Abstract
Effective clinical decision support (CDS) is essential for addressing healthcare performance improvement imperatives, but care delivery organizations (CDO) typically struggle with CDS deployment. Ensuring safe and effective medication delivery to patients is a central focus of CDO performance improvement efforts, and this article provides an overview of best-practice strategies for applying CDS to these goals. The strategies discussed are drawn from a new guidebook, co-published and co-sponsored by more than a dozen leading organizations. Developed by scores of CDS implementers and experts, the guidebook outlines key steps and success factors for applying CDS to medication management. A central thesis is that improving outcomes with CDS interventions requires that the CDS five rights be addressed successfully. That is, the interventions must deliver the right information, to the right person, in the right format, through the right channel, at the right point in workflow. This paper provides further details about these CDS five rights, and highlights other important strategies for successful CDS programs.
Keywords: Clinical decision support, medication management, performance improvement
Medications are a central element in the healthcare toolkit, but their use is fraught with well-documented errors of omission and commission. In the hospital setting, where many safety studies have been conducted, approximately 47 percent of medical errors are medication related. The 2007 IOM report on prevention of medication error estimates that a hospital patient may be subjected to at least one medication error per day.1
Such errors exact a high toll on patient safety and healthcare costs, resulting in approximately 7,000 deaths per year and $2 billion from inpatient adverse drug events (ADE).1 The prevalence of medication errors and high cost associated with these errors has compelled healthcare regulators, payors, accreditors, the public and others to make safe and effective medication management a key performance improvement imperative for all care delivery organizations.
For example, The American Recovery and Reinvestment Act (ARRA) of 2009 provides billions of dollars of incentives and penalties to hospitals and physician practices that use “qualified electronic health records” to support care delivery, and associates clinical decision support (CDS) with the term. Implementation of EHRs allows for physician order entry; to capture and query information relevant to healthcare quality; and to exchange electronic health information with and integrate such information from other sources—each function typically focused heavily on medication use providing further opportunities for CDS. Even though meaningful use of an EHR has not been defined in detail as of this writing, it is very likely that ARRA will provide strong incentives for providers to embrace CDS and execute it effectively.
HOW CAN CDS HELP?
Getting clinical knowledge and intelligently filtered patient data to the patient and care delivery team offers great promise for addressing these challenges.2 Many studies have reported successful results from applying CDS to the medication-related challenges noted above. One such CDS-supported medication dosing process produced a 21-percent increase in appropriate medication prescribing in renal sufficiency which ultimately resulted in a reduced length of stay3; the same study also demonstrated an 86-percent absolute reduction in non-intercepted serious medication errors. Another form of CDS for empiric preoperative antibiotic selection decreased postoperative wound infections.4
Despite these successes, studies show that even with advanced clinical systems and CDS, ADEs can persist.5 To help ensure that CDS is successfully deployed on a widespread basis to improve medication use and outcomes, many diverse organizational and individual stakeholders came together to synthesize CDS implementation best practices.6 This paper presents an overview of several key strategies compiled in Improving Medication Use and Outcomes with Clinical Decision Support: A Step-by-Step Guide> (HIMSS, 2009).
A central premise of the recommended implementation approach is that successfully applying CDS to address targeted objectives requires that the CDS five rights’ must be addressed. That is, the CDS intervention must deliver the right information, to the right person, in the right format, through the right channel, at the right point in workflow. We explain below each of these CDS five rights. Although these “rights” may seem like common sense, many problems encountered in CDS deployments can be traced to failure in appropriately addressing one or more of these elements.
In addition, we will briefly outline other best practices that complement the CDS five rights approach in setting up effective CDS programs focused on medication management.
THE CDS FIVE RIGHTS OVERVIEW
CDS interventions can be applied throughout the medication management cycle to optimize medication safety and other pertinent outcomes, and the CDS five rights approach is a useful framework for this. Note that the five rights of medication use—i.e., ensuring that the right patient gets the right drug at the right dose via the right route at the right time7—was the conceptual springboard for the CDS five rights, but is different.
The CDS Five Rights model states that we can achieve sustainable CDS-supported improvements in desired healthcare outcomes if we communicate:
The right information: evidence-based, suitable to guide action, pertinent to the circumstance;
to the right person: considering all members of the care team, including clinicians, patients and their caretakers;
in the right CDS intervention format: such as an alert, order set or reference information to answer a clinical question;
through the right channel: for example, a clinical information system (CIS) such as an electronic medical record (EMR), personal health record (PHR) or a more general channel such as the internet or a mobile device;
at the right time in workflow: for example, at time of decision/ action/need.
MEDICATION MANAGEMENT CYCLE
Because the CDS five rights are focused on achieving specific objectives, it is important to understand the medication management process and the objectives associated with each step. Figure 1 outlines these workflow steps and objectives that can be supported with CDS to ensure safe and effective medication use.
Fig. 1.
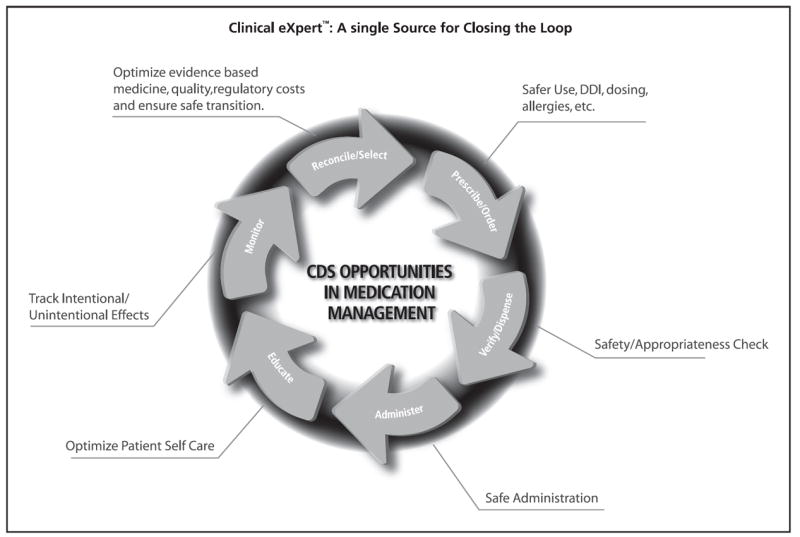
CDS opportunities in the medication management loop. [Reprinted with permission, Thomson Reuters]
Each step in the medication management process outlined above anchors the ‘right point in workflow’ element of the CDS five rights. Below we consider how each of the CDS five rights can be addressed to optimize effectiveness, safety and resource use at each step, and for overall outcomes associated with medication use.
THE CDS FIVE RIGHTS IN DETAIL
Right information
Although it may seem self-evident that the core of a good CDS intervention is providing the right information, many CDS interventions fail because this element isn’t addressed appropriately. For example, one reason for the very high rate at which recipients override certain interruptive alerts is because the information presented to users in the alert isn’t relevant.8 Components of the right information include the following:
Pertinent to clinician and patient at hand: e.g., displaying a recommended lower dose during order entry for a medication that is renally excreted, along with the evidence that patient has decreased kidney function (e.g., as suggested by a low creatinine clearance value) or the fact that patient has not had this measurement made.9
Address specific information need or action at hand. Kawamoto et al. found in a meta-analysis that CPOE systems with CDS “that provided a recommendation [such as “Patient is at high risk of coronary artery disease; recommend initiation of blocker therapy]” were significantly more likely to succeed than systems that provided only an assessment of the patient (such as “Patient is at high risk of coronary artery disease”) [rate difference 35 percent] (confidence interval: 8 percent to 58 percent).”10
Current, believable and trusted by recipient. All information provided to practitioners and patients should be current, relevant to the patient, evidence-based and from trusted sources. To help reinforce information validity, literature citation(s), and ideally direct link to pertinent sources, can be provided. These links also can help in on-going CDS knowledge and intervention maintenance.11
Contain the appropriate level of detail—not too much, not too little. For example, it is important to keep alert text clear and concise, but it should be easy to access more supporting evidence at a later time.12
Right person
The healthcare team is comprised of several key players—such as physicians, nurses, pharmacists and others—each of which play different patient care roles, and therefore has different decision support needs and opportunities. The patient, of course, plays a central role in the care process, and is a powerful but often overlooked target for CDS interventions.
Depending on his/her role, each team member may need certain (right) data/information that would be most meaningful to them. Identifying the connection between this right information and the right person is the basis of this second CDS right. Examples: 1.) Showing the patient’s latest creatinine clearance (kidney function test) result to a pharmacist verifying/dispensing a medication whose dosing depends on kidney function; 2.) Showing all of the past INRs (measures of anticoagulation) when a physician is ordering Coumadin (an anticoagulant).
Right CDS intervention format
CDS interventions are the basic tools in the CDS toolkit, and come in a variety of types or formats. There are various taxonomies, and in this paper we use to ones presented in the Roadmap for National Action on CDS13 and in guidebooks noted above. Of note, deployed interventions may consist of various combinations of these individual intervention format elements. For example, reference information may be provided within an order set, or alerts may link to orderable items.
Just as you wouldn’t build a house with only hammer, it is important to consider the full portfolio of CDS intervention types in addressing specific CDS objectives. Typically, organizations deploying CDS will focus very heavily on interruptive alerts—especially to physicians via CPOE. Although alerts can be a powerful CDS intervention, they tend to be used excessively and inappropriately, resulting in commonly reported CDS problems such as alert overrides14 and physician frustration and backlash.
A more nuanced and effective approach requires careful assessment of the role for each of the potentially applicable CDS intervention types (as well the other four CDS rights) in addressing the specific objectives at hand.
Right channel
The next CDS right addresses the communication channel through which the right information will hopefully be conveyed to the right person in the right format. Because it is the starting point for many important healthcare actions—and a powerful opportunity to provide CDS interventions such as alerts and order sets—the CPOE channel is often a heavy focus for CDS efforts.
Although this intense focus is warranted, it shouldn’t come at the expense of other important channels for conveying CDS interventions. These may include other clinical applications, such as eMAR, EHRs, PHRs (and other at-home applications and devices used by patients), departmental systems, etc., but also handheld devices, e-mail/Internet, pagers, text messaging, etc.
It is important to keep in mind that not all CDS intervention formats can be presented or are available in all potential communication channels. Nonetheless, the full spectrum of available channels should be considered in mapping out combinations of CDS five rights to achieve a particular objective. When dealing with patient information it is important to keep in mind the confidentiality issues one may have with certain channels.
Right point in the workflow
A rich understanding of the workflow that a planned CDS intervention is designed to support is absolutely essential for intervention success—but often overlooked. These workflow details not only provide most of the information needed to flesh out the other four of the CDS five rights, they also provide must-have clues to intervention configuration, deployment and support features. Below are some important details on workflow/process mapping—a tool to help implementers address the ‘right point in workflow’ step.
Workflow/process mapping is a method by which activities pertinent to a specific objective are identified and represented in a visual manner. In the medication-management cycle, the maps depict how information and tasks flow from one stakeholder to another. They help to identify how data originate and are reused and highlight potential areas of vulnerability and improvement related to outcomes, such as care quality, safety, and efficiency. Gathering objective data (for example, based on directly observing care activities) is an important foundation for understanding steps participants actually take in a medication management activity.
Techniques for gathering the data that underpin creation of workflow maps include structured interviews and observations. Include structured interviews and direct observation. Structured interviews involve conducting team sessions or one-on-one interviews to identify individual tasks performed by each person in the clinical process. Asking about other participants in tasks related to key medication use processes that are CDS targets can help ensure that everyone in the clinical process is included. As an example, pharmacists play a central role in processing of medication orders and are a logical starting point for analyzing this task.
It is important at some point to directly observe all the participants involved in a particular clinical task and document workflow and information flow related to the improvement target at hand. The method can also include discussion with those being observed to address observer questions that arise about workflow activities and issues. If you have conducted interviews prior to direct observation, remain alert for any differences between results gleaned from the two methods.
Interviews and observations should complement each other in creating an accurate “before picture’ into which CDS-enabled improvement will be introduced. Smoothing out an identified inconsistencies or rough spots prior to CDS launch can help increase intervention success.
PUTTING THE CDS FIVE RIGHTS TOGETHER
To demonstrate how one could put the CDS five rights together to address a particular objective, we present an example of how one organization approached CDS to support renal dosing for Enoxaparin, a drug commonly used for DVT/PE prophylaxis. CDS is important in this setting because Enoxaparin can be very dangerous if not given in a dosage appropriate for a patient’s kidney function.
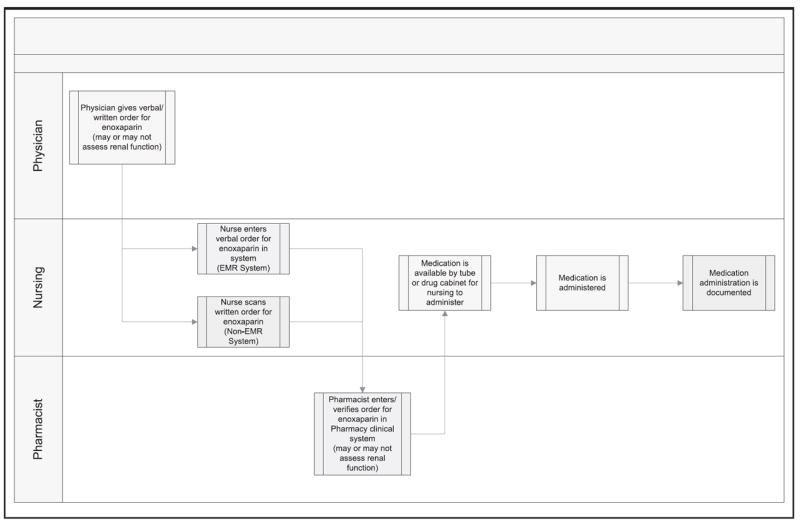
With the help of workflow mapping the following clinical stakeholders and their clinical processes were identified:
The physicians may not be considering renal function (typically assessed based on the patient’s creatinine clearance or CrCl) prior to ordering Enoxaparin.
The pharmacists are primarily responsible for evaluating renal function prior to dispensing Enoxaparin, but obtaining this information requires a manual, inefficient process prone to errors of omission.
Nursing staff may or may not pay attention to the CrCl before administration of the medication.
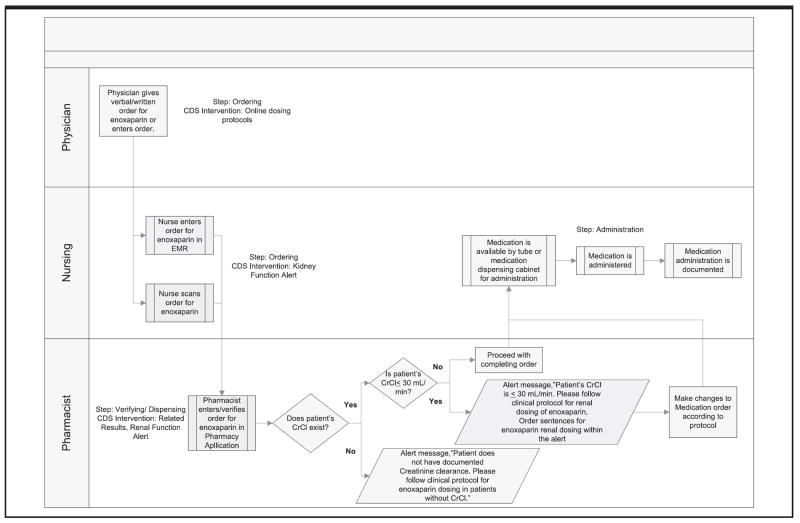
This is represented in Figure 2.
Fig. 2.
Workflow mapping clinical stakeholders and their clinical processes. [Reprinted with permission, Memorial Hermann Healthcare System]
As a result of the workflow analysis, the following CDS five rights were delineated:
Right information: the patient’s creatinine clearance is a critical yet often overlooked piece of information needed by the clinicians to ensure appropriate dosing
Right person: After discussion with the key stakeholders of the process, it was decided that the pharmacists would be the gatekeeper for the appropriate dosing of Enoxaparin
- Right intervention format:
- The physicians would like to see the adjusted dose for Enoxaparin embedded within the context of order sentences.
- The pharmacists would like to be able to view the CrCl seamlessly in the medication verification process (relevant data display) and would like to receive an alert when ordering a normal dosage for a patient with a reduced CrCl or if the baseline CrCl has not be determined.
- The nursing staff was comfortable having the physicians and pharmacists play the key roles in the dosage of the medication.
Right channel: Since the physicians were using a combination of paper and electronic order entry, the right channel consists of order sentences in a paper format or in the context of CPOE. Because the pharmacists’ workflows were entirely electronic, in their case, the right channel is the pharmacy system.
Right point in workflow: For the physicians, the right point in workflow is at the ordering step. For the pharmacists, the right point in workflow is at the point of verification/dispensing.
The ideal future state workflow is depicted in Figure 3. This example was borrowed from an organization that is in the process of implementing this new CDS-supported workflow.
Fig. 3.
The ideal future state workflow. [Reprinted with permission, Memorial Hermann Healthcare System]
OTHER PEARLS FOR SUCCESSFUL CDS PROGRAMS
The CDS Five Rights approach outlined above speaks to setting up one or more CDS interventions to address particular performance improvement objectives. This work unfolds in the context of broader organizational programs focused on CDS. The CDS guide on which this paper is based speaks extensively to success factors in setting up these programs, as well in individual interventions.
Below is a brief overview of selected best practices for setting up a successful CDS program to address medication use, and other priority targets as well. Of note, many of these lessons for CDS resonate strongly with best practices that have been defined more broadly for HIT, such as EHR and CPOE implementation. Readers interested in exploring these synergies further might begin by examining application criteria for the HIMSS Davies Awards of Excellence, established to recognize best practices for EHR implementation and derived value (www.himss.org/davies).
Establish a solid foundation for CDS efforts
CDS interventions and related activities are a means to an end, and successful programs begin with a shared understanding among key stakeholders about both the ends and (at least at a high level) the means. Solid governance structures and operating mechanisms are invaluable in building consensus around the goals of greatest mutual interest to key stakeholders (e.g., medical staff and hospital administration). They likewise play a central role in creating a shared understanding of basic concepts and approaches (e.g., the CDS five rights) and of improvement and intervention opportunities and strategies. Another key foundation component is quantifying baseline performance levels around the targets to be addressed with CDS—and producing some commitment to targeted benefits. This enables improvements to be tracked quantitatively against pre-set goals, which both justifies CDS investments to the organization, and provides a springboard for further program enhancements.
In each of these activities, it is important to engage all the pertinent stakeholders involved in the clinical and related processes, and gain their commitment to ensuring that the organizational performance improvements imperatives are realized. Successful organizations form a high-profile CDS steering committee populated with committed key leaders; for example from informatics (e.g., chief medical information officer), quality/patient safety, pharmacy, nursing, practicing physicians/thought leaders and other key functional areas. This CDS governance should be aligned with the organization’s leadership structure; for example, through formal connections with committees on EHR Oversight, Quality/Patient Safety, Medical Executive, and others.
Deploy CDS for maximum acceptance
Do CDS with users and not to them. This approach is a hallmark of the most successful CDS programs, and many CDS implementation disasters are the result of the opposite approach. When end-users are involved in developing CDS interventions that become tools that can help them achieve their important goals (or are at least aligned in some way with them), the resulting interventions will be much more likely to be used and useful.
Consider the full spectrum of potential CDS stakeholders and interventions. Observe and document relevant workflow before and after interventions to help optimize intervention acceptance and drive inevitable post-launch modifications. Prior to and surrounding intervention launch, communicate with and educate the affected users. This includes helping them understand how the CDS interventions will impact their daily activities and how they can provide feedback to implementers. Provide on-site support initially as needed to help with any immediate issues that may occur.
Devote adequate attention and resources to measurement
A well accepted adage of performance improvement is that you can’t improve what you can’t measure. Nonetheless, many CDS programs do not devote adequate attention to measuring intervention effects—both intended and unintended – and thereby miss important improvement opportunities. As noted above, a foundation for CDS efforts is establishing performance baselines and targeted improvement levels, so measurement starts well before intervention deployment. Intervention development should include formal planning for when and how intervention effects will be monitored to assess their effects. Issues to track for CDS interventions include structural measures (e.g., what interventions have we deployed?); processes measures (e.g., how are these interventions affecting decisions and workflow?); and outcome measures (e.g., to what extend are the interventions helping achieve the organization’s care quality, safety and efficacy goals?).
To the extent the goals targeted by the CDS program align with organizational priorities (such as increasing performance in core measures or decreasing “never events”), resources should be available for CDS-related process measurements as a component of the broader efforts to address those priorities. Likewise, broader organizational mechanisms for measuring and reporting outcome performance against targets can be leveraged for assessing results from the CDS component of these efforts.
Manage knowledge asset and decisions proactively
As an organization’s CDS program evolves over time, there is an increasing volume of knowledge assets—e.g. CDS interventions such as order sets, rules, referential content, etc. – as well as decisions related to those assets—e.g., why CDS targets were selected, how interventions were configured, etc. It is therefore essential to proactively manage these; for example, to ensure that the currency, consistency, evidence base and value of CDS knowledge assets remains optimized over time.
Since this type of CDS knowledge management is relatively early in its evolution, there aren’t inexpensive, robust, off-the-shelf tools to support these activities. Pioneering organizations use various online collaboration tools, web-based knowledge portals, and related mechanisms to help keep track of CDS assets and decisions related to them. In any case, a systematic, proactive approach to knowledge management will help organizations avoid a tangled, inconsistent mass of interventions, and potentially save significant time and energy as the program grows and matures.
CONCLUSION
The stakes are high and growing for successful CDS focused on medication management. For example, in addition to increasing pressures from many sectors to increase care quality, safety and efficiency, significant financial incentives—such as those associated with meaningful EHR use under ARRA—create significant incentives for effective CDS use. CDS implementers, experts and other stakeholders are coming together to synthesize best practices to increase the efficiency and effectiveness CDS deployments in general, and for those focused on medication management more specifically.
The CDS five rights approach provides a framework for successfully linking CDS intervention features with specific objectives the interventions are intended to address. This paper provides an overview of these CDS five rights with the hope that implementers and others involved in CDS will find them valuable in their efforts. In addition, other success factors in developing
CDS programs focused on improving priority goals such as medication use are presented. These include establishing a foundation for CDS, deploying CDS for maximum acceptance, measuring CDS effects and proactively managing CDS knowledge assets and decisions.
Readers are encouraged to participate in ongoing collaborative processes to develop, disseminate and improve the successful application of CDS to improving outcomes. HIMSS is one organization facilitating such collaborations—keep an eye on www.himss.org/cdsguide for further information.
Table 1.
Different CDS intervention formats. [Reprinted with permission, HIMSS]
| Format Type | Examples | Useful Tips |
|---|---|---|
| Relevant Data Presentation | Flowsheets, Surveillance | Simply providing the user with appropriate information prior to a decision can often address needs, and may be the preferred intervention in these instances. |
| Order Creation Facilitators | Order Sentences, Order Sets | Order sets are particularly effective when there is a focused problem or condition being addressed with orders, for example, admitting a patient to the hospital with pneumonia. |
| Reference Information | Infobuttons, Web links | Consider providing at points in the workflow and clinical applications (e.g. problem and medication list review) for which users may seek reference information to guide decisions |
| Unsolicited Alerts | Interruptive Warnings | These should generally be the intervention of last resort, used as a safety net when other ‘upstream’ CDS intervention types have failed to produce (or avoid) a specific action. |
| Documentation Templates | Patient History, Visit Note | Like order sets, are particularly effective when there are clearly defined clinical documentation tasks and needs. |
| Protocol Support | Pathways | Helpful when there are formal policies guiding coordinated care team activities. For example, managing patients who are post-operative from hip replacement surgery |
Acknowledgments
The authors wish to acknowledge Loius Penrod, MD who is director of Clinical Decision Support for the University of Pittsburgh Medical Center eRecord project for his valuable feedback in reviewing this manuscript. JHIM
Contributor Information
Anwar M Sirajuddin, Clinical Informaticist whose primary focus is implementation of CDS to drive quality and patient safety at Memorial Hermann Healthcare System in Houston.
Jerome A. Osheroff, Chief Clinical Informatics Officer for the Healthcare business of Thomson Reuters, and clinical faculty and staff at the University of Pennsylvania Health System.
Dean F. Sittig, University of Texas School of Health Information Sciences. His research centers on the design, development, implementation, and evaluation of clinical information systems.
John Chuo, Neonatal Quality Informatics Officer and a practicing Neonatologist at the Children’s Hospital of Philadelphia. His primary focus is to implement a quality improvement framework to drive and sustain a quality improvement and patient safety culture for CHOP’s neonatal network.
Ferdinand Velasco, Chief Medical Information Officer for Texas Health Resources.
David A. Collins, Healthcare Information Systems, for HIMSS, overseeing the Davies Awards of Excellence and the Patient Safety & Quality Outcomes Steering Committee.
References
- 1.Institute of Medicine. Preventing Medication Errors, Quality Chasm Series. IOM. 2007 [Google Scholar]
- 2.Osheroff JA, Pifer EA, Teich JM, et al. Improving Outcomes with Clinical Decision Support: An Implementer’s Guide. HIMSS; Chicago: 2005. [Google Scholar]
- 3.Teich JM, Merchia PR, Schmiz JL, et al. Effects of computerized physician order entry on prescribing practices. Arch Intern Med. 2000;160(18):2741–2747. doi: 10.1001/archinte.160.18.2741. [DOI] [PubMed] [Google Scholar]
- 4.Evans RS, Classes DC, Pestotnik SL, et al. Improving empiric antibiotic selection using computer decision support. Arch Intern Med. 1994;154(8):878–884. [PubMed] [Google Scholar]
- 5.Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF. High rates of adverse drug events in a highly computerized hospital. Arch Intern Med. 2005;165:1111–1116. doi: 10.1001/archinte.165.10.1111. [DOI] [PubMed] [Google Scholar]
- 6.Osheroff JA. Improving Medication Use and Outcomes with Clinical Decision Support: A Step by Step Guide. HIMSS; Chicago: 2009. [Google Scholar]
- 7.Federico F. The five rights of medication administration. Institute for Healthcare Improvement. [online]. Available at: www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/ImprovementStories/FiveRightsofMedicationAdministration.htm.
- 8.Shah NR, Seger AC, Seger DL, Fiskio JM, Kuperman GJ, Blumenfeld B, Recklet EG, Bates DW, Gandhi TK. Improving acceptance of computerized prescribing alerts in ambulatory care. JAMIA. 2006;13(1):5–11. doi: 10.1197/jamia.M1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatient with renal insufficiency. JAMA. 2001;286(22):2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim J, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, Tang PC. Clinical decision support systems for the practice of evidence-based medicine. JAMIA. 2001;8(6):527–34. doi: 10.1136/jamia.2001.0080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein A, Simon SR, Schneider J, Krall M, Laferriere D, Smith DH, Sittig DF, Soumerai SB. How to design computerized alerts to safe prescribing practices. Jt Comm J Qual Saf. 2004;30(11):602–13. doi: 10.1016/s1549-3741(04)30071-7. [DOI] [PubMed] [Google Scholar]
- 13.Osheroff JA, Teich JM, Middleton B, et al. A roadmap for national action on clinical decision support. JAMIA. 2007;14(2):141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sittig D, Wright A, Osheroff J, Middleton B, Teich J, Ash A, Campbell E, Bates D. Grand challenges in clinical decision support. J Bio Inform. 2008;41:387–392. doi: 10.1016/j.jbi.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]