiving Dil lipoproteins or Hoechst 33342 was subtracted from all groups, and Dil-lipoprotein fluorescence was normalized to Hoechst 33342 to correct for differences in cell number.
Statistical Analyses
Data were log-transformed if necessary to stabilize variance. For endogenous levels of LXR agonists identified by LC-MS/MS, differences were analyzed by one-way ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Raw data for Q-PCR, Western blot, cholesterol efflux, and lipoprotein uptake experiments in dispersed luteal cells were subjected to one-way repeated-measures ANOVA followed by posthoc comparisons with the Student-Newman-Keuls test. When only two groups were compared, data were analyzed by paired t-test. Differences were considered significant at P < 0.05.
RESULTS
Individual Endogenous LXR Ligand Concentrations in the Rhesus Macaque CL Through the Luteal Phase
Linear standard curves (R2 > 0.99) developed from increasing quantities (0–3 ng/μl) of authentic standards were obtained for all sterols of interest. Extraction efficiencies calculated from the isotopically labeled standards were: 22ROH, 79%; 24SOH, 77%; 25OH, 85%; 27OH, 85%; 24,25-epoxy, 86%; and desmosterol, 100%. Recovery of endogenous sterols and/or isotopically labeled standards from 2-fold increasing quantities of rhesus macaque liver, as well as 2-fold increasing amounts of purified sterols spiked into PBS, was linear for all sterols analyzed (R2 > 0.97; Supplemental Fig. S1, available online at www.biolreprod.org). Calibrators generated using authentic standards were included with each sample set to obtain an overview of the precision and accuracy of detection with the LC-MS/MS method. Acceptable reproducibility (intraassay n = 3 per assay, and interassay n = 2; <18% coefficient of variation) was obtained for analysis of oxysterols across the range of 0.08–1 ng/μl.
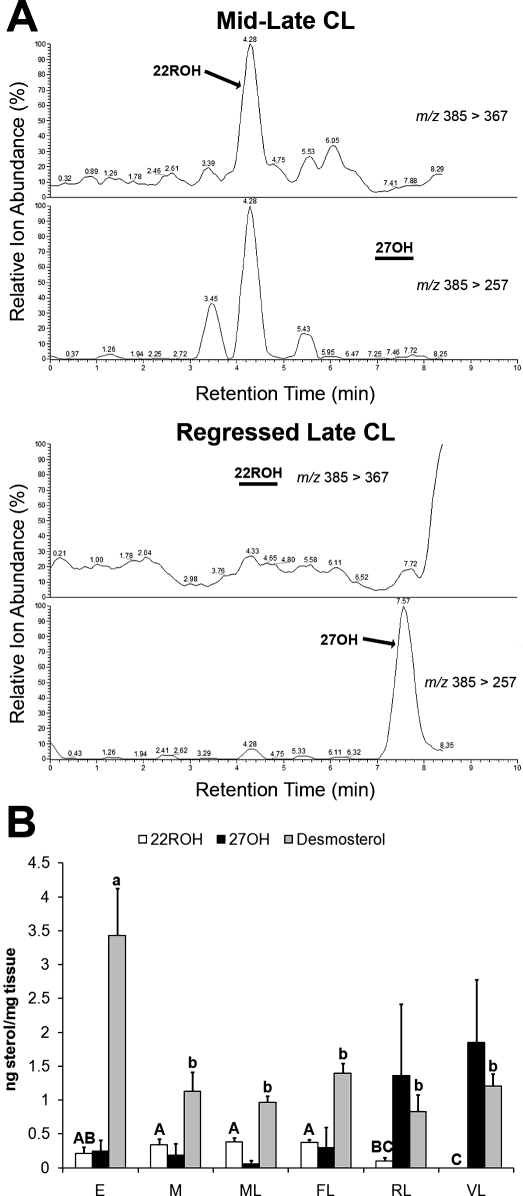
Among the natural LXR agonists evaluated, 22ROH, 27OH, and desmosterol were consistently detected within macaque CL during either the entire luteal phase or at specific stages (Fig. 1). Levels of 22ROH paralleled serum P4 levels, with regressed late and very late CL having significantly (P < 0.05) lower levels of 22ROH than mid, mid-late, or functional late CL. In contrast, mean concentrations of 27OH were low in early, mid, mid-late, and functional late CL, but trended toward increased concentrations in regressed late CL and very late CL. Differences in 27OH were not statistically significant because the data failed to pass normality. Desmosterol levels in early CL were significantly (P < 0.05) higher than in all other stages.
FIG. 1.
Concentrations of endogenous LXR agonists in rhesus macaque CL throughout the natural luteal phase. A) Extracted ion chromatograms from a representative mid-late CL indicating the presence of a peak for 22ROH, and the absence of a peak for 27OH (black bar). A representative regressed late CL is shown, indicating the absence of 22ROH (black bar) and presence of 27OH. B) Concentrations of 22ROH, 27OH, and desmosterol in individual CL (n = 7) collected throughout the luteal phase (E, early; M, mid; ML, mid-late; FL, functional late; RL, regressed late; VL, very late). Error bars indicate one SEM. Different letters of the same case indicate significant differences between stages of the luteal phase for a particular endogenous LXR ligand (P < 0.05).
LXR Ligands Induce the Expression of RCT Components in Rhesus Macaque Luteal Cells
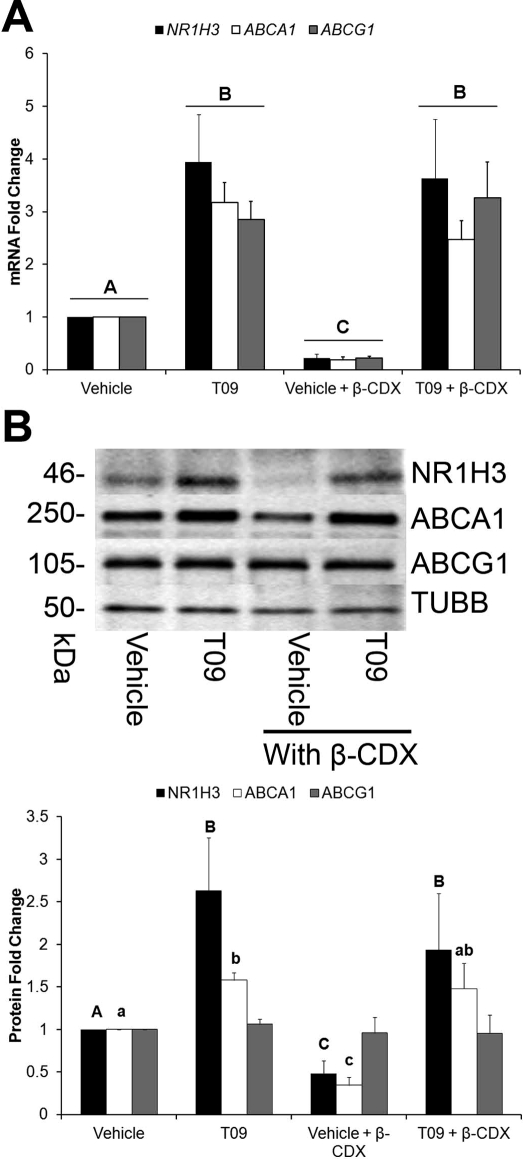
Messenger RNA levels of the known LXR target genes LXRα (NR1H3), ABCA1, and ABCG1 were significantly (P < 0.05) increased by the synthetic agonist T09 in the presence or absence of β-CDX (Fig. 2A). Also, β-CDX itself, which depletes cells of sterols when incubated in the absence of sterols in the media [15], caused a significant (P < 0.05) decrease in the basal expression of these three genes. Corresponding changes in protein levels of LXRα (NR1H3) and ABCA1, but not ABCG1, were observed following T09 and β-CDX treatments (Fig. 2B).
FIG. 2.
LXR target gene expression in rhesus macaque luteal cells following treatment with a synthetic LXR agonist and β-CDX. A) The Q-PCR data for the three LXR target genes—LXRα (NR1H3), ABCA1, and ABCG1—following a 2 × 2 factorial treatment with T09 and β-CDX for 24 h. B) Representative Western blots for the proteins encoded by the mRNA quantified in A, as well as results from the corresponding densitometry analysis of individual replicates (n = 7) normalized to TUBB. Error bars indicate one SEM. Different letters indicate significant differences (P < 0.05).
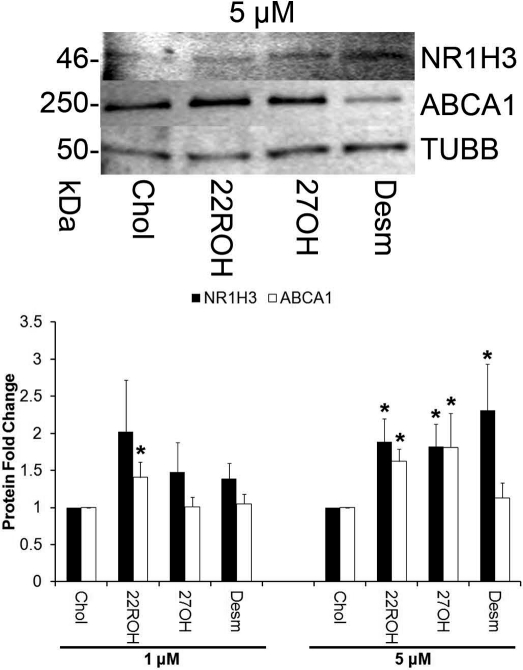
All three endogenous agonists tested (22ROH, 27OH, desmosterol) significantly increased (P < 0.05) luteal cell protein levels for LXRα (NR1H3) at 5 μM doses; 22ROH increased ABCA1 at both 1 and 5 μM doses; and 27OH increased ABCA1 at the 5 μM dose (Fig. 3). There were no changes in ABCG1 protein expression in any of the groups (data not shown).
FIG. 3.
Protein expression of LXR target genes in rhesus macaque luteal cells following treatment with endogenous LXR agonists. The image shows representative Western blots of luteal cell homogenates for LXRα (NR1H3) and ABCA1 following a 24-h exposure to a 5 μM dose of the various treatments, as well as results from the corresponding densitometry analysis of individual replicates (n = 8) normalized to TUBB to account for differences in total protein loaded. Error bars indicate one SEM. Asterisks denote significant difference from cholesterol-only control cultures (P < 0.05).
LXR Ligands Inhibit LDLR Protein Expression in Rhesus Macaque Luteal Cells
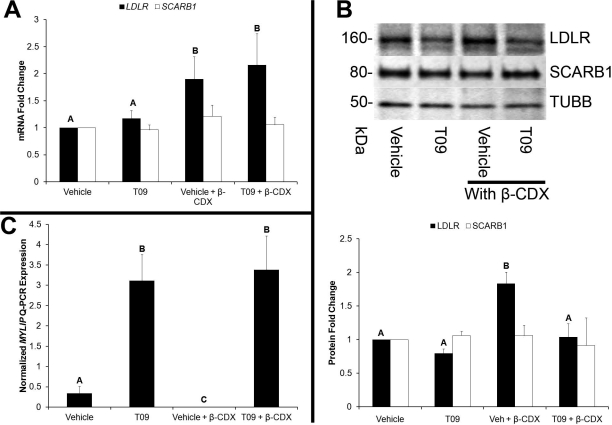
There was no effect of T09 on LDLR or SCARB1 mRNA, whereas β-CDX significantly (P < 0.05) increased LDLR but not SCARB1 mRNA (Fig. 4A). Levels of LDLR protein were significantly increased by β-CDX and decreased by T09 in the presence of β-CDX (P < 0.05). There was no effect of any treatment on SCARB1 protein (Fig. 4B). Levels of IDOL (MYLIP) mRNA were significantly increased by T09, and basal expression was decreased (P < 0.05) by β-CDX (Fig. 4C).
FIG. 4.
Control of lipoprotein receptor levels in rhesus macaque luteal cells by a synthetic LXR agonist and β-CDX. A) The Q-PCR results for quantification of LDLR and SCARB1 mRNA levels following treatment of luteal cells with T09 in the presence or absence of β-CDX. B) Representative Western blot images of LDLR and TUBB, as well as results from densitometry analysis of individual replicates (n = 7) for LDLR and SCARB1 protein levels normalized to TUBB to account for differences in total protein loaded. C) IDOL (MYLIP) mRNA expression. Error bars indicate one SEM. Different letters denote significant (P < 0.05) differences.
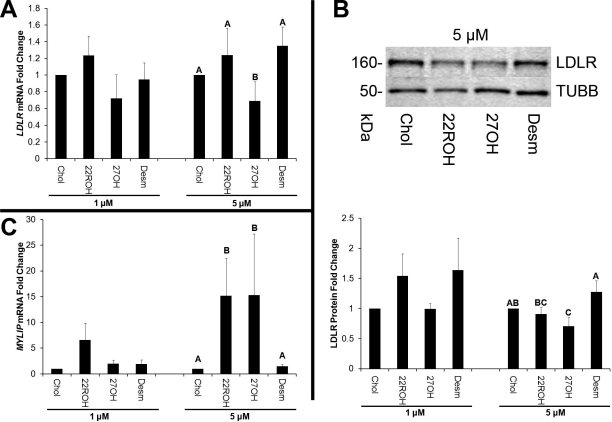
The 5 μM dose of 27OH significantly (P < 0.05) decreased LDLR mRNA compared with an equal dose of cholesterol (Fig. 5A). Levels of LDLR protein were significantly reduced by 5 μM 27OH (Fig. 5B). There was no effect of any treatment on SCARB1 mRNA or protein (data not shown). Both 22ROH and 27OH significantly increased MYLIP expression compared with cholesterol at 5 μM doses (Fig. 5C).
FIG. 5.
Control of LDLR and IDOL (MYLIP) mRNA, as well as LDLR protein levels by endogenous luteal LXR agonists. A) The Q-PCR results for the quantification of LDLR mRNA levels in luteal cells treated with 1 or 5 μM doses of 22ROH, 27OH, desmosterol, and cholesterol as the negative control. B) Representative Western blot images, as well as results from densitometry analysis of individual replicates (n = 8) for LDLR protein levels normalized to TUBB to account for differences in total protein loaded. C) The Q-PCR results for IDOL (MYLIP) mRNA expression in the same cells from A. Error bars indicate one SEM. Different letters denote significant (P < 0.05) differences.
LXR Activation Induces Cholesterol Efflux and Inhibits Lipoprotein Uptake in Rhesus Macaque Luteal Cells
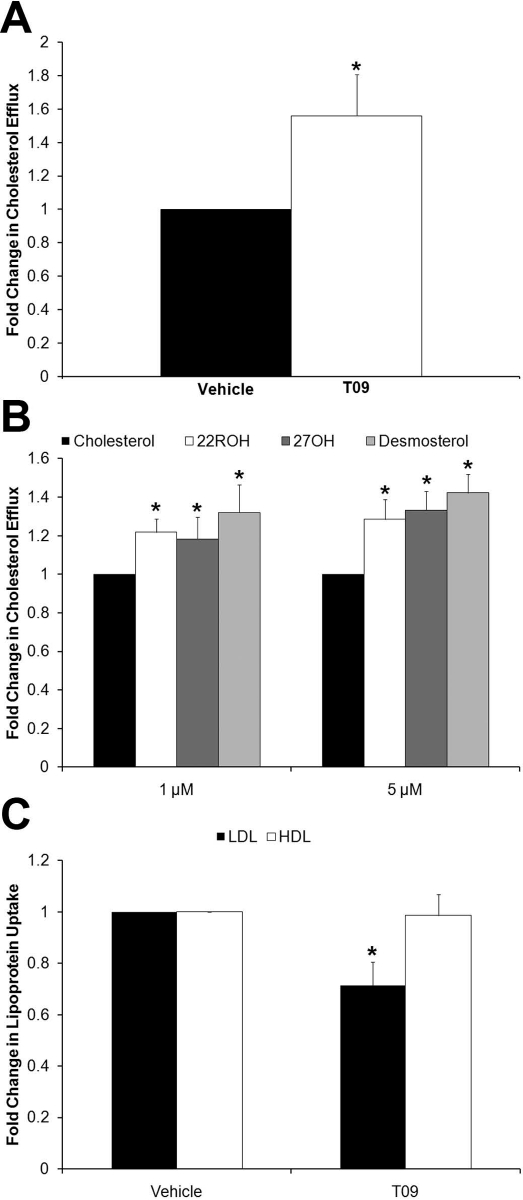
Treatment with T09 caused a significant increase (P < 0.05) in BODIPY-cholesterol efflux from luteal cells compared with the DMSO vehicle control (Fig. 6A). Pretreatment with all three endogenous agonists (22ROH, 27OH, desmosterol) resulted in significantly (P < 0.05) higher rates of BODIPY-cholesterol efflux compared with cells pretreated with an equal concentration of cholesterol at both 1 and 5 μM doses (Fig. 6B). There was a significant decrease (P < 0.05) in LDL uptake in cells treated with T09 in the presence of β-CDX, but there was no effect of T09 on HDL uptake (Fig. 6C). No significant differences in LDL or HDL uptake were detected between the endogenous LXR agonists and equal concentrations of cholesterol (data not shown).
FIG. 6.
Effects of LXR activation by synthetic or endogenous LXR agonists on luteal cell cholesterol efflux and extracellular lipoprotein uptake. A) Results of cholesterol efflux assays performed in luteal cells treated with DMSO or T09. BODIPY-cholesterol efflux from luteal cells treated with 1 or 5 μM doses of cholesterol, 22ROH, 27OH, and desmosterol is displayed in B. Extracellular LDL and HDL uptake in luteal cells treated with DMSO or T09 is shown in C. All cholesterol efflux and lipoprotein uptake experiments were performed with 0.5% β-CDX present during the initial 24-h treatment period, and during the 1-h BODIPY-cholesterol loading period for the efflux assay. However, β-CDX was not included during the 2-h period when cholesterol efflux and lipoprotein uptake were quantified. Error bars indicate one SEM. Asterisks denote statistically significant (P < 0.05) differences (n = 6–7 per experiment).
DISCUSSION
Our data indicate that at least three known LXR ligands [9] are present in the primate CL during different stages of the luteal phase. Concentrations of 22ROH (nanograms per milligram [ng/mg] tissue) closely paralleled serum concentrations of P4 (nanograms per milliliter [ng/ml]), which was expected, considering that 22ROH is a byproduct of the conversion of cholesterol to pregnenolone via cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) [9]. Concentrations of 22ROH displayed the opposite pattern relative to 27OH, with the former at peak levels in functional CL and the latter more prevalent in CL undergoing luteolysis. Our previously published microarray databases revealed cytochrome P450, family 27, subfamily A, polypeptide 1 (CYP27A1) mRNA, which encodes the enzyme responsible for the synthesis of 27OH from cholesterol, was significantly (P < 0.05) increased in regressed late and very late CL relative to all other stages [5, 11]. In addition to serving as an LXR ligand, a second possible function of 27OH is to serve as an alternate mechanism for cholesterol efflux from the CL, because it has been demonstrated that cholesterol is removed from macrophages via an RCT-independent pathway involving CYP27A1-induced hydroxylation of cholesterol [17]. Desmosterol was present throughout the luteal phase, but its concentrations were invariant during functional and structural regression.
We have previously reported that there appears to be an acute induction of LXR activity associated with spontaneous functional regression of the macaque CL [4], with the first evidence of increased transcription of LXR target genes occurring between the mid-late and functional late stages of the luteal phase. We did not detect a significant increase in any endogenous LXR ligands during this period. Mean levels of 27OH were higher during luteolysis, but this appeared to occur either simultaneously with or immediately following functional regression and was not statistically significant because of nonnormality of the data. Therefore, an increase in endogenous LXR ligands is likely not the cause of increased LXR signaling that appears to occur preceding functional regression. We previously reported an increase in LXRα (NR1H3) mRNA and protein levels during the mid-late to functional late transition [4], which by binding existing endogenous ligands could account for increased LXR signaling. Although LXRα (NR1H3) itself is an LXR target gene [18], expression of LXRα (NR1H3) can increase through non-LXR-mediated mechanisms, including peroxisome proliferator-activated receptors α and γ, insulin, and interleukin 10 [9], some of which may be responsible for the increase in LXRα (NR1H3) mRNA and protein levels just prior to functional regression in the rhesus macaque CL. Additionally, the ability of LXRα (NR1H3) to regulate transcription is, in some cases, independent of changes in receptor or ligand levels. For example, protein kinase A (PKA) phosphorylation of LXRα (NR1H3) has been shown to inhibit its transcriptional activity [19]. In luteal cells, LH binds to its receptor and activates PKA [20], which is critical for maintaining primate luteal function. Toward the end of the primate luteal phase, LH pulses become less frequent in primates [21] and luteal LH sensitivity is diminished [14, 22, 23], potentially leading to enhanced LXRα (NR1H3) signaling due to decreased PKA activity. Indeed, luteal expressions of several known LXR target genes increase significantly (P < 0.05) following blockade of LH secretion and are returned to near control levels with LH replacement during the mid-late luteal phase of rhesus macaques, as determined via a previously published microarray database [24].
To determine the functional effects of LXR signaling, CL were collected from rhesus macaques during the mid luteal phase for dispersed luteal cell cultures. This corresponds to the period of peak P4 synthesis and is approximately 1 wk before functional regression normally occurs. Luteal cells were treated with either a potent synthetic LXR agonist or endogenous LXR ligands discovered by LC-MS/MS analyses. As expected, T09 caused significant increases in the mRNA levels of the known LXR target genes LXRα (NR1H3), ABCA1, and ABCG1. Interestingly, β-CDX itself caused a significant decrease in their mRNA levels. Because β-CDX is known to deplete cells of sterols and presumably endogenous LXR ligands if present in excess relative to extracellular sterols [15], this indicates that there is a significant level of basal LXR transcription occurring in these cells that is dependent on endogenous sterols. This finding was supported by results obtained from luteal cells treated with the endogenous LXR ligands 22ROH, 27OH, and desmosterol, whereby all three caused a significant increase in protein levels of LXRα (NR1H3), and both 22ROH and 27OH significantly increased ABCA1 protein.
With regard to the net effect of changes in cholesterol transporter mRNA or protein expression on cholesterol efflux, the synthetic LXR agonist T09 significantly increased luteal cell cholesterol efflux as expected. Luteal cells treated with 1 or 5 μM 22ROH, 27OH, and desmosterol had significantly higher rates of cholesterol efflux compared with cells treated with 1 or 5 μM cholesterol. It was somewhat surprising that desmosterol induced significant increases in cholesterol efflux, given that it appeared to be less potent than either 22ROH or 27OH in stimulating expression of LXR target genes. Desmosterol did significantly increase LXRα (NR1H3) protein levels, and its net effect on cholesterol efflux may be partially the result of an undefined mechanism. The 1 and 5 μM doses of endogenous agonists were selected to approximate the physiologic range of endogenous LXR ligands detected in the primate CL by LC-MS/MS (micrograms of sterol per gram of tissue = micrograms of sterol per milliliter of media). Thus, at doses approximating physiologic levels, all endogenous LXR ligands tested are able to induce cholesterol efflux from functional primate luteal cells.
We found no evidence that LXR signaling mediates the decrease in SCARB1 mRNA and protein that occurs in the primate CL during spontaneous functional regression [4, 5], indicating that LXR signaling does not directly regulate HDL uptake in the primate CL. However, LDL uptake may be more important with regard to functional regression because the primate CL preferentially uses LDL-derived cholesterol for steroidogenesis [3]. Both T09 and 27OH were able to significantly reduce LDLR protein levels. In the case of T09, this decrease in LDLR protein was associated with a significant decrease in extracellular LDL uptake, and occurred in spite of the fact that T09 did not reduce LDLR mRNA levels. This effect is likely mediated through induction of IDOL (MYLIP) mRNA, which has previously been shown to be an LXR-induced gene encoding an E3 ubiquitin ligase that specifically targets the LDLR for proteolytic digestion [7]. Interestingly, when intracellular sterol concentrations are low, there is an increase in LDLR transcription mediated by sterol-regulatory elements on the LDLR promoter [25]. This is consistent with our findings that β-CDX itself, which depletes cells of sterols when present in excess of extracellular sterols [15], increased LDLR mRNA and protein. Thus, reduced intracellular cholesterol levels resulting from increased RCT may cause a compensatory increase in LDLR transcription, but LXR-mediated increases in IDOL (MYLIP) expression may cause an overall inhibitory effect on cellular LDLR protein levels by ensuring receptor degradation. The ability of 27OH to lower LDLR protein levels may be not only due to induction of IDOL (MYLIP) expression, but also the result of reduced LDLR transcription, because 27OH was the only LXR agonist among either the endogenous or synthetic agonists tested that reduced LDLR mRNA levels. This may also explain why 27OH was the only endogenous agonist to significantly reduce LDLR protein, even though 22ROH also significantly increased IDOL (MYLIP) expression. The effect of 27OH on LDLR transcription is likely not an LXR-mediated effect and is more likely a 27OH-specific effect, because it has previously been reported that 27OH reduces LDLR transcription during periods when steroidogenesis is suppressed [26, 27]. The P4 and pregnenolone produced during steroidogenesis inhibit CYP27A1 expression and activity, resulting in decreased 27OH synthesis, which in turn maintains LDLR expression and cholesterol uptake for steroidogenesis [26, 27]. Our LC-MS/MS data are consistent with this scenario because mean levels of 27OH were the highest among LXR ligands present in the rhesus macaque CL during the regressed late and very late stages of the luteal phase, the stages following the loss of P4 synthesis when LDLR mRNA and protein are at their lowest levels [4, 5].
The role of the LXRs and their effects on regulating steroidogenesis, particularly in reproductive tissues, are less clear. Increased steroidogenesis and accumulation of cholesterol esters is observed in the adrenals of Nr1h3 knockout mice [28], whereas human adrenal cells treated with a synthetic LXR agonist have reduced steroid secretion [6]. In luteinized human granulosa cells, in addition to increasing the expression of known LXR target genes and cholesterol efflux, LXR agonists also inhibit P4 production [29]. Furthermore, in macaque granulosa cells an ovulatory bolus of human chorionic gonadotropin decreases LXRα (NR1H3) and its target genes ABCA1 and ABCG1 within 3 h, whereas an LXR agonist inhibited human chorionic gonadotropin-induced effects on LXR target gene expression and P4 production, indicating that a suppression of LXR function is necessary for the dramatic increase in steroidogenesis that occurs during luteinization of the ovulatory follicle [30]. Collectively, these studies support an antisteroidogenic effect of LXR activity. Our data indicate that both synthetic and physiologically relevant concentrations of endogenous LXR agonists increase cholesterol efflux, whereas the synthetic agonist reduces extracellular cholesterol uptake from LDL in luteal cells. In rhesus macaque luteal cells, 27OH significantly inhibited LDLR protein expression but not LDL uptake. A longer treatment period may eventually lead to reduced LDL uptake. Because the primate CL is a highly steroidogenic gland that has been estimated to secrete 40 mg of P4 per day in humans [31], and the primate CL preferentially uses LDL to supply cholesterol for steroidogenesis [3], increasing cholesterol efflux and reducing LDL uptake may deprive the primate CL of cholesterol, ultimately serving as a mediator of functional regression. Future studies are needed to determine whether limiting cholesterol availability via increased LXR activity is the trigger for the onset of luteal regression in the primate CL.
ACKNOWLEDGMENT
The authors would like to thank Ms. Melinda Murphy for general laboratory technical assistance. We would also like to thank the Oregon National Primate Research Center Division of Animal Resources for surgical procedures, and the Molecular and Cellular Biology Core for Q-PCR support and cell culture reagents (Dr. Eliot Spindel, director; Yibing Ja; and Corey Singleton). Finally, we would like to thank the Bioanalytical Shared Resource/Pharmacokinetics Core at the Oregon Health & Science University main campus for LC-MS/MS support (Dr. Dennis Koop, director).
Footnotes
Supported by National Institutes of Health grants K99 HD067678 to R.L.B., R01 HD42000 to J.D.H., U54 HD55744 to J.D.H., and RR00163 to J.D.H.
REFERENCES
- Gwynne JT, Strauss JF., III The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev 1982; 3: 299 329 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000; 80: 1 29 [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Structure, function, and regulation of the corpus luteum. : Knobil E, Neill JD. (eds.), The Physiology of Reproduction. New York: Raven; 2006: 475 526 [Google Scholar]
- Bogan RL, Hennebold JD. The reverse cholesterol transport system as a potential mediator of luteolysis in the primate corpus luteum. Reproduction 2010; 139: 163 176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Hennebold JD. Dynamic changes in gene expression that occur during the period of spontaneous functional regression in the rhesus macaque corpus luteum. Endocrinology 2009; 150: 1521 1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Stulnig TM, Lin CY, Yeo AL, Nowotny P, Liu ET, Steffensen KR. Liver X receptors regulate adrenal steroidogenesis and hypothalamic-pituitary-adrenal feedback. Mol Endocrinol 2007; 21: 126 137 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009; 325: 100 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 2000; 16: 459 481 [DOI] [PubMed] [Google Scholar]
- Wójcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online) 2007; 61: 736 759 [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996; 383: 728 731 [DOI] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol 2008; 22: 1260 1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JG, Thompson BM, McCrum EC, Russell DW. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol 2007; 432: 145 170 [DOI] [PubMed] [Google Scholar]
- DeBarber AE, Lutjohann D, Merkens L, Steiner RD. Liquid chromatography-tandem mass spectrometry determination of plasma 24S-hydroxycholesterol with chromatographic separation of 25-hydroxycholesterol. Anal Biochem 2008; 381: 151 153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannian JD, Stouffer RL. Progesterone production by monkey luteal cell subpopulations at different stages of the menstrual cycle: changes in agonist responsiveness. Biol Reprod 1991; 44: 141 149 [DOI] [PubMed] [Google Scholar]
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res 1997; 38: 2264 2272 [PubMed] [Google Scholar]
- Holtta-Vuori M, Uronen RL, Repakova J, Salonen E, Vattulainen I, Panula P, Li Z, Bittman R, Ikonen E. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic 2008; 9: 1839 1849 [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Andersson O, Diczfalusy U, Sevastik B, Xiu RJ, Duan C, Lund E. Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc Natl Acad Sci U S A 1994; 91: 8592 8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte BA, Joseph SB, Walczak R, Pei L, Wilpitz DC, Collins JL, Tontonoz P. Autoregulation of the human liver X receptor alpha promoter. Mol Cell Biol 2001; 21: 7558 7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Shimano H, Inoue N, Nakagawa Y, Matsuzaka T, Takahashi A, Yahagi N, Sone H, Suzuki H, Toyoshima H, Yamada N. Protein kinase A suppresses sterol regulatory element-binding protein-1C expression via phosphorylation of liver X receptor in the liver. J Biol Chem 2007; 282: 11687 11695 [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 2002; 23: 141 174 [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Norman RL, Spies HG. Changing frequency of pulsatile luteinizing hormone and progesterone secretion during the luteal phase of the menstrual cycle of rhesus monkeys. Biol Reprod 1984; 31: 714 722 [DOI] [PubMed] [Google Scholar]
- Cameron JL, Stouffer RL. Gonadotropin receptors of the primate corpus luteum. II. Changes in available luteinizing hormone- and chorionic gonadotropin-binding sites in macaque luteal membranes during the nonfertile menstrual cycle. Endocrinology 1982; 110: 2068 2073 [DOI] [PubMed] [Google Scholar]
- Eyster KM, Ottobre JS, Stouffer RL. Adenylate cyclase in the corpus luteum of the rhesus monkey. III. Changes in basal and gonadotropin-sensitive activities during the luteal phase of the menstrual cycle. Endocrinology 1985; 117: 1571 1577 [DOI] [PubMed] [Google Scholar]
- Bishop CV, Hennebold JD, Stouffer RL. The effects of luteinizing hormone ablation/replacement versus steroid ablation/replacement on gene expression in the primate corpus luteum. Mol Hum Reprod 2009; 15: 181 193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW. Cholesterol biosynthesis and metabolism. Cardiovasc Drugs Ther 1992; 6: 103 110 [DOI] [PubMed] [Google Scholar]
- Rennert H, Fischer RT, Alvarez JG, Trzaskos JM, Strauss JF., III Generation of regulatory oxysterols: 26-hydroxylation of cholesterol by ovarian mitochondria. Endocrinology 1990; 127: 738 746 [DOI] [PubMed] [Google Scholar]
- Takagi K, Strauss JF., III Control of low density lipoprotein receptor gene expression in steroidogenic cells. Can J Physiol Pharmacol 1989; 67: 968 973 [DOI] [PubMed] [Google Scholar]
- Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, Caira F, Veyssiere G, Mangelsdorf DJ, Lobaccaro JM. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest 2006; 116: 1902 1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouineaud V, Sagot P, Garrido C, Logette E, Deckert V, Gambert P, Jimenez C, Staels B, Lagrost L, Masson D. Inhibition of progesterone production in human luteinized granulosa cells treated with LXR agonists. Mol Hum Reprod 2007; 13: 373 379 [DOI] [PubMed] [Google Scholar]
- Puttabyatappa M, Vandevoort CA, Chaffin CL. hCG-induced down-regulation of PPARgamma and liver X receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology 2010; 151: 5865 5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett MB. Steroid hormones. : Yen SC, Jaffe RB. (eds.), Reproductive Endocrinology. Philadelphia: WB Saunders; 1978: 80 92 [Google Scholar]