ABSTRACT
Doxorubicin (DXR) is a frontline chemotherapy agent implicated in unintended ovarian failure in female cancer survivors. The fertility preservation techniques currently available for cancer patients are often time and cost prohibitive and do not necessarily preserve endocrine function. There are no drug-based ovary protection therapies clinically available. This study provides the first investigation using dexrazoxane (Dexra) to limit DXR insult in ovarian tissue. In KK-15 granulosa cells, a 3-h DXR treatment increased double-strand (ds) DNA breaks 40%–50%, as quantified by the neutral comet assay, and dose-dependent cytotoxicity. Dexra exhibited low toxicity in KK-15 cells, inducing no DNA damage and less than 20% cell loss. Cotreating KK-15 cells with Dexra prevented acute DXR-induced dsDNA damage. Similarly, Dexra attenuated the DXR-induced 40%–65% increase in dsDNA breaks in primary murine granulosa cells and cells from in vitro cultured murine ovaries. DXR can cause DNA damage either through a topoisomerase II-mediated pathway, based on DXR intercalation into DNA, or through oxidative stress. Cotreating KK-15 cells with 2 μM Dexra was sufficient to prevent DXR-induced, but not H2O2-induced, DNA damage. These data indicated the protective effects are likely due to Dexra's inhibition of topoisomerase II catalytic activity. This putative protective agent attenuated downstream cellular responses to DXR, preventing H2AFX activation in KK-15 cells and increasing viability as demonstrated by increasing the DXR lethal dose in KK-15 cells 5- to 8-fold (LD20) and primary murine granulosa cells 1.5- to 2-fold (LD50). These data demonstrate Dexra protects ovarian cells from DXR insult and suggest that it is a promising tool to limit DXR ovarian toxicity in vivo.
Keywords: doxorubicin, female infertility, granulosa cells, ovary, premature ovarian failure
Dexrazoxane protects ovarian cells from DXR-induced DNA damage, subsequent H2AX activation, and cell death by topoII inhibition, providing a novel tool for protection of the ovary from chemotherapy insult.
INTRODUCTION
The numbers of people surviving cancer are progressively rising with advances in early detection and treatment, making unintended, long-term consequences of chemotherapy an immediate challenge for the health care community. According to the American Cancer Society, there will be roughly 740 000 new female cancer cases nationwide this year. Breast cancer is the most prevalent among these cases, and up to 50% of breast cancer survivors will develop premature ovarian insufficiency as a result of their chemotherapy treatment [1]. In addition, approximately 1 in 800 female adults will be a survivor of childhood cancer by the year 2020 [2], roughly 7% of whom will experience ovarian insufficiency [3]. Ovarian insufficiency in turn increases the survivor's risk for infertility, osteoporosis, and heart disease. It is therefore imperative to find ways to preserve ovarian function for cancer survivors and thereby enable a better quality of life.
The American Society of Clinical Oncology recommends physicians offer fertility preservation options to female cancer patients of reproductive age. The options currently available include embryo, oocyte, and ovarian tissue cryopreservation, and ovarian suppression [4]. The recommended therapies, however, are expensive with no guaranteed success, delay cancer therapy, and are not available for prepubescent patients. In addition, oocyte and embryo cryopreservation provide limited future fertility options, but they do not protect ovarian endocrine function and prevent premature menopause or osteoporosis. New chemotherapy-shielding approaches must be developed to protect the function of the ovary as a whole.
Doxorubicin (DXR), an anthracycline, was first used in clinical trials in the 1960s and is still a cornerstone agent in chemotherapy regiments for breast and childhood cancers [5]. Although DXR treatment causes infertility in cancer patients due to follicular attrition [4], the mechanism(s) of DXR-induced cell death in the ovary are undefined. We and others have shown DXR induces acute apoptosis (within 12 h) in the mouse ovary [6–8] (Roti Roti, Leisman, Salih, personal communication); this acute cell death occurs primarily in the granulosa cells of growing follicles as opposed to stroma cells and primordial follicles [6–8].
DXR can induce cellular toxicity via two distinct mechanisms, depending upon cell type and growth rates. This intercalating drug kills rapidly dividing cancer cells by preventing resealing of topoisomerase II (TOP2)-mediated double-strand (ds) DNA breaks that occur during normal DNA replication [9, 10]. Cells respond to unrepaired DNA damage by committing to apoptotic death. In the heart, where DXR causes dose-limiting toxicity, the drug's primary mode of insult occurs when DXR interacts with iron to induce oxidative stress [11–15]. Blocking oxidative stress is sufficient to prevent acute DXR insult in the heart but is not enough to prevent long-term DXR cardiotoxicity, which requires TOP2 inhibition as well [16]. Whether one or both of these mechanisms functions in the ovary is not well-defined.
Dexrazoxane (Dexra) (also known as ICRF-187) is a chemoprotectant currently used to protect the heart and skin from DXR-induced extravasation and cardiotoxicity [17–21]. Dexra can protect cells via two mechanisms, both of which correspond to the known modes of DXR-induced toxicity. Dexra, a member of the bisdioxopiperazine family [22, 23], specifically inhibits the closed-clamp conformation of TOP2, keeping TOP2 bound to the DNA after resealing the strand breaks, thereby preventing the next enzymatic cycle of TOP2-mediated dsDNA breaks [16, 23–28]. Cells cleave Dexra during normal metabolism, creating an ethylenediaminetetraacetic acid (EDTA) derivative that chelates iron to reduce oxidative stress, providing the mechanism by which it protects the heart from acute DXR injury [29, 30]. Importantly, Dexra does not prevent DXR antitumor activity in breast cancer nor pediatric leukemia cases, and does not increase the risk for secondary malignant neoplasm in leukemia cases [17, 31–41].
This study is the first to test Dexra as an ovarian chemoprotectant agent and demonstrates that Dexra prevents DXR-induced DNA damage in KK-15 granulosa cells at doses that do not limit H2O2-induced DNA damage, suggesting that the mechanism of DXR insult in these cells is largely based on TOP2 activity. The putative protective agent also blocks DXR-induced H2AFX activation and attenuates DXR toxicity, right-shifting the kill curve 5- to 8-fold. Similarly, Dexra attenuated the DNA damage response to DXR for cells from in vitro murine ovary culture and primary murine granulosa cells, where Dexra right-shifted the DXR kill curve ∼2-fold. The data suggest Dexra is a promising drug to develop as a protective agent to attenuate DXR injury in the ovary. This approach has the advantage over current fertility preservation methods in that it may be applicable to prepubescent patients, limits downstream genetic consequences following chemotherapy, and circumvents the prohibitive time and expense involved in traditional fertility preservation options and hormone replacement therapy.
MATERIALS AND METHODS
Chemicals and Drugs
Complete protease inhibitors were obtained from Roche. All the other chemicals were obtained from Fisher. DXR was obtained as a gift from the UW-Madison Chemotherapy Pharmacy as a 2 mg/ml aqueous solution. Dexra (Sigma) was solubilized in dimethyl sulfoxide (DMSO) (stock = 80 mM).
Cell Line
KK-15 (mouse immortalized granulosa-derived cell line) cells were cultured in 50/50 Dulbecco modified Eagle medium (DMEM)/F12 (Cellgro) supplemented with 2.5 mM l-glutamine, 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10% heat-inactivated fetal bovine serum, and penicillin 1000 units/ml; Cellgro and streptomycin 1000 μg/ml; (Cellgro) at 37°C with 5% CO2.
Ovary Culture
This study was conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act and its subsequent amendments. All the animal procedures were reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin-Madison. The University of Wisconsin Animal Care Facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care as part of the University of Wisconsin-Madison Graduate School. Animals were housed in the University of Wisconsin Animal Care Facility and provided standard care with free access to food and water. Female CD1 mice were purchased from Charles River Laboratories through the University of Wisconsin Animal Care Facility. Ovaries were harvested from 4-wk-old female CD1 mice euthanized with CO2 per approved animal protocol. Ovaries were cleansed of bursa and attached fat in minimal essential media (minus phenol red). One ovary from each mouse was transferred to a filter basket (Millipore) in a well containing ovary culture media (Ham F12/DMEM minus phenol red, 0.01 mg/ml bovine serum albumin [BSA], 1 mg/ml albumin, 0.05 mg/ml ascorbic acid, 5 units/ml penicillin, 5 μg/ml streptomycin, and 0.0275 mg/ml transferrin) plus Dexra or DMSO (vehicle control) and incubated for 1 h at 37°C. Ovaries were then transferred to baskets in new wells containing DXR or DXR plus Dexra at the concentrations given in the figures. Ovaries were incubated an additional 3 h at 37°C and then processed for the neutral comet assay (NCA; see below). Granulosa cells and oocytes were released by gently puncturing the follicles. Stroma cells were isolated by 30-min treatment with 0.25% collagenase at 37°C followed by cell dispersion using a 23-gauge needle. While this cell-separation technique does not provide pure cell populations, it is expected to provide two populations that are enriched in granulosa and theca/stroma cells, respectively. We will refer to these enriched populations as granulosa cells and stroma/theca cells throughout.
Primary Granulosa Cell Culture
The second ovary from each mouse (above) was incubated for 30 minutes at 37°C in dissociation media (DMEM/F12, 0.5 M sucrose, and 10 mM ethylene glycol tetraacetic acid). Granulosa cells were released by gently puncturing the follicles and dispersed into single-cell suspensions using a 23-gauge needle. Viable cells were counted using trypan blue exclusion, and for the NCA, cells were plated in 6-well dishes at 2 × 105 cells/well and cultured overnight at 37°C in 5% CO2. The cells were then pretreated with DMSO (carrier control) or 20 μM Dexra for 1 h at 37°C. DXR was then added at the given concentrations to the media containing either DMSO or Dexra as indicated in the figures. After a 3-h incubation with DXR, the cells were processed for the NCA.
Neutral Comet Assay
KK-15 cells at 50% confluency were treated with drugs (see the figure legends). The cells were pipetted to achieve single-cell suspensions in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4), and blind samples were subsequently used. The cells were diluted 1:10 in 1% low-melting agarose/PBS and plated onto microscope slides (precoated with 1% agarose in double-distilled [dd] H2O) with coverslips and incubated 10 min on ice. Coverslips were removed, and the slides were incubated for 1 h at 4°C in the NCA lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10.0, 1% Triton X-100, and 10% DMSO). The slides were then equilibrated 30 min at 4°C in 90 mM Tris base, 90 mM boric acid, and 2 mM EDTA, and subjected to 30 min electrophoresis at 25 volts. The slides were rinsed twice with ddH2O and dried overnight at room temperature. Nuclei were stained by incubating the slides for 20 min in 2 μg/ml propidium iodide in PBS. The slides were washed twice with ddH2O and imaged on an Olympus microscope fit with a 20× objective and a live charge-coupled device camera (University of Wisconsin-Madison Flow Cytometry Facility). Images were collected using Spot Advanced Plus software. Olive moments [42] were measured using CometScore software (Autocomet.com), collecting at least 50 cells per condition. Postanalysis, the samples were unblinded. One-way ANOVA analysis with a Bonferroni means comparison was carried out using OriginLab.
Lysate Preparation
KK-15 cells were plated at 1 × 106 cells per 100 mm dish 24 h prior to drug treatment. Following drug treatment, the cells were washed twice with PBS, scraped into 1 ml PBS, and collected in microcentrifuge tubes. All the steps for nuclear lysate preparation were conducted at 4°C. Cells were harvested by centrifugation for 5 min at 900 × g. Pelleted cells were resuspended in 250 μl hypotonic solution—10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 1× complete protease inhibitors (PIs) (Roche)—and incubated 15 min on ice. Cells were physically disrupted using 40 strokes of a size B Dounce homogenizer. Lysates were centrifuged 5 min at 230 × g to pellet the nuclei and membranes. The nuclear pellet was resuspended in 200 μl of 0.25 M sucrose, 10 mM MgCl2, 1× PIs, and 1 mM sodium orthovanadate; layered over 200 μl 0.88 M sucrose, 0.5 mM MgCl2, 1× PIs, and 1 mM sodium orthovanadate; and centrifuged 10 min at 2800 × g with no brake to enrich for nuclei. Nuclear pellet was solubilized in 100 μl of radioimmunoprecipitation assay buffer (100 mM Tris, pH 7.3, 500 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.2% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 100 mM NaF, and 1× PIs). Samples were sonicated five times for 10 sec on ice with a 5-sec rest between each burst. Lysates were stored on ice overnight at 4°C and then analyzed via Western blots.
Western Blot Analysis
Protein quantification was determined using the BioRad DC Protein Assay per the manufacturer's instructions. Lysates were prepared in Laemmli sample buffer (63 mM Tris HCl, 10% glycerol, 2% sodium dodecyl sulfate, 0.0025% bromophenol blue, and 50 μM dithiothreitol, pH 6.8) and heated for 5 min at 95°C. Approximately 10 μg total protein was loaded per lane and samples were size separated on a 4%–20% gradient gel (BioRad) under reducing conditions. Proteins were transferred to polyvinylidene fluoride membranes optimized for fluorescence (Millipore) where the membranes were preblocked in TBS-T (20 mM Tris base, 137 mM NaCl, and 1M HCl) plus 5% BSA for 1 h at room temperature. Blots were probed with rabbit anti-phospho γH2AFX antibody (1:500; Abcam) and mouse anti-β actin (1:1000; Sigma) in TBS-T plus 5% BSA overnight at 4°C. Blots were washed with TBS-T and then probed with donkey anti-rabbit Alexa 680 (1:15 000; Molecular Probes) and donkey anti-mouse IRdye 800 (1:15 000; LiCor) in TBS-T for 1 h at room temperature. Blots were washed with TBS-T, dried, and scanned using the LiCor Odyssey System (University of Wisconsin Small Molecule Screening Facility). Density measurements were taken using the Odyssey software.
Cytotoxicity
KK-15 cells were plated in a 96-well dish, 5000 cells/well, 24 h prior to drug treatments. Primary granulosa cells were plated in a 96-well dish at 1.5 × 104 cells/well 24 h prior to drug treatments. Triplicate samples were processed using the CellTiter-Glo kit (Promega) per the manufacturer's protocol, and the luminescence was read on a Synergy plate reader (Typhoon, University of Wisconsin Small Molecule Screening Facility). Graphs were generated in Origin. Two-way ANOVA was done using OriginLab.
RESULTS
DNA Damage and Cytotoxicity Profiles of DXR and Dexra in KK-15 Cells
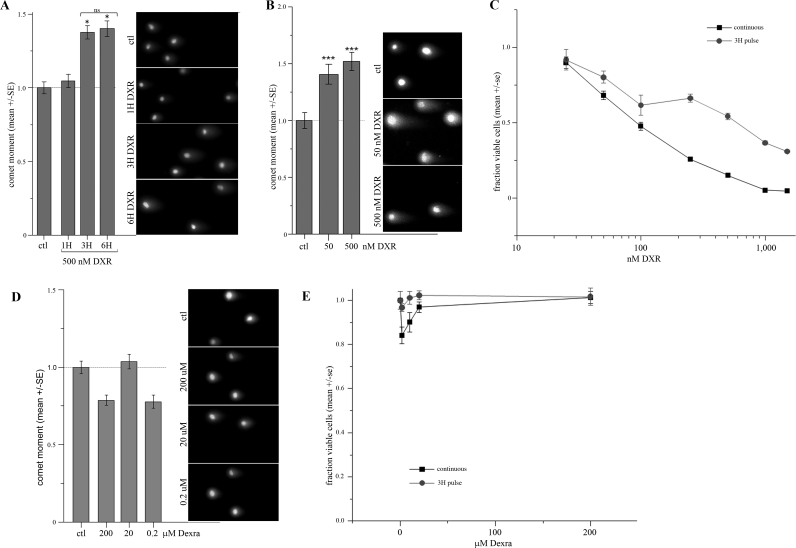
Testing Dexra as a putative protective agent required defining the onset of DXR-induced DNA damage in our murine granulosa-derived cell line model, KK-15. We used the NCA to quantify acute DXR-induced DNA damage in KK-15 cells. This is a sensitive single-cell assay of DNA damage where ds breaks are measured as the OM [42]. Time-course experiments revealed that 3 h was the earliest time at which 500 nM DXR exposure induced measurable DNA damage (OM), with no further significant increase at 6 h (Fig. 1A). The 3-h point was therefore utilized in subsequent experiments. Treating for 3 h with either 50 or 500 nM DXR, to encompass the range of circulating blood serum concentrations in patients (100–400 nM) [43], induced a 40%–55% increase in the extent of DNA damage in KK-15 cells (Fig. 1B).
FIG. 1.
Acute DNA damage and cytotoxicity profiles of DXR and Dexra in KK-15 cells. A) KK-15 cells were treated with 500 nM DXR for 1, 3, and 6 h, and the cells processed for the NCA. OM measurements demonstrate ∼45% increase in DNA damage over control at 3- and 6-h treatments (n = 3, *P < 0.01, one-way ANOVA). B) Treatment with 50 or 500 nM DXR for 3 h induced a 40%–50% increase in DNA damage (OM) in the NCA (n = 5, one-way ANOVA, P < 0.005). C) Treating KK-15 cells with a 3-h DXR pulse induced a dose-dependent cytotoxicity response that was less severe than but parallel to continuous exposure (two-way ANOVA, P < 0.001). The fraction viable cells was assessed by mean ATP-based luminescence (CellTiter-Glo assay) normalized to control (n = 3). D) Treatment with 0.2–200 μM Dexra for 3 h did not induce any DNA damage (OM) in KK-15 cells over the controls (DMSO carrier treated) as measured by the NCA (n = 4, one-way ANOVA, P > 0.05). E) Dexra did not cause significant toxicity in KK-15 cells. The fraction viable cells plotted for 3-h pulse or continuous 24-h exposure to Dexra (n = 3). Original magnification ×200 (A, B, D).
A 3-h pulse with DXR was also sufficient to cause dose-dependent KK-15 cell death as demonstrated by cytotoxicity curves. Cells were treated with DXR at the indicated doses either continuously for the entire duration of the experiment or for only 3 h; the media was then replaced with drug-free media. The percentage of viable KK-15 cells 48-h postdrug treatment was quantified using the ATP-based CellTiter-Glo kit. The cytotoxicity curve generated from a 3-h pulse of DXR paralleled that from continuous exposure (Fig. 1C), indicating that DNA damage measured after 3 h (Fig. 1B) is sufficient to induce dose-dependent cell death.
KK-15 cells were treated with Dexra alone to determine whether the putative protective agent caused any measurable DNA damage or cytotoxicity. Cells were treated for 3 h with indicated Dexra concentrations, and DNA damage was measured using the NCA. Summary data demonstrated the OM never rose above baseline levels (Fig. 1D), indicating that Dexra did not cause any dsDNA damage. In addition, treating KK-15 cells for 24 h with a 100-fold range of Dexra doses induced less than 20% cell loss as measured by the ATP-based viability assay (Fig. 1E). A 3-h pulse exposure caused less than 5% cell loss (Fig. 1E). These data indicate that Dexra causes minimal toxicity in KK-15 granulosa cells.
Dexra Provided Protection from DXR-Induced DNA Damage and H2AFX Activation
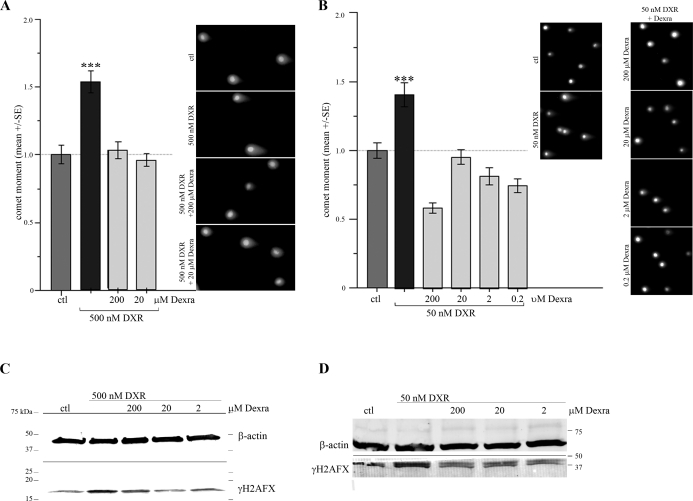
To test the hypothesis that Dexra protects KK-15 cells from DXR-induced DNA damage, dsDNA breaks in cells exposed to DXR for 3 h, with or without a 1-h Dexra preincubation and continued cotreatment, were measured using the NCA. OM measurements quantified in Figure 2A demonstrated that cotreatment with 20 or 200 μM Dexra prevented DNA damage induced by 500 nM DXR. In addition, Dexra (0.2–200 μM) reduced dsDNA breaks resulting from 50 nM DXR treatment to at or below control levels (Fig. 2B). Dexra pretreatment is required to afford protection from DXR in KK-15 cells because adding Dexra and DXR to the cells simultaneously did not provide protection (data not shown). These data demonstrate that cotreating KK-15 cells with a 1000-fold range of Dexra concentrations prevented the onset of DXR-induced DNA damage in a dose-independent manner.
FIG. 2.
Dexra prevented DXR-induced DNA damage and H2AFX activation. A, B) Pretreating KK-15 cells for 1 h with indicated Dexra doses (vs. DMSO carrier for control and DXR-alone treatments) along with its continued presence in the culture media prevented DNA damage caused by 3-h 500 nM (A) or 50 nM (B) DXR treatment, as measured by the NCA. Bar graph summarizes OM quantification (n = 4, ***P < 0.001, one-way ANOVA). Original magnification ×200. C, D) Western blots of lysates from cells treated with 500 nM (C, n = 5) or 50 nM (D, n = 3) with and without 1-h pretreatment and continued incubation with the indicated Dexra doses (or DMSO for control and DXR-only samples) were probed with anti-phospho γH2AFX and anti-β actin. Dexra attenuated γH2AFX phosphorylation at both DXR doses.
We tested the hypothesis that Dexra also attenuated DXR-induced H2AFX activation, the earliest cellular reporter of DNA damage. KK-15 cells were exposed to 500 or 50 nM DXR for 3 h (Fig. 2, C and D, respectively) with or without a 1-h, 2−200 μM pre- and cotreatment with Dexra. Nucleus-enriched lysate fractions were analyzed on Western blots probed for S139-phosphorylated (activated) H2AFX and β-actin (loading control) (Fig. 2, C and D). Dexra attenuated H2AFX phosphorylation in response to DXR at every dose tested, consistent with the DNA-damage protection afforded by Dexra in the NCA.
Dose-Dependent Dexra Protection from H2O2-Induced DNA Damage
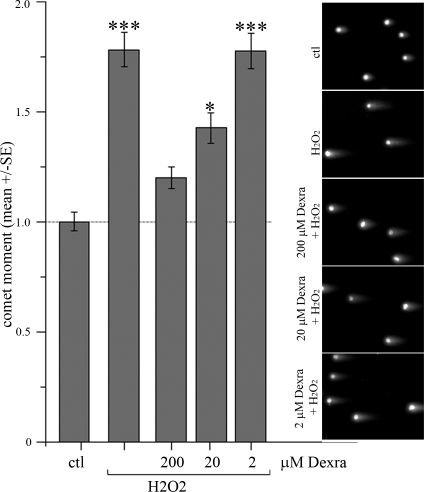
Dexra is an inhibitor of TOP2 catalytic activity, but it is also an EDTA derivative and can limit oxidative stress. To help determine which mechanism plays the primary role in preventing DXR insult in granulosa cells, we assessed Dexra's ability to prevent oxidative stress-induced DNA damage in our model system. KK-15 cells were treated with H2O2 (an oxidative stress-inducing agent) in the presence or absence of Dexra. The DNA damage response to the H2O2 treatment appeared greater than the 3-h DXR response, but the two were not significantly different. OM measurements revealed that a 1-h pre- and cotreatment with Dexra attenuated H2O2-induced DNA damage in a dose-dependent manner (Fig. 3). While 200 μM Dexra prevented and 20 μM Dexra diminished the DNA damage response to H2O2, 2 μM Dexra had no effect (Fig. 3). In contrast, both 2 and 20 μM Dexra completely eliminated DXR-induced DNA damage (Fig. 2B), suggesting that at these doses, the anti-oxidant effect is not sufficient to account for Dexra-mediated protection from DXR-induced DNA damage and that the protection may predominantly occur through inhibition of TOP2 catalytic activity. We attempted to copurify Dexra bound to TOP2 from the treated KK-15 cells to measure the extent of inhibition achieved in the cells during the Dexra treatments. Dexra does not bind covalently to TOP2, however, and is washed out during the stringent protein purification process required to purify and assay TOP2 activity (Brian Hasinoff, personal communication). Because we were unable to copurify Dexra-TOP2 complexes from KK-15 cells, we cannot rule out the involvement of undefined ovarian-specific mechanisms for Dexra-mediated protection in these cells.
FIG. 3.
Dose-dependent Dexra inhibition of H2O2-induced DNA damage. KK-15 cells were treated for 1 h with the indicated Dexra doses (or DMSO for control and H2O2-only samples) prior to a 5-min incubation with 100 mM H2O2; all the samples were then processed for the NCA. A summary graph reveals that Dexra attenuated H2O2-induced DNA damage (OM) in a dose-dependent manner (n = 3, ***P < 0.005, two-way ANOVA); however, 2 μM Dexra, which was sufficient to prevent DXR-induced damage, did not attenuate H2O2 damage. Original magnification ×200.
Dexra Rescued KK-15 Cell Viability in Response DXR
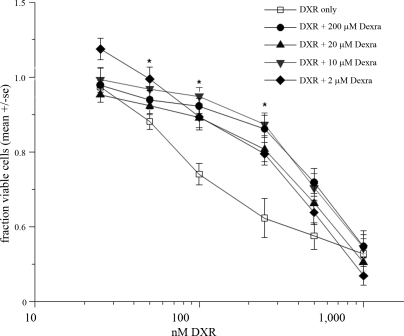
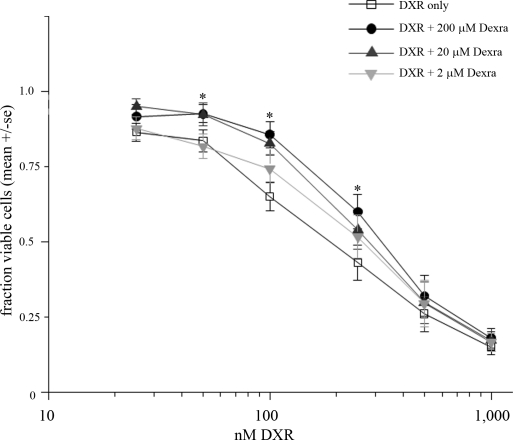
To determine whether attenuating acute DNA damage translated into increased cell viability, we assessed changes in DXR's cytotoxicity dose-response curve in the presence or absence of Dexra. KK-15 cells were pretreated for 1 h with Dexra or DMSO (carrier control) followed by continuous exposure to DXR with or without Dexra as indicated in Figure 4. At 24 h post-DXR treatment, every Dexra dose examined right shifted the cytotoxicity curve, resulting in a 5- to 8-fold increase in the LD20 (Fig. 4). The LD50 would not have been an accurate measurement because 50% cell loss was reached only at the highest DXR dose with 24-h treatment, and the LD50 was therefore not calculated. Treatment with 10 μM Dexra afforded the best protection, inducing the largest LD20 shift. These data demonstrate Dexra preserves KK-15 cell viability in the presence of DXR.
FIG. 4.
Dexra limited DXR's cytotoxicity in KK-15 cells. The fraction of viable cells was measured using the ATP-based CellTiter-Glo assay 24 h postdrug treatment. The graph depicts summary of four experiments (± SEM). The cells were pretreated for 1 h with Dexra or DMSO followed by the addition of DXR to the media already containing Dexra or DMSO. The presence of Dexra shifted the LD20 for 24-h exposure to DXR 5- to 8-fold for all Dexra doses tested (P < 0.01, two-way ANOVA). The maximum shift was seen with 10 μM Dexra, but there was no statistically significant difference between the protective effect across the Dexra doses (two-way ANOVA, P > 0.05).
Dexra Prevents DXR-Induced DNA Damage in Primary Granulosa as well as Granulosa and Theca/Stroma Cells from Cultured Ovaries
The ovary is an organ with a complex structure, consisting of multiple cell types. To determine whether Dexra could protect ovarian cells from DXR insult in a more nativelike environment, murine ovaries were cultured in vitro and treated with DXR with and without Dexra. Both stroma/theca and granulosa cell populations exhibited a 40%–65% increase in dsDNA breaks, measured by the NCA, in response to a 3-h exposure to a range of DXR doses as summarized in Figure 5, A and B. In contrast, oocytes did not exhibit DXR-induced damage in response to the 3-h treatment with DXR (Fig. 5C). Cotreatment with 20 μM Dexra (1-h pretreatment and maintained in the media with DXR) prevented or significantly decreased DXR-induced dsDNA breaks in stroma/theca (Fig. 5A) and granulosa cells (Fig. 5B) at doses up to 10 μM DXR. Dexra had no protective effect on the oocytes because of the inherent absence of DXR-induced DNA damage (Fig. 5C). Cotreatment with 20 μM Dexra also prevented the DXR-induced 20%–40% increase in DNA damage across the same range of doses in primary murine granulosa cells (Fig. 5D). These data suggest that Dexra can protect the ovary as a whole from DXR insult.
FIG. 5.
Dexra prevented DXR-induced DNA damage in stroma/theca and granulosa cells from in vitro cultured murine ovaries and primary murine granulosa cells. Bar graphs summarize the OM quantification from four experiments (*P < 0.05, one-way ANOVA). Example images are given to the right of each graph. In vitro cultured ovaries or primary granulosa cells were pretreated with 20 μM Dexra or DMSO (the vehicle carrier control) followed by the addition of DXR for 3 h in the continued presence of Dexra. A) Stroma/theca cells: Dexra prevented or reduced the 45%–65% increase in DNA damage induced by 50 nM, 500 nM, or 10 μM DXR (vs. DMSO carrier for control) in stroma/theca cells from in vitro culture ovaries. B) Granulosa cells: Dexra eliminated the 45%–65% increase in DNA damage caused by exposure to 50 nM, 500 nM, or 10 μM DXR in granulosa cells from in vitro cultured ovaries. C) Oocytes: Oocytes from ovaries treated in vivo for 3 h with DXR did not show significant DNA damage response in the NCA (P > 0.05, one-way ANOVA), and the presence of 20 μM Dexra did not alter the comet moment (OM). D) Primary murine granulosa cell culture: Dexra eliminated the 20%–40% increase in DNA damage induced by 50 nM, 500 nM, or 10 μM DXR in primary murine granulosa cells. Original magnification ×200.
Dexra Rescued Primary Granulosa Cell Viability in Response to DXR
To determine whether Dexra could increase the viability of primary granulosa cells to the same level as that seen with KK-15 cells, we assessed changes in DXR's cytotoxicity dose-response curve in the presence or absence of Dexra. Primary granulosa cells were pretreated for 1 h with Dexra or DMSO (carrier control) followed by continuous exposure to DXR with or without Dexra as indicated in Figure 6. After 24-h continuous DXR treatment, 20 and 200 μM Dexra right shifted the cytotoxicity curve for DXR, resulting in a ≥2-fold increase in the LD20 and 1.5- to 2-fold increase in the LD50 (Fig. 6), respectively. Treatment with 2 μM Dexra did not significantly shift the cytotoxicity curve (Fig. 6). These data demonstrate Dexra doses ranging from 20 to 200 μM preserve primary murine granulosa cell viability in the face of DXR insult.
FIG. 6.
Dexra limited DXR's cytotoxicity in primary granulosa cells. The fraction of viable cells was measured using the ATP-based CellTiter-Glo assay 24-h postdrug treatment. The graph depicts summary of four experiments (± SEM). Cells were pretreated for 1 h with Dexra or DMSO followed by the addition of DXR. The presence of either 20 or 200 μM Dexra right shifted the cytotoxicity curve for DXR (P < 0.05, two-way ANOVA, Tukey means comparison). There was no significant difference in protection afforded by the two Dexra doses. Dexra shifted the LD20 for DXR ≥2-fold, and the LD50 by 1.5- to 2-fold for 20 and 200 μM Dexra, respectively.
DISCUSSION
This study is the first to identify Dexra as a drug-based protection mechanism to prevent anthracycline toxicity in the ovary and to implicate Dexra as a promising candidate for continued in vivo experiments to prevent DXR-induced ovarian insult. In the mouse model granulosa cell line, a wide range of Dexra doses eliminated acute DNA damage and H2AFX activation in response to DXR. Similar reductions in DNA damage were obtained using Dexra to protect mouse primary granulosa cells and in vitro mouse ovary cultures from DXR insult. The putative protective agent also significantly increased cell viability following DXR treatment.
This study is also the first to identify an ovarian protective agent that prevents both the primary DNA insult and ensuing toxicity, rather than inhibiting only apoptosis that occurs subsequent to the initial insult. In the ovary, an organ dedicated to supplying, protecting, and maturing oocytes for reproduction, genetic fidelity is critical. Oocytes have extraordinary DNA repair machinery, and simply blocking apoptosis in response to radiation or cisplatin is sufficient to preserve follicles and viable oocytes [44, 45]. Oocytes exposed in vitro, however, cannot repair DNA damage after premature removal from DXR treatment [46], so preventing the initial insult is critical to maintaining their genetic fidelity. Over the time course of our 3-h in vitro ovary treatment, oocytes did not exhibit DXR-induced DNA damage (Fig. 5). This is consistent with a TOP2-mediated damage model because a vast majority of oocytes in the ovary are meiotically quiescent. Whether oocytes would exhibit DXR-induced DNA damage, however, if followed over a longer period and as they mature remains to be seen. Future studies will determine whether Dexra protects oocyte DNA integrity in vivo in the face of DXR insult and whether the protection can be maintained to preserve development and maturation prior to ovulation and meiotic competence through to fertilization.
Maintaining the integrity of the other cell types comprising the ovary is also of paramount importance in preserving oocyte health. Granulosa cells sustain the oocyte; they are the primary site of DXR-induced apoptosis [6] and therefore must also be protected to preserve follicular and oocyte integrity. Furthermore, granulosa cells produce estrogen that promotes endometrial proliferation for embryo implantation and have a role in protecting from osteoporosis and urogenital atrophy [47]. Likewise, stroma/theca cells are integral to preserving ovarian structure and function, playing a key role in steroidogenesis and hormone synthesis. Data presented here show that Dexra prevented DXR insult in primary cultured murine granulosa cells and granulosa and stroma/theca cells from in vitro ovarian culture; future studies will determine whether Dexra also protects each ovarian cell type from DXR insult in vivo, thereby preserving follicular health and oocyte viability. The lack of Dexra protection of the oocytes in this study was due to the inherent lack of DXR toxicity on the oocyte in our model of ex vivo ovarian culture. While others have shown denuded oocytes cultured in vitro with DXR exhibit DNA damage [46], we did not observe induced DNA damage in our ex vivo ovarian culture, suggesting the surrounding follicular cells and stromal tissue may play a role in protecting the oocyte from DXR toxicity. The potential to protect the ovary as a whole from chemotherapy, regardless of cell type, makes Dexra a promising tool as an ovarian shield.
DXR can cause cellular toxicity via two main mechanisms: oxidative stress and TOP2-mediated dsDNA breaks following DXR intercalation into DNA. If DNA damage occurs via the TOP2-dependent mechanism, we predict that Dexra-afforded protection will be solely dependent upon Dexra's inhibitory constant for TOP2 and therefore independent of DXR concentration. In agreement with this prediction, we found that DNA damage caused by 50 or 500 nM DXR was prevented by Dexra under identical pretreatment conditions (Figs. 2 and 5). If the tested Dexra dose is sufficient to completely inhibit TOP2 activity, the observed protection will also be independent of Dexra concentration. While we could not copurify TOP2-Dexra complexes from treated KK-15 cells, studies from multiple groups have demonstrated that Dexra and other members of the bisdioxopiperazine family inhibit the enzymatic activity of purified TOP2 [48, 49] and have identified residues in the N-terminus of TOP2 critical for Dexra binding through mutation and crystallography studies [25, 26]. Our data fit the model of TOP2 dependency for dsDNA breaks because every tested Dexra concentration afforded similar protection from DXR-induced DNA damage (Fig. 2). In contrast, Dexra prevented oxidative stress-induced DNA damage in a dose-dependent manner. In addition, doses of 2 and 20 μM Dexra were sufficient to completely prevent DXR-induced DNA damage in KK-15 and murine granulosa cells, but either failed or only partially decreased H2O2-induced DNA damage. We therefore propose a model in which DXR causes acute DNA damage in granulosa cells primarily in a TOP2-dependent manner and KK-15 cells are protected from that insult mechanism by Dexra pretreatment. Dexra-afforded protection from DXR-induced cytotoxicity, however, may be more complicated. In primary murine granulosa cells, 20 and 200 μM Dexra increased cell viability in response to DXR, where 2 μM Dexra did not. This dose dependence suggests that DXR cell demise may include an oxidative stress response or other unknown ovarian-specific mechanisms. In addition, Dexra did not completely prevent DXR-induced cell demise at DXR concentrations above 100 nM, consistent with the possible role of multiple DXR-toxicity mechanisms or indicating that higher doses of Dexra may be required to completely eliminate DXR-induced cytotoxicity.
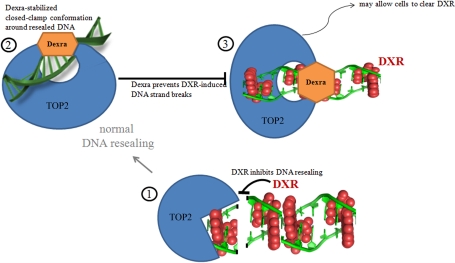
Dexra pretreatment is required to afford protection from DXR in our cell system, just as in the heart and skin; adding Dexra and DXR to the cells simultaneously did not provide protection. While DXR is labeled a TOP2 poison, Dexra is an inhibitor of TOP2 catalytic activity, and the two drugs act at different points in the enzymatic cycle of TOP2 as illustrated in steps 1 and 2 of the model (Fig. 7). DXR, and other TOP2 poisons, act early in the enzymatic cycle, preventing resealing of TOP2 dsDNA breaks based on the drug's presence in the DNA strands (step 1 of the model, Fig. 7) [16, 27, 28, 50]. During normal DNA processing, in the absence of TOP2 poisons, TOP2 seals the strand breaks and then releases the DNA (step 2 of the model, Fig. 7). Dexra acts by stabilizing the closed-clamp conformation in which TOP2 holds the DNA after resealing breaks [16, 23, 27, 28, 50]. In this model, pretreating cells with Dexra locks TOP2 in the closed-clamp conformation, preventing DNA release from the TOP2 complex and making the enzyme unavailable to cleave the DNA when cells are subsequently treated with DXR (step 3 of the model, Fig. 7). We hypothesize that this allows the cells to clear DXR, providing long-term increase in cell viability. The effects of Dexra are reversible, making it well-tolerated in slow- or nondividing cells.
FIG. 7.
Model for TOP2-mediated DXR insult and Dexra protection. Red ball structures represent DXR intercalated into the DNA. Step 1 illustrates DXR intercalation into DNA; the structure is from Protein Data Bank 1D12 [56]. The presence of DXR prevents resealing of TOP2-mediated strand breaks. Step 2 illustrates Dexra bound to the N-terminal TOP2 active site, stabilizing the closed-clamp conformation after strand resealing but prior to releasing the DNA from the enzyme. Step 3 illustrates DXR can still intercalate into DNA when Dexra is bound to the TOP2 active site. The enzyme cannot cleave DNA and thus the presence of DXR does not result in strand breaks. This may allow the cells to clear DXR and limit the drug's toxicity.
Whether only one of both of the DXR-insult mechanisms occurs in the intact ovary in vivo remains to be seen. The ovary is a unique, heterogeneous organ within which follicles reside in various developmental stages ranging from rapidly growing antral follicles to quiescent primordial follicles. It is possible that these distinct follicle populations respond differently to DXR based on cell division rates and metabolic activity. One model is that primordial follicles may respond to DXR via the oxidative stress pathway, in a manner similar to nondividing heart cells, while growing follicles may succumb to TOP2-mediated DXR insult. Given this inherent heterogeneity of the organ, however, our data indicate that Dexra may still be effective in protecting the ovary as a whole. Dexra similarly protected granulosa and stroma/theca cells with no evidence for separate populations exhibiting differential responses. These data therefore suggest Dexra provides a unique tool that may be well-suited to protecting a heterogeneous organ like the ovary because it can prevent both mechanisms of DXR toxicity and appears effective across granulosa and stroma/theca cell types. Future studies may also determine whether other members of the bisdioxopiperazine family can similarly protect the ovary from chemotherapy insult.
Dexra may provide a time- and cost-efficient way to attenuate DXR insult in the ovary and prevent premature menopause and associated health risks without decreasing the effectiveness of cancer therapy as evidenced by its clinical application to prevent both DXR-induced cardiotoxicity and extravasation [17–21]. Dexra does not diminish DXR antitumor activity in pediatric leukemia cases; while it may slow the response, it does not diminish survival in breast cancer cases; and nor does Dexra increase the risk for secondary malignant neoplasm in leukemia cases [17, 31–41]. One possible explanation for this dichotomy, protecting healthy tissue but not cancer cells, was provided in a mechanistic study by Yan et al. [51]. Their work demonstrated that while Dexra blocked DXR-induced dsDNA breaks in a human fibrosarcoma cell line, it did not prevent DXR-induced apoptosis that occurred via glutathione depletion in a TOP2-independent manner. Dexra can have synergistic effects when combined with other chemotherapy agents in cancer treatment. Dexra impairs the development of DXR resistance in the leukemia cell line K562 [52] and is synergistic with docetaxel or docetaxel plus DXR in treating MCF7 wild-type or resistant breast cancer cells and BT474 breast cancer cells [53]. Combined therapy incorporating Dexra with DXR allows an increase in the maximal DXR dosage used [40]. This may be beneficial for patients with triple-negative breast cancer, whose cancer is aggressive and DXR is part of the frontline chemotherapy regime [38, 41, 54]. Similarly, Dexra pretreatment allows increased doses of etoposide in a mouse model by preventing not only cadiotoxicity, but preventing etoposide-induced decreases in white blood cell, platelet, and absolute neutrophil cell counts [55], suggesting that Dexra has the potential to prevent insult across a range of TOP2 poisons.
Utilizing a chemical ovarian shield like Dexra presents a clear advantage over traditional hormone replacement therapy and current fertility preservation approaches. Traditional fertility preservation options are both time and cost prohibitive. While they can provide the option for a female cancer survivor to have biologically related children in the future, they do not protect endocrine function of the ovary. This leaves survivors susceptible to premature ovarian insufficiency and associated health complications, including osteoporosis and cardiovascular disease. Ovarian hyperstimulation and in vitro fertilization cycles required for oocyte and embryo banking for cancer patients can delay cancer therapy and promote the growth of tumors that are hormone responsive. In addition, oocyte and embryo cryopreservation are not treatment options for prepubescent patients. Pretreating patients with Dexra prior to DXR may provide a time- and cost-effective way to preserve not only fertility, but endocrine function as a whole. It is a therapy that should be equally effective in pediatric as well as adult cancer patients and bypass the pitfalls of hormone stimulation. This therapy therefore has the potential to increase the quality of life for female cancer patients without compromising their cancer treatment.
ACKNOWLEDGMENT
The authors are extremely grateful to Dr. David Abbott for ongoing mentorship and aid with statistical analysis, manuscript review, and scientific input; Scott Leisman for scientific input; Trever Greene, Polly Godfrey, Abby Forss, and Lydia Weyenberg for technical assistance; and Sharon Moses for providing doxorubicin from the University of Wisconsin-Pharmacy.
Footnotes
Supported by startup funds from the University of Wisconsin-Madison and NIH training grant K12HD0558941 to S.M.S., and by the UW-Madison Comprehensive Cancer Center grant NIH/NCI P30 CA014520.
REFERENCES
- Oktem O, Oktay K. Fertility preservation for breast cancer patients. Semin Reprod Med 2009; 27: 486 492 [DOI] [PubMed] [Google Scholar]
- Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: analyses of the 1992 National Health Interview Survey. J Natl Cancer Inst 1999; 91: 1480 1486 [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006; 91: 1723 1728 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24: 2917 2931 [DOI] [PubMed] [Google Scholar]
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsk DA. Daunomycin an antitumor antibiotic in treatment of neoplastic disease—clinical evaluation with special reference to childhood leukemia. Cancer 1967; 20: 333 353 [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I, Bar-Joseph H, Tzarfaty G, Kuchinsky L, Rizel S, Stemmer SM, Shalgi R. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol 2010; 8: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 1997; 3: 1228 1232 [DOI] [PubMed] [Google Scholar]
- Bar-Joseph H, Ben-Aharon I, Rizel S, Stemmer SM, Tzabari M, Shalgi R. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod Toxicol 2010; 30: 566 572 [DOI] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog Nucleic Acid Res Mol Biol 2000; 64: 221 253 [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727 741 [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Schroeder PE, Patel D. The metabolites of the cardioprotective drug dexrazoxane do not protect myocytes from doxorubicin-induced cytotoxicity. Mol Pharmacol 2003; 64: 670 678 [DOI] [PubMed] [Google Scholar]
- Swift LM, Sarvazyan N. Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. Am J Physiol Heart Circ Physiol 2000; 278: H982 H990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi MM, Algharably NM, Alshabanah OA, Albekairi AM, Osman AMM, Tawfik HN. Prevention of doxorubicin-induced myocardial and hematological toxicities in rats by the iron chelator desferrioxamine. Cancer Chemother Pharmacol 1992; 31: 200 204 [DOI] [PubMed] [Google Scholar]
- Hershko C, Link G, Tzahor M, Kaltwasser JP, Athias P, Grynberg A, Pinson A. Anthracycline toxicity is potentiated by iron and inhibited by deferoxamine—studies in rat-heart cells in culture. J Lab Clin Med 1993; 122: 245 251 [PubMed] [Google Scholar]
- Voest EE, Vanacker S, Vandervijgh WJF, Vanasbeck BS, Bast A. Comparison of different iron chelators as protective agents against acute doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol 1994; 26: 1179 1185 [DOI] [PubMed] [Google Scholar]
- Martin E, Thougaard AV, Grauslund M, Jensen PB, Bjorkling F, Hasinoff BB, Tjornelund J, Sehested M, Jensen LH. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology 2009; 255: 72 79 [DOI] [PubMed] [Google Scholar]
- Herman EH, Ferrans VJ. Examination of the potential long-lasting protective effect of ICRF-187 against anthracycline-induced chronic cardiomyopathy. Cancer Treat Rev 1990; 17: 155 160 [DOI] [PubMed] [Google Scholar]
- Mouridsen HT, Langer SW, Buter J, Eidtmann H, Rosti G, de Wit M, Knoblauch P, Rasmussen A, Dahlstrom K, Jensen PB, Giaccone G. Treatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studies. Ann Oncol 2007; 18: 546 550 [DOI] [PubMed] [Google Scholar]
- Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase II beta-mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 2007; 67: 8839 8846 [DOI] [PubMed] [Google Scholar]
- Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol 2004; 130: 1 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, Jones SE, Wadler S, Desai A, Vogel C, Speyer J, Mittelman A, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997; 15: 1318 1332 [DOI] [PubMed] [Google Scholar]
- Fattman CL, Allan WP, Hasinoff BB, Yalowich JC. Collateral sensitivity to the bisdioxopiperazine dexrazoxane (ICRF-187) in etoposide (VP-16)-resistant human leukemia K562 cells. Biochem Pharmacol 1996; 52: 635 642 [DOI] [PubMed] [Google Scholar]
- Renodon-Corniere A, Jensen LH, Nitiss JL, Jensen PB, Sehested M. Analysis of bisdioxopiperazine dexrazoxane binding to human DNA topoisomerase II alpha: decreased binding as a mechanism of drug resistance. Biochemistry 2003; 42: 9749 9754 [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Kuschak TI, Yalowich JC, Creighton AMA. QSAR study comparing the cytotoxicity and DNA topoisomerase-II inhibitory effects of bisdioxopiperazine analogs of ICRF-187 (dexrazoxane). Biochem Pharmacol 1995; 50: 953 958 [DOI] [PubMed] [Google Scholar]
- Classen S, Olland S, Berger JM. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci U S A 2003; 100: 10629 10634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen S, Olland S, Berger JM. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci U S A 2003; 100: 14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AK, Eseargueil AE, Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther 2003; 99: 167 181 [DOI] [PubMed] [Google Scholar]
- Sehested M, Jensen PB. Mapping of DNA topoisomerase II poisons (etoposide, clerocidin) and catalytic inhibitors (aclarubicin, ICRF-187) to four distinct steps in the topoisomerase II catalytic cycle. Biochem Pharmacol 1996; 51: 879 886 [DOI] [PubMed] [Google Scholar]
- Diop NK, Vitellaro LK, Arnold P, Shang MY, Marusak RA. Iron complexes of the cardioprotective agent dexrazoxane (ICRF-187) and its desmethyl derivative, ICRF-154: solid state structure, solution thermodynamics, and DNA cleavage activity. J Inorg Biochem 2000; 78: 209 216 [DOI] [PubMed] [Google Scholar]
- Hasinoff BB. Dexrazoxane (ICRF-187) protects cardiac myoctes against hypoxia-reoxygenation damage. Cardiovasc Toxicol 2002; 2: 111 118 [DOI] [PubMed] [Google Scholar]
- Pouillart P. Evaluating the role of dexrazoxane as a cardioprotectant in cancer patients receiving anthracyclines. Cancer Treat Rev 2004; 30: 643 650 [DOI] [PubMed] [Google Scholar]
- Pearlman M, Jendiroba D, Pagliaro L, Keyhani A, Liu BS, Freireich EJ. Dexrazoxane in combination with anthracyclines lead to a synergistic cytotoxic response in acute myelogenous leukemia cell lines. Leuk Res 2003; 27: 617 626 [DOI] [PubMed] [Google Scholar]
- Styczynski J, Wysocki M, Balwierz W, Kowalczyk JR. Dexrazoxane has no impact on sensitivity of childhood leukemic blasts to daunorubicin. Leukemia 2002; 16: 820 825 [DOI] [PubMed] [Google Scholar]
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, Clavell LA, Larsen EC, Moghrabi A, Samson Y, Schorin MA, Cohen HJ, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol 2008; 26: 1106 1111 [DOI] [PubMed] [Google Scholar]
- Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, Cole PD, Kelly KM, Larsen EC, Laverdiere C, Michon B, Schorin M, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer 2011; 47: 1373 1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007; 25: 493 500 [DOI] [PubMed] [Google Scholar]
- Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, Samson Y, Schorin M, Dalton VK, Lipshultz SE, Neuberg DS, Gelber RD, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 2007; 109: 896 904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Dexrazoxane Study G. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 2006; 17: 614 622 [DOI] [PubMed] [Google Scholar]
- Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, Dubin N, Ferrans V, Stecy P, Zeleniuchjacquotte A, Wernz J, Feit F, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast-cancer. N Engl J Med 1988; 319: 745 752 [DOI] [PubMed] [Google Scholar]
- Speyer JL, Green MD, Zeleniuchjacquotte A, Wernz JC, Rey M, Sanger J, Kramer E, Ferrans V, Hochster H, Meyers M, Blum RH, Feit F, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast-cancer. J Clin Oncol 1992; 10: 117 127 [DOI] [PubMed] [Google Scholar]
- Testore F, Milanese S, Ceste M, de Conciliis E, Parello G, Lanfranco C, Manfredi R, Ferrero G, Simoni C, Miglietta L, Ferro S, Giaretto L, et al. Cardioprotective effect of dexrazoxane in patients with breast cancer treated with anthracyclines in adjuvant setting. Am J Cardiovasc Drugs 2008; 8: 257 263 [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res 1990; 122: 86 94 [PubMed] [Google Scholar]
- Speth PAJ, Vanhoesel Q, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet 1988; 15: 15 31 [DOI] [PubMed] [Google Scholar]
- Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, Ilagan A, Hunt PA, Morgan WF, Tilly JL, Kolesnick R. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med 2002; 8: 901 902 [DOI] [PubMed] [Google Scholar]
- Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, Mattei M, Candi E, De Felici M, Melino G, Cesareni G. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15: 1179 1185 [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Lee HJ, D'Estaing SG, Tilly J, Perez GI. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ 2006; 13: 1466 1474 [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu XM, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol 2004; 276: 64 73 [DOI] [PubMed] [Google Scholar]
- Barnabe N, Hasinoff BB. High-throughput fluorescence flow-injection topoisomerase II inhibition assay. J Chromatogr B 2001; 760: 263 269 [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Creighton AM, Kozlowska H, Thampatty P, Allan WP, Yalowich JC. Mitindomide is a catalytic inhibitor of DNA topoisomerase II that acts at the bisdioxopiperazine binding site. Mol Pharmacol 1997; 52: 839 845 [DOI] [PubMed] [Google Scholar]
- Wu X, Hasinoff BB. The antitumor anthracyclines doxorubicin and daunorubicin do not inhibit cell growth through the formation of iron-mediated reactive oxygen species. Anticancer Drugs 2005; 16: 93 99 [DOI] [PubMed] [Google Scholar]
- Yan TD, Deng SW, Metzger A, Godtel-Armbrust U, Porter ACG, Wojnowski L. Topoisomerase II alpha-dependent and -independent apoptotic effects of dexrazoxane and doxorubicin. Mol Cancer Ther 2009; 8: 1075 1085 [DOI] [PubMed] [Google Scholar]
- Sargent JM, Williamson CJ, Yardley C, Taylor CG, Hellmann K. Dexrazoxane significantly impairs the induction of doxorubicin resistance in the human leukaemia line, K562. Br J Cancer 2001; 84: 959 964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman DR, Calabro A. In vitro search for synergy and antagonism: evaluation of docetaxel combinations in breast cancer cell lines. Breast Cancer Res Treat 2002; 74: 41 46 [DOI] [PubMed] [Google Scholar]
- Andoh T, Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta 1998; 1400: 155 171 [DOI] [PubMed] [Google Scholar]
- Hofland KF, Thougaard AV, Sehested M, Jensen PB. Dexrazoxane protects against myelosuppression from the DNA cleavage-enhancing drugs etoposide and daunorubicin but not doxorubicin. Clin Cancer Res 2005; 11: 3915 3924 [DOI] [PubMed] [Google Scholar]
- Frederick CA, Williams LD, Ughetto G, Vandermarel GA, Vanboom JH, Rich A, Wang AHJ. Structural comparison of anticancer drug DNA complexes—adriamycin and daunomycin. Biochemistry 1990; 29: 2538 2549 [PubMed] [Google Scholar]