Abstract
Background
Management of frailty is the cornerstone of geriatric medicine, but there remains a need to identify biomarkers that can predict early death, and thereby lead to effective clinical interventions. We aimed to study the combination of C-reactive protein (CRP), albumin, gamma-glutamyl transferase (GGT), and HDL to predict mortality.
Methods
A total of 44,457 persons aged 50+ whose levels of CRP, albumin, GGT, and HDL were measured at baseline were selected from the Swedish Apolipoprotein MOrtality RISk (AMORIS) study. A mortality score, ranging from 0 to 4, was created by adding the number of markers with abnormal values according to the clinical cut-off (CRP > 10 mg/L, albumin < 35 mg/L, GGT > 36 kU/L, HDL < 1.04 mmol/L). Mortality was studied with multivariate Cox proportional hazards models.
Results
2,245 persons died from cancer, 3,276 from circulatory disease, and 1,860 from other causes. There was a positive trend between mortality score and all-cause mortality as well as cancer and circulatory disease-specific death (e.g. HR for all-cause mortality: 1.39 (95%CI: 1.32-1.46), 2.04 (1.89-2.21), and 3.36 (2.87-3.93), for score=1, 2, and 3+, compared to score=0). Among cancer patients with no other co-morbidities (n=1,955), there was a positive trend between the score and mortality (HR: 1.24 (95%CI: 1.0.-1.49), 2.38 (95%CI: 1.76-3.22), and 5.47 (95%CI: 2.98-10.03) for score=1, 2, and 3+ compared to score=0).
Conclusions
By combining biomarkers of different mechanisms contributing to patient frailty, we found a strong marker for mortality in persons aged 50+. Elevated risks among cancer patients with no other co-morbidities prior to biomarker assessment call for validation in other cohorts and testing of different combinations and cut-offs than those used here, in order to aid decision-making in treatment of older cancer patients.
Keywords: Frailty, mortality, albumin, HDL-cholesterol, C-reactive protein, gamma-glutamyltransferase
Introduction
The European Organization for Research and Treatment of Cancer (EORTC) Elderly Task Force recently expressed the need for an assessment of functional rather than chronological age when treating cancer patients [1]. This is of special importance in light of predictions of an increased cancer burden in many countries with aging cancer populations suffering from concomitant disease at time of cancer diagnosis. The concept of “frailty” was introduced in this context and mainly describes a physical and functional decline which may occur as a consequence of certain diseases, but often also in the absence of identifiable specific disease [2]. Frailty thus indicates which individuals have a higher susceptibility to adverse outcomes such as hospitalization or mortality [3-4]. The pathophysiological causal understanding of frailty is that multi-system reduction in reserve capacity leads to physiological and metabolic changes that drive progressive physical and cognitive impairments resulting in loss of functional capacity, often augmented by acute or chronic disease [5]. The benefit of evaluating those underlying biological drivers is that early (or even subclinical) identification of frailty would then permit proactive clinical intervention to reduce adverse outcomes. The current study aims to combine several blood biomarkers in order to strengthen the prediction of mortality.
Since systemic low level inflammation is strongly associated with frailty and early death, inflammatory parameters have been used to study it [2]. In the context of cancer prognosis, the inflammation-based Glasgow Prognostic Score (GPS) was introduced as a predictor of survival, independent of tumor stage, performance status and treatment [6]. This score is derived from the acute-phase proteins C-reactive protein (CRP) and albumin: patients with both abnormal CRP (>10mg/L) and albumin (<35g/L) levels are allocated a score of two, patients in whom only one of these abnormalities is present are allocated a score of one, and patients in whom neither of these markers show abnormal levels are allocated a score of zero [6]. For instance, in a study of 65 patients with colorectal cancer GPS was found to be a significant independent predictor of cancer survival (HR: 1.65 (95%CI: 1.10-2.47))[7]. The link between inflammation and frailty in older patients has been shown in several other studies [1-4]. Biomarkers of the hepatic function are another way of assessing frailty in older patients; however the potential significance of liver function in aging has not been studied extensively. Gamma-glutamyl transferase (GGT) is a blood marker of liver injury and liver disease and is correlated with alanine transaminase (ALT) activity, which is an enzyme released from damaged hepatocytes that marks fatty liver disease and is linked to obesity and increased mortality and morbidity [8]. While a study of 1,673 community-dwelling men aged 70 years or older did not find a statistically significant association between GGT and frailty [9], a prospective study in the Austrian Vorarlberg Health Monitoring and Promotion Programme did show that high levels of GGT (>28 mg/dL) were associated with increased risk of all cause mortality (HR: 1.66, 1.65, and 1.48 in age groups <50, 50-64, and 65+, respectively) [10].
Another marker that can be used to assess frailty and poor prognosis among the older patients is high-density lipoprotein cholesterol (HDL) due to its correlation with obesity and dyslipidemia [11,12]. HDL-cholesterol is also associated with inflammation [12]. A prospective cohort study based on 4,128 persons aged 70 and older showed that after adjustment for age and gender, persons with low total cholesterol had significantly higher mortality than those with normal and high total cholesterol. Moreover, it was shown that a combination of low total cholesterol and low albumin levels was even worse for survival, particularly among those who also had low HDL-cholesterol [13].
Thus, several biomarkers and combinations of biomarkers have been suggested to predict poor prognosis and mortality. To our knowledge, no large prospective cohort study has yet examined whether a combination of these markers that are easily measurable in clinical practice is a strong predictor for mortality in older persons. We aimed to study a combination of CRP, albumin, GGT, and HDL in a Swedish cohort of 44,457 persons aged 50 and over.
Methods
Study population and data collection
The Central Automation Laboratory (CALAB) database (1985-1996), includes data obtained from 351,487 men and 338,101 women, mainly from the greater Stockholm area (Sweden). All individuals were either healthy individuals referred for clinical laboratory testing as part of a general health check-up or outpatients referred for laboratory testing. No individuals were inpatients at the time their blood samples were taken and none were excluded due to disease symptoms or because of treatment. Apart from the information on blood testing, no personal data were included in the CALAB database [14]. This database was linked to several Swedish national registries such as the National Cancer Register, the Hospital Discharge Register, the Cause of Death Register, the consecutive Swedish Censuses during 1970-1990, and the National Register of Emigration by using the Swedish 10-digit personal identity number to provide information on socio-economic status (SES), vital status, cancer diagnosis prior to baseline measurements, and emigration. The linkage of national registers to the CALAB database is called the AMORIS study and it has been described in detail elsewhere [14-20]. This study complied with the Declaration of Helsinki, and the ethics review board of the Karolinska Institute approved the study.
For the current study, we selected a sub-cohort of all persons aged 50 years or older, whose levels of CRP, albumin, GGT, and HDL were measured at baseline and took the following information from the CALAB database: CRP (mg/L), albumin (g/L), GGT (U/L), HDL (mmol/L), age at diagnosis, and gender (n=44,457). From the other registries, we collected information regarding SES, co-morbidity, and death. Charlson co-morbidity index (CCI) was calculated by using the information from the Hospital Discharge Register. The CCI consists of 18 groups of diseases with a specific weight assigned to each disease category (1, 2, 3, and 6). These weights were then summed to obtain an overall score, resulting in five comorbidity levels (0, 1, 2, 3, and 4+) indicating a scale ranging from no comorbidity to severe comorbidity. Follow-up time started at time of measurement and ended at time of event (i.e. death), emigration, or end of follow-up (31 December 2002), whichever occurred first.
The quantitative determination of CRP was done with an immunoturbidimetric method (reagents from Orion Diagnostics, Finland; coefficient of variation (CV) 12% at CRP level 40 mg/L) and albumin was measured with a bromcresolgreen method (CV < 1.8%). The concentration of HDL was calculated and the validation procedures have been reported [14]. An enzymatic colorimetric test using L-γ-glutamyl- 3-carboxy-4-nitroanilide as donor substrate was conducted to measure levels of GGT (reagents from Randox Laboratories Ltd, UK; CV ≤ 6.0%) [8]. All methods were fully automated with automatic calibration and accredited laboratory facilities [15].
Data analysis
A mortality score, ranging from 0 to 4, was calculated as the number of biomarkers with abnormal values according to their clinical cut-offs (CRP > 10 mg/L, albumin < 35 g/L, GGT > 35 kU/L, and HDL < 1.03 mmol/L) [6,21-22]. Due to the small number of subjects with mortality score = 4, the score was truncated at 3. Multivariate Cox proportional hazards regression was used to investigate this mortality score in relation to all-cause mortality as well as cancer-specific and cardiovascular-specific death (ICD10: I00-I99). The analysis was conducted for the overall age group as well as for different age categories (< 65, 65-74, 75+). All models took into account age, SES, gender, and the CCI. To ascertain the affect of gender, age, and ongoing comorbidities, stratified analyses were conducted for men and women, age-groups 50-64, 65-74, and 75+, and different scores of the CCI. We also assessed the association between the mortality score and the CCI by calculation the correlation coefficient and kappa’s coefficient of agreement between both measurements. A sensitivity-analysis was conducted in which those who had < 1 year of follow- up were deleted in order to assess reverse causation. To assess and illustrate clinical relevance of our mortality score, we calculated the hazard ratios for all-cause death among cancer patients with no other co-morbidities (n=1,955). In addition, we calculated the sensitivity and specificity for the different values of the mortality score after 1, 2, and 3 years of follow-up in the same group of cancer patients [23]. All analyses were conducted with Statistical Analysis Systems (SAS) release 9.1.3 (SAS Institute, Cary, NC).
Results
A total of 7,381 persons died during follow-up time, of whom 2,245 (30.4 %) died of cancer and 3,276 (44.4%) of circulatory disease. All the population characteristics are shown in Table 1. A higher CCI was observed for those who died during follow-up than for those who were alive at the end of follow-up (4.73% versus 0.68% with CCI=4+) (Table 1).
Table 1.
Descriptive statistics of study population by vital status.
| Alive (N=37,076) | All-cause Death (N=7,381) | Cancer specific Death (N=2,245) | Circulatory disease Death (N=3,276) | |
|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | |
| Mean Age (years) (SD) | 60.46 (7.87) | 69.99 (10.28) | 66.50 (9.45) | 71.72 (10.03) |
| Gender | ||||
| Men | 18899 (50.97) | 3987 (54.02) | 1245 (55.46) | 1843 (56.26) |
| Women | 18177 (49.03) | 3394 (45.98) | 1000 (44.54) | 1433 (43.74) |
| SES | ||||
| White collar | 16302 (43.97) | 2088 (28.89) | 817 (36.39) | 840 (25.64) |
| Blue collar | 15460 (41.70) | 2111 (28.60) | 770 (34.30) | 854 (26.07) |
| Not gainfully employed/Missing | 5314 (14.33) | 3182 (43.11) | 658 (29.31) | 1582 (48.29) |
| Charlson Comorbidity Index | ||||
| 0 | 31574 (85.16) | 4523 (61.28) | 1499 (66.77) | 1874 (57.20) |
| 1 | 2790 (7.53) | 1142 (15.47) | 203 (9.04) | 623 (19.02) |
| 2 | 1997 (5.39) | 1026 (13.90) | 388 (17.28) | 419 (12.79) |
| 3 | 464 (1.25) | 341 (4.62) | 75 (3.34) | 180 (5.49) |
| 4+ | 251 (0.68) | 349 (4.73) | 80 (3.56) | 180 (5.49) |
| Cancer prior to measurement | 905 (2.44) | 936 (12.68) | 433 (19.29) | 325 (9.92) |
| Circulatory disease prior to measurement | 5246 (14.15) | 2227 (30.17) | 460 (20.49) | 1265 (38.61) |
| Mean follow-up time (years) (SD) | 9.51 (2.82) | 5.56 (3.61) | 5.58 (3.59) | 5.39 (3.62) |
| CRP (mg/l) | ||||
| Mean (SD) | 5.27 (9.56) | 7.82 (18.16) | 8.30 (19.55) | 7.42 (16.31) |
| >10 | 2041 (5.50) | 912 (12.36) | 283 (12.61) | 402 (12.27) |
| Albumin (g/l) | ||||
| Mean (SD) | 42.57 (2.60) | 41.17 (2.98) | 41.26 (3.05) | 41.19 (2.83) |
| <35 | 61 (0.16) | 138 (1.87) | 47 (2.09) | 46 (1.40) |
| HDL-cholesterol (mmol/L) | ||||
| Mean (SD) | 1.55 (0.43) | 1.47 (0.45) | 1.49 (0.44) | 1.42 (0.45) |
| <1.03 | 3680 (9.93) | 1154 (15.63) | 304 (13.54) | 600 (18.32) |
| Gamma-glutamyltransferase (U/L) | ||||
| Mean (SD) | 34.61 (42.00) | 46.60 (94.54) | 48.13 (106.50) | 41.80 (59.86) |
| >36 | 10009 (27.00) | 2309 (31.28) | 663 (29.53) | 1048 (31.99) |
| Mortality score | ||||
| 0 | 23772 (64.12) | 3975 (53.85) | 1273 (56.70) | 1700 (51.89) |
| 1 | 11039 (29.77) | 2480 (33.60) | 706 (31.45) | 1137 (34.71) |
| 2 | 2048 (5.52) | 762 (10.32) | 214 (9.53) | 363 (11.08) |
| 3 | 212 (0.57) | 147 (1.99) | 45 (2.00) | 71 (2.17) |
| 4 | 5 (0.01) | 17 (0.23) | 7 (0.31) | 5 (0.15) |
A multivariate Cox proportional hazards model including continuous variables of CRP, albumin, GGT, and HDL (adjusted for age, gender, SES, and CCI) showed that each variable was statistically significantly associated with all-cause mortality: HR or one unit increase: 1.08 (95%CI: 1.06-1.11), 0.94 (0.93-0.94), 1.37 (1.33-1.41), and 0.78 (95%CI: 0.73-0.82), respectively. Note that CRP and GGT were log-transformed due to their skewed distributions.
When using the summary mortality score, a clear statistically significant positive trend was observed between the score and all-cause mortality as well as cancer and circulatory disease- specific death (e.g. HR for all-cause mortality: 1.39 (95%CI: 1.32-1.46), 2.04 (95%CI: 1.89-2.21), and 3.36 (95%CI: 2.87-3.93) for score=1, 2, and 3 compared to score=0). Stratification by gender showed similar patterns for men and women (Table 2).
Table 2.
Hazard Ratio (HR) and 95% Confidence Intervals (CI) for risk of all-cause, cancer-specific, and circulatory disease death. All models were adjusted for age, gender, socio-economic status, and Charlson Comorbidity Index.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause N=7,381 | Cancer N=2,245 | Circulatory N=3,276 | All-cause N=3,987 | Cancer N=1,245 | Circulatory N=1,843 | All-cause N=3,394 | Cancer N=1,000 | Circulatory N=1,433 | |
| HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | |
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.39 (1.32-1.46) | 1.20 (1.09-1.32) | 1.47 (1.36-1.58) | 1.29 (1.20-1.38) | 1.07 (0.95-1.21) | 1.40 (1.27-1.55) | 1.53 (1.41-1.65) | 1.42 (1.23-1.64) | 1.57 (1.43-1.81) |
| Score=2 | 2.04 (1.89-2.21) | 1.77 (1.53-2.05) | 2.19 (1.95-2.46) | 1.90 (1.72-2.10) | 1.65 (1.37-1.97) | 2.11 (1.83-2.44) | 2.25 (1.97-2.56) | 2.10 (1.63-2.71) | 2.18 (1.79-2.66) |
| Score=3 | 3.36 (2.87-3.93) | 3.49 (2.64-4.62) | 3.40 (2.70-4.28) | 3.46 (2.86-4.18) | 3.30 (2.34-4.65) | 3.62 (2.75-4.77) | 2.94 (2.22-3.89) | 3.77 (2.32-6.12) | 2.71 (1.76-4.19) |
To identify whether these associations were affected by age, a stratified analysis by age groups was conducted (Table 3). The same pattern as seen in Table 2 was observed in Table 3. However, the hazard ratios were slightly higher among those in the youngest age group (e.g. HR for overall death when score=3: 4.79 (95%CI: 3.68-6.22), 3.28 (95%CI: 2.45-4.38), and 2.55 (95%CI: 1.95-3.33) for persons aged 50-65, 65-74, and 75+, respectively).
Table 3.
Age-group specific Analysis: Hazard Ratio (HR) and 95% Confidence Intervals (CI) for risk of all cause, cancer-specific, and circulatory disease death. All models were adjusted for age, gender, socio-economic status, and Charlson Comorbidity Index.
| Age-group 50-64 (N=30,359) | Age-group 65-74 (N=8,709) | Age-group 75+ (N=5,389) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All cause N=2,530 | Cancer N=1,071 | Circulatory N=3,276 | All cause N=2,110 | Cancer N=683 | Circulatory N=938 | All cause N=2,741 | Cancer N=491 | Circulatory N=1,445 | |
| HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | |
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.47 (1.35-1.61) | 1.28 (1.12-1.46) | 1.56 (1.35-1.84) | 1.45 (1.32-1.60) | 1.16 (0.98-1.38) | 1.64 (1.42-1.89) | 1.27 (1.17-1.39) | 1.11 (0.90-1.37) | 1.31 (1.17-1.47) |
| Score=2 | 2.29 (2.01-2.61) | 1.86 (1.50-2.30) | 2.58 (2.09-3.18) | 2.12 (1.83-2.45) | 1.55 (1.18-2.05) | 2.66 (2.16-3.27) | 1.78 (1.55-2.04) | 2.00 (1.48-2.71) | 1.67 (1.38-2.02) |
| Score=3 | 4.79 (3.68-6.22) | 3.83 (2.44-6.00) | 5.10 (3.33-7.80) | 3.28 (2.45-4.38) | 2.77 (1.65-4.65) | 3.88 (2.57-5.87) | 2.55 (1.95-3.33) | 3.85 (2.35-6.31) | 2.44 (1.68-3.54) |
In order to compare our mortality score with CCI, we also calculated the HRs for the association between CCI and all-cause mortality, which resulted in similar risks (HR: 1.86 (95%CI: 1.74- 1.98), 2.10 (95%CI: 1.96-2.25), 2.58 (95%CI: 2.31-2.89), and 3.59 (95%CI: 3.57-4.46) for CCI=1, 2, 3, and 4+ compared to CCI=0). The effect of co-morbidity was then assessed with a stratified analysis by values of the CCI (Table 4). The patterns observed in Table 2 were seen in each stratum of CCI, even among those with CCI=0. For instance among those with CCI=2 the risk of cancer-specific death was 1.08 (95%CI: 0.86-1.36), 2.16 (95%CI: 1.53-3.04), and 5.10 (95%CI: 2.83-9.19) for score=1, 2, and 3, compared to score=0 (Table 4). The univariate association between our mortality score and the CCI indicated that there was not a strong correlation between both measurements (correlation coefficient: 0.11; p<0.0001 and kappa’s coefficient of agreement: 0.05; p: 0.003).
Table 4.
Hazard Ratio (HR) and 95% Confidence Intervals (CI) for risk of all-cause, cancer-specific, and cardiovascular death, stratified by Charlson comorbidity index. All models were adjusted for age, gender, socio-economic status. *Also adjusted for history of cancer prior to measurement. ^Also adjusted for history of circulatory disease prior to measurement.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All cause | Cancer* | Circulatory^ | All cause | Cancer* | Circulatory^ | All cause | Cancer* | Circulatory^ | |
| HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | |
| Charlson = 0 | |||||||||
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.40 (1.31-1.49) | 1.30 (1.16-1.46) | 1.42 (1.28-1.57) | 1.33 (1.22-1.44) | 1.21 (1.05-1.40) | 1.35 (1.18-1.54) | 1.51 (1.36-1.67) | 1.45 (1.21-1.73) | 1.54 (1.32-1.80) |
| Score=2 | 2.14 (1.92-2.39) | 1.89 (1.57-2.29) | 2.18 (1.84-2.58) | 2.11 (1.85-2.41) | 1.82 (1.44-2.29) | 2.33 (1.91-2.85) | 2.09 (1.72-2.54) | 1.89 (1.32-2.71) | 1.67 (1.21-2.31) |
| Score=3 | 3.49 (2.74-4.45) | 3.39 (2.22-5.18) | 3.62 (2.52-5.21) | 3.90 (2.91-5.22) | 3.22 (1.89-5.49) | 4.63 (3.04-7.04) | 2.71 (1.74-4.22) | 3.57 (1.77-7.18) | 1.96 (0.93-4.13) |
| Charlson = 1 | |||||||||
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.33 (1.17-1.52) | 1.29 (0.95-1.74) | 1.25 (1.04-1.49) | 1.24 (1.04-1.47) | 1.13 (0.76-1.69) | 1.31 (1.04-1.65) | 1.48 (1.21-1.80) | 1.52 (0.95-2.43) | 1.14 (0.85-1.53) |
| Score=2 | 1.80 (1.49-2.18) | 1.37 (0.85-2.20) | 2.06 (1.62-2.62) | 1.65 (1.30-2.09) | 1.10 (0.59-2.06) | 1.95 (1.44-2.65) | 2.09 (1.52-2.88) | 1.90 (0.92-3.90) | 2.37 (1.59-3.55) |
| Score=3 | 2.49 (1.74-3.56) | 1.91 (0.76-4.78) | 2.43 (1.50-3.92) | 2.45 (1.62-3.69) | 1.10 (0.34-3.56) | 2.57 (1.50-4.38) | 2.18 (1.03-4.62) | 15.98 (3.64-70.10) | 1.83 (0.58-5.74) |
| Charlson = 2 | |||||||||
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.38 (1.20-1.58) | 1.08 (0.86-1.36) | 1.60 (1.30-1.98) | 1.19 (0.98-1.45) | 0.80 (0.57-1.13) | 1.52 (1.33-2.03) | 1.57 (1.30-1.90) | 1.41 (1.04-1.92) | 1.70 (1.24-2.32) |
| Score=2 | 2.22 (2.82-2.72) | 2.16 (1.53-3.04) | 2.13 (1.55-2.93) | 2.02 (1.53-2.67) | 2.26 (1.45-3.52) | 1.92 (1.25-2.97) | 2.43 (1.81-3.26) | 2.04 (1.17-3.54) | 2.30 (1.43-3.69) |
| Score=3 | 3.19 (2.18-4.65) | 5.10 (2.83-9.19) | 2.69 (1.49-4.86) | 2.61 (1.58-4.28) | 4.43 (2.03-9.67) | 2.01 (0.92-4.39) | 4.04 (2.26-7.22) | 6.24 (2.54-15.31) | 4.21 (1.71-10.36) |
| Charlson = 3 | |||||||||
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.27 (0.99-1.61) | 1.31 (0.76-2.27) | 1.30 (0.93-1.81) | 1.05 (0.76-1.45) | 0.79 (0.37-1.68) | 0.94 (0.61-1.44) | 1.56 (1.09-2.23) | 2.01 (0.93-4.35) | 2.14 (1.28-3.57) |
| Score=2 | 1.71 (1.25-2.33) | 3.72 (2.02-6.85) | 1.35 (0.87-2.10) | 1.18 (0.79-1.76) | 2.27 (0.96-5.34) | 0.98 (0.57-1.67) | 3.44 (2.12-5.57) | 10.05 (4.11-24.59) | 2.51 (1.17-5.39) |
| Score=3 | 2.80 (1.57-4.98) | 8.69 (2.90-26.05) | 2.09 (0.89-4.89) | 2.71 (1.38-5.34) | 5.90 (1.64-21.17) | 1.79 (0.69-4.64) | 2.63 (0.82-8.44) | 48.72 (5.04-471.16) | 1.90 (0.26-14.20) |
| Charlson = 4 | |||||||||
| Score=0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Score=1 | 1.34 (1.04-1.73) | 0.79 (0.46-1.36) | 1.67 (1.16-2.40) | 1.28 (0.89-1.83) | 0.82 (0.39-1.72) | 1.60 (0.97-2.64) | 1.44 (1.00-2.06) | 0.78 (0.35-1.75) | 1.82 (1.08-3.10) |
| Score=2 | 1.78 (1.32-2.40) | 1.47 (0.80-2.70) | 1.93 (1.26-2.97) | 1.70 (1.13-2.56) | 1.82 (0.87-3.79) | 1.74 (0.97-3.14) | 1.98 (1.26-3.12) | 1.05 (0.29-3.74) | 2.22 (1.17-4.22) |
| Score=3 | 4.24 (2.68-6.71) | 4.62 (2.12-10.53) | 4.50 (2.22-9.09) | 5.67 (3.08-10.43) | 8.76 (3.30-23.25) | 4.22 (1.53-11.64) | 2.88 (1.36-6.09) | 1.03 (0.13-7.91) | 5.13 (1.89-13.90) |
The sensitivity analysis in which those with follow- up of < 1 year were excluded showed similar, but attenuated, patterns to those observed in Table 2, however the results were still statistically significant (results not shown). For instance, the hazard ratios for all-cause death increased with the values of our mortality score: 1.23 (95%CI: 1. 21-1.35), 1.66 (95%CI: 1.43-1.94), and 2.05 (95%CI: 1.48-2.85), for score=1, 2, and 3 compared to score=0.
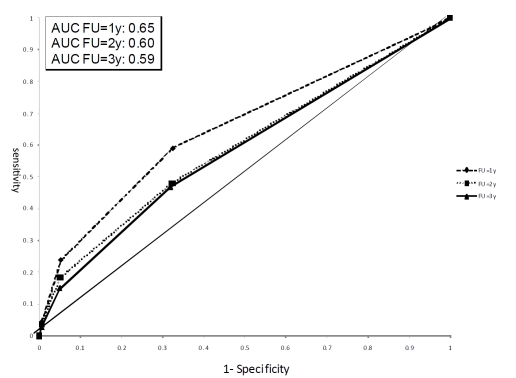
To assess and illustrate clinical relevance of our mortality score, we calculated the hazard ratios for all-cause death among cancer patients with no other co-morbidities (n=1,955). We found a clear statistically significant positive trend between the score and all-cause mortality (e.g. HR: 1.24 (95%CI: 1.0.-1.49), 2.38 (95%CI: 1.76-3.22), and 5.47 (95%CI: 2.98-10.03) for score=1, 2, and 3 compared to score=0). When assessing death after one year of follow-up, this resulted in a specificity of 0.68 and a sensitivity of 0.59 for a mortality score with a cut-off of 0 versus ≥ 1. The area under the curve (AUC) was 0.65 when using the mortality score as a predictor of death within one year of follow-up (Figure 1).
Figure 1.
ROC curve for the mortality score after 1, 2, and 3 years of follow-up among cancer patients with no other co-morbidities.
Discussion
In this large prospective cohort study, the combination of CRP, albumin, GGT, and HDL was associated with mortality in persons older than 50. The hazard ratios of our mortality score were of a similar magnitude for all-cause mortality, cancer-specific and circulatory disease mortality. The score was shown to be predictive among patients for whom the CCI was low.
The principle focus of geriatric medicine is to identify, assess and treat frail older people in order to reduce their elevated risks of adverse health outcomes and loss of independence. Age and chronic-disease related activation of inflammation and neuroendocrine dysregulation and metabolic changes leading to physiological frailty (e.g anorexia, anaemia) and clinical frailty (e.g. falls) is a known concept in geriatric medicine. There remains however a need for combined clinical-biomarker predictor tools to identify people at risk of poor prognosis in whom clinical intervention may proactively reduce risk of adverse health events. This is especially relevant in patients undergoing extra stressors of e.g. surgery or cancer treatment [2,24].
Chronic inflammation is suggested to play a role in the pathophysiology of poor prognosis [3]. A combination of abnormal values of albumin (< 3.8 g/dL), cholesterol (< 170 mg/dL), IL-6 (> 3.8 pg/mL), and CRP (> 2.65 mg/L) was studied in a prospective cohort study of 870 high-functioning persons aged 70-79. Those with three or four abnormal values of inflammatory markers were 6.6 and 3.2 time more likely to die after 3 and 7 years of follow-up, respectively. Even though pro-inflammatory markers are the most commonly studied biomarkers in terms of frailty and poor prognosis [1,4], markers of liver dysfunction can also add predictive information for patient frailty [9]. GGT is one of the commonly used markers for chronic liver disease, but it has also been associated with all-cause mortality, cardiovascular disease and death, as well as diabetes and cancer incidence death [22,25-26]. A recent study in NHANES III, based on 14,950 adult participants, showed that those with elevated GGT levels (> 51 U/L in men or > 33 U/L in women) were 1.5 times more likely to die from all causes of death as well as from cancer. No statistically significant results were found for death due to cardiovascular disease [25]. Related to liver dysfunction and early death are also markers of the lipid metabolism as several of them are produced by the liver. An Italian prospective study that collected data on 359 persons aged 80 years and older showed a statistically significant difference in mean HDL cholesterol between those who died after 2 years of follow-up and those who did not (36.7 mg/dL versus 43.4 mg/dL) [11].
Our study corroborates the above findings as we found a significant positive association between abnormal values of inflammatory, hepatic, as well as lipid biomarkers and mortality. However, by calculating a summary mortality score we also aimed to assess the combination of different mechanisms that might contribute to patient frailty in order to get a more precise prediction. All our analyses for mortality score showed a strong positive trend and hazard ratios of a magnitude that is of clinical relevance, especially for those having a mortality score of three or more. In the analyses stratified by age group the hazard ratios were slightly higher for the youngest age group. This may be due to the fact that the score is more specifically indicating medical problems in younger persons, while in older subjects it has less precision to separate normal ageing from real frailty. It is of importance to note that the association between the mortality score and mortality remained even after stratifying by CCI, which may suggest that this score adds predictive information on mortality to the CCI. This was also seen when calculating the risk of dying among cancer patients with no other co-morbidities.
The major strength of this analysis lies in the large number of persons with prospective measurements of albumin, CRP, GGT, and HDL in AMORIS, all measured at the same clinical laboratory. Use of national health registers provided complete follow-up for each person as well as detailed information on cancer diagnosis, time of death, and emigration. Furthermore, assessments of exposure and outcome variables were conducted in an accurate manner. The AMORIS population was selected by analysing blood samples from health check-ups in non-hospitalized individuals. The AMORIS cohort is similar to the general working population of Stockholm county in terms of SES and ethnicity. During the study period all-cause mortality was about 14% lower in the AMORIS population than in the general population of Stockholm county when taking age, gender, and calendar year into account [27]. This selection of a healthy cohort does however not affect the internal validity of our study. A potential limitation of our analyses is the lack of information on possible confounders such as smoking habits or obesity. However it is likely that some of the effect of these behavioural risk factors was captured by the disease groups in the CCI. Another limitation is that we did not have information on tumour severity or type for those persons diagnosed with cancer.
Conclusions
By combining easily measured and inexpensive biomarkers of the different mechanisms that might contribute to early death, this study found a marker for mortality in persons aged 50+ years. A clear association, of a magnitude that is difficult to explain by confounding only, was found for all-cause mortality as well as cancer and circulatory disease-specific death. The elevated hazard ratios among cancer patients with no other co-morbidities call for validation in other cohorts, testing combinations with other markers, and testing other cut-offs than the clinically accepted levels used here, with a view to informing decision-making in treatment of older patients with cancer.
Acknowledgement
MVH, HG, and LH contributed to the conception and design as well as analysis and interpretation of data. IG, GW, NH, DH and ML contributed in the acquisition of data. MVH drafted the manuscript and all authors contributed to the critical revision of the manuscript for important intellectual content. MVH and HG conducted the statistical analysis. LH, IG, NH, and ML obtained funding. The study was supported by grants from the Gunnar and Ingmar Jungner Foundation for Laboratory Medicine (Stockholm) the Swedish Cancer Society (#2010/608), and Cancer Research-UK.
References
- 1.Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, Lacombe D, Monfardini S, Scalliet P, Wildiers H. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer. 2010;46:1502–1513. doi: 10.1016/j.ejca.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 5.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 7.Crumley AB, Stuart RC, McKernan M, McDonald AC, McMillan DC. Comparison of an inflammation- based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol. 2008;23:e325–329. doi: 10.1111/j.1440-1746.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 8.Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer - a narrative review. Clin Chem Lab Med. 2010;48:147–157. doi: 10.1515/CCLM.2010.031. [DOI] [PubMed] [Google Scholar]
- 9.Le Couteur DG, Blyth FM, Creasey HM, Handelsman DJ, Naganathan V, Sambrook PN, Seibel MJ, Waite LM, Cumming RG. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci. 2010;65:712–717. doi: 10.1093/gerona/glq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulmer H, Kelleher C, Diem G, Concin H. Why Eve is not Adam: prospective follow-up in 149650 women and men of cholesterol and other risk factors related to cardiovascular and all-cause mortality. J Womens Health (Larchmt) 2004;13:41–53. doi: 10.1089/154099904322836447. [DOI] [PubMed] [Google Scholar]
- 11.Landi F, Russo A, Pahor M, Capoluongo E, Liperoti R, Cesari M, Bernabei R, Onder G. Serum high-density lipoprotein cholesterol levels and mortality in frail, community-living elderly. Gerontology. 2008;54:71–78. doi: 10.1159/000111381. [DOI] [PubMed] [Google Scholar]
- 12.Kaysen GA. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S56–63. doi: 10.2215/CJN.03090509. [DOI] [PubMed] [Google Scholar]
- 13.Volpato S, Leveille SG, Corti MC, Harris TB, Guralnik JM. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49:1142–1147. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 14.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 15.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 16.Holme I, Aastveit AH, Jungner I, Walldius G. Relationships between lipoprotein components and risk of myocardial infarction: age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2008;264:30–38. doi: 10.1111/j.1365-2796.2008.01925.x. [DOI] [PubMed] [Google Scholar]
- 17.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2009;265:275–287. doi: 10.1111/j.1365-2796.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 18.Walldius G, Jungner I, Kolar W, Holme I, Steiner E. High cholesterol and triglyceride values in Swedish males and females: increased risk of fatal myocardial infarction. First report from the AMORIS (Apolipoprotein related MOrtality RISk) study. Blood Press. (Suppl 1992; 4):35–42. [PubMed] [Google Scholar]
- 19.Van Hemelrijck M, Garmo H, Binda E, Hayday A, Karagiannis SN, Hammar N, Walldius G, Lambe M, Jungner I, Holmberg L. Immunoglobulin E and cancer: a meta-analysis and a large Swedish cohort study. Cancer Causes Control. 2010;21:1657–1667. doi: 10.1007/s10552-010-9594-6. [DOI] [PubMed] [Google Scholar]
- 20.Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, Lambe M. Prostate cancer risk in the Swedish AMORIS study: The interplay between triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 21.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Strasak AM, Pfeiffer RM, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H. Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902–1906. doi: 10.1002/ijc.23714. [DOI] [PubMed] [Google Scholar]
- 23.Glasser S. Research Methodology for Studies of Diagnostic Tests. In: Glasser S, editor. Essentials of Clinical Research. Springer Science + business Media B.V.; 2008. pp. 254–256. [Google Scholar]
- 24.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 25.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H. Association of gamma- glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–3977. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzmann M, Jungner I, Walldius G, Ivert I, Nordqvist T, östergren J. Apolipoproteins B and A-I, standard lipid measures and incidence of myocardial infarction in men and women, with or without chronic kidney disease. Study IV in Thesis for doctorial degree (PhD) In: Holzmann M, editor. Renal insufficiency, mortality and myocardial infarction. Stockholm: Karolinska Institutet; 2008. [Google Scholar]