Abstract
The gene expression of human brain microvascular endothelial cells (HBMEC) in response to 4 h of infection by Listeria monocytogenes was analyzed. Four hours after infection, the expression of 456 genes of HBMEC had changed (p < 0.05). We noted that many active genes were involved in the formyl-methionyl-leucyl-phenylalanine pathway in infected HBMEC. In the upregulated genes, mRNA levels of interleukin-8 and interleukin-15 in infected cells increased according to microarray and real-time reverse transcription – PCR analyses. Since both cytokines are regarded as potent chemotactic factors, the results suggest that HBMEC are capable of recruiting cells of innate and adaptive immune responses during early L. monocytogenes infection.
Keywords: Listeria, HBMEC, gene expression, microarray, real-time PCR, central nervous system
Listeria monocytogenes is a facultative intracellular bacterium that can lead to fatal infection in humans if it invades the central nervous system (CNS). Human listeriosis infection of the CNS can manifest in many ways, including meningitis, rhombencephalitis, and choriomeningitis (Gray and Killinger 1966). Direct invasion of vascular endothelial cells by L. monocytogenes is one of the major mechanisms for CNS infection (Drevets et al. 2004). The survival of the invading pathogen depends on its virulence and the responses of the host cells to the pathogen. During the past decade, significant progress has been made to elucidate the host cell responses to L. monocytogenes (Cohen et al. 2000; Rose et al. 2001; Baldwin et al. 2002; Schmeck et al. 2006). The varied responses by different types of host cells to L. monocytogenes indicates the dynamic nature of host–pathogen interactions with some degree of specificity. Cohen et al. (2000) used oligonucleotide arrays to study the interaction between human promyelocytic THP-1 cells and L. monocytogenes EGD. They reported 74 upregulated and 23 downregulated host cell genes attributed to listerial infection. On the other hand, Rose et al. (2001) found that human umbilical vein endothelial cells respond by increasing nitric oxide synthesis and by generating proinflammatory cytokines and upregulating the cytokines interleukin (IL)-6, IL-8, and granulocyte-macrophage colony-stimulating factor. The typical primary site for CNS infection is a layer of human brain microvascular endothelial cells (HBMEC), part of the blood–brain barrier (Betz 1985). It is known that the virulence factors of L. monocytogenes facilitate the invasion of HBMEC (Greiffenberg et al. 1998); however, little is known about how HBMEC responds to the infection. In this study, we used a whole-genome microarray to identify the transcriptome changes of HBMEC in response to in vitro invasion by L. monocytogenes, and we validated a subset of the affected genes using real-time reverse transcription – PCR (RRT–PCR).
HBMEC (Cell Systems, Kirkland, Washington) were cultured in CSC-Complete medium (Cell Systems) at 37 °C with 5% CO2, according to the manufacturer's instructions. To control the consistency of the experiments, passage 12 HBMEC cells were used throughout the entire study. When HBMEC reached 70% confluence, the cells were inoculated with 100:1 multiplicity of infection (MOI) of L. monocytogenes F2365 serovar 4b, which is known to cause CNS infections in humans. Forty-five minutes after infection, the infected cells were treated with gentamicin (50 μg/mL) to kill the extracellular bacteria (Greiffenberg et al. 1998). For a control group, HBMEC was not infected with Listeria but was treated with gentamicin. Immediately following the gentamicin treatment, we incubated both cell groups for an additional 3 h and 15 min. RNA (Qiagen, Valencia, California) was later added to stabilize the integrity of mRNA. We then extracted cellular RNA using an RNeasy mini kit from Qiagen by following the manufacturer's instructions.
A human whole-genome microarray (Amersham Codelink, Piscataway, New Jersey) that targets ~55 000 transcripts and expressed sequence tags was used to determine the differential gene expression between infected and uninfected cells. Five and four biological replicates were used for infected and uninfected cells, respectively. RNA was extracted from the pelleted cells and primed for reverse transcription by a DNA oligonucleotide containing the T7 RNA polymerase promoter 5′ to a d(T)24 sequence. After second-strand cDNA synthesis, we used cDNA as the template for an in vitro transcription reaction to produce the target cRNA in the presence of biotinylated nucleotides to label the target RNA. We then performed hybridization overnight and stained with streptavidin-Cy5 conjugate. We scanned the microarray with an Axon GenePix Scanner and analyzed it with Axon Acuity 3.0 analysis software. Data were further evaluated through bioinformatics and statistical analysis.
Image analysis and data acquisition from the microarray were performed using CodeLink Expression Analysis version 4.1 software (Wu et al. 2005). Functional annotation and classification of selected genes were performed using the public annotation information provided at the NCBI databases and the DAVID system (Dennis et al. 2003). Statistically enriched genes belonging to particular functional categories and molecular interaction pathways were identified using gene ontology (Ashburner et al. 2000).
To validate the microarray-derived data, RRT–PCR was performed in a Bio-Rad iCycler (Hercules, California). Cellular RNA used in RRT–PCR was harvested independently from that used in the microarray experiments. Three biological replicates were performed. Both cDNA synthesis and RRT–PCR amplification were carried out by using the Super-Script III Platinum Two-Step qRT-PCR kit (Invitrogen), according to the manufacturer's instructions. For internal standard, β-actin (BACT) mRNA was used to normalize each sample. The data were analyzed using Optical System software version 3.1 (BioRad) and presented as the ratios of target mRNA to BACT mRNA. All the mRNA samples from the three biological repeats were prepared in two experimental replicates and presented in the form of mean fold change ± standard error compared with control cell groups. A Student's t test was used for statistical analysis, with a significance level of p < 0.05. Primers of each target mRNA for amplifications were designed using the Invitrogen D-LUX Designer software. The sequences of the IL-8 primers were 5′-CTCTCTTGGCAGCCTTCCTGAT-3′ (sense) and 5′-CGCCTTTACAATAATTTCTGTGTTGGCG-3′ (antisense), with an expected product of 188 bp. The sequences of the IL-15 primers were 5′-CGAAATGGCTTTGAGTAATGAGAATTTCG-3′ (sense) and 5′-TTAGGAAGCCCTGCACTGAAAC-3′ (antisense), with an expected product of 151 bp. The sequences of the GNB1 primers were 5′-CGTAACATGAGTGAGCTTGACCAGTTACG-3′ (sense) and 5′-GGCGTCTCGAATCTGGTTCTTA-3′ (antisense), with an expected product of 69 bp. The BACT primers were directly purchased from Invitrogen (catalogue No. 101H-01).
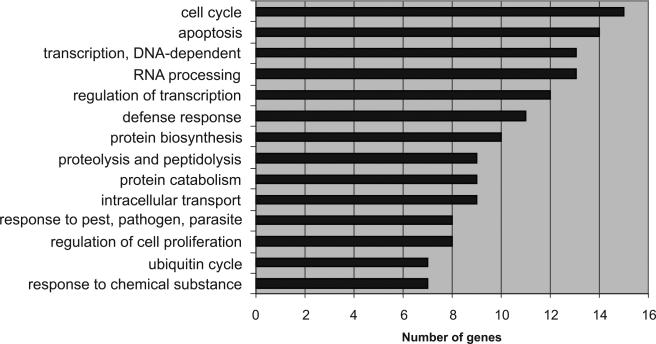
Host transcriptional responses of HBMEC infected with L. monocytogenes 4b F2365 at 4 h postinfection were determined using a human whole-genome microarray consisting of 55K gene probes. Based on Cyber-T algorithm analysis (Baldi and Long 2001), 456 genes changed significantly in response to the bacterial infection. Based on gene ontology analysis, 310 genes were upregulated. Most of the upregulated genes were involved in cell cycles, apoptosis or antiapoptosis (mostly antiapoptosis at this stage), transcription, protein synthesis, and defense responses in reactions to pathogens and chemicals (Fig. 1). In contrast, most of the 146 downregulated genes were involved in apoptosis or antiapoptosis, drug resistance, cytoskeleton-associated organelle transport, ion transport, cell arrest and DNA damage repair, host defense system, brain cell adhesion and differentiation, cell–cell interaction, genomic stability, and oxidative stress response (Fig. 2). The suppression of these activities provides evidence that the basic functions of HBMEC were damaged by the listerial infection.
Fig. 1.
Gene ontology analysis showing the functional categories with at least seven genes upregulated.
Fig. 2.
Gene ontology analysis showing the functional categories with at least seven genes downregulated.
Gene set enrichment analysis and the molecular signature database (Subramanian et al. 2005) were further used to identify all active genes that are associated with specific pathways in response to bacterial infection. The microarray data was transformed into 18 126 unique genes, each characterized using the modified T static obtained from the Cyber-T program. Through this program, 32 pathways (many with overlapping genes) were relatively enhanced with at least 25 induced genes having a score of >0.50. This score was a weighted Kolmogorov–Smirnov-like statistic that measured deviation from a random (normal) distribution. Among these pathways, the N-formyl-methionine-leucine-phenylalanine (fMLP) pathway (a potent mediator for innate inflammatory reactions) received the highest score of 0.648, indicating that it was the most active pathway.
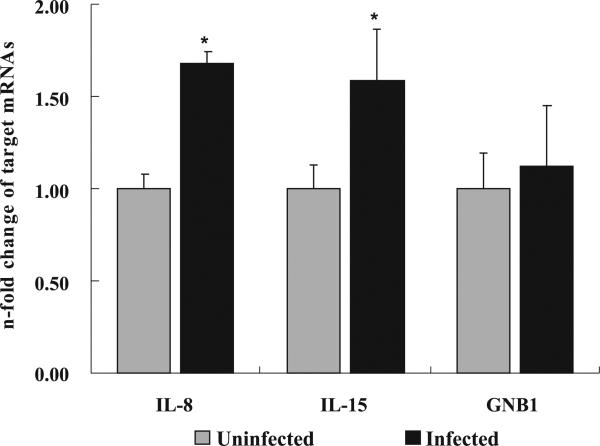
To verify the microarray transcription data, the RRT–PCR with three biological and two experimental replicates was performed. After normalizing the expression level with an internal control housekeeping gene, BACT, the relative mRNA expression levels between the infected and noninfected HBMEC groups of tested genes were calculated using the comparative threshold cycle method. Two proinflammatory-related cytokine genes, IL-8 and IL-15, which were upregulated in the microarray assays, were further confirmed using the RRT–PCR assay; the test samples were independent for each assay. To further test the consistency between the microarray and RRT–PCR results, the GNB1 gene, which was not altered in the microarray assay, was also tested using RRT–PCR. As expected, the result showed the same expression level between uninfected and infected cells. IL-8 and IL-15 mRNA levels rose significantly in infected HBMEC compared with noninfected cells. The mRNA relative expression levels of IL-8 to BACT showed a 1.7-fold elevation (p < 0.05) at 4 h postinfection, IL-15 to BACT showed a 1.6-fold increase (p < 0.05), and GNB1 to BACT exhibited almost no change (p > 0.05) (Fig. 3).
Fig. 3.
Interleukin-8 (IL-8), interleukin-15 (IL-15), and GNB1 in human brain microvascular endothelial cells (HBMEC) infected with Listeria monocytogenes relative to that in uninfected HBMEC (control). Data are expressed as n-fold change compared with values obtained for control HBMEC. Samples were analyzed in duplicate from three independent replications, and data are expressed as mean fold change ± SE. The asterisk (*) indicates a significant difference between uninfected and infected HBMEC, at a p value of <0.05.
HBMEC response to L. monocytogenes invasion showed that 310 genes upregulated and 146 downregulated at 4 h postinfection. To the best of our knowledge, these findings represent the first study delineating global changes in gene expression for human CNS endothelial cells in response to L. monocytogenes infection. The gene expression profiles indicate that there is a large scale of reprogrammed gene expression combating this intracellular bacterium. The results suggest that gene transcription in HBMEC was more activated than suppressed with infection by pathogenic L. monocytogenes 4b. This disproportionate ratio between upregulated and downregulated genes of host cell responses to pathogens was consistent with other studies (Ichikawa et al. 2000; Doran et al. 2003). However, the magnitude of mRNA expression changes in HBMEC was relatively lower than those observed in Caco-2 and THP-1 cells in response to L. monocytogenes infection (Cohen et al. 2000; Baldwin et al. 2002). In our microarray data, there were statistically significant 1.5- and 1.3-fold elevations of IL-8 and IL-15 mRNA, respectively, whereas, 1.7- and 1.6-fold changes were observed in the RRT–PCR assay. Despite the use of an MOI of 10:1 to infect macrophages and intestinal cells with L. monocytogenes, an MOI at 100:1 was much better than MOI at 10:1 to attach or infect HBMEC endothelial cells based on confocal images (data not shown). The relative lower gene expression levels might be associated with the cell type (macrophages versus endothelial cells), infection stage, and (or) MOI.
Among the 32 enhanced pathways that responded to the infection, the fMLP signaling pathway was shown to be most activated. As the fMLP pathway became activated, chemotactic cytokines were expressed, resulting in activation of neutrophils and migration to the inflammatory site for the destruction of the pathogens (Heit et al. 2002; Crossley 2003). This pathway is considered an essential part of the host innate immune system. Gao and his colleagues (Gao et al. 1999) demonstrated that mice lacking an fMLP receptor suffered from impaired host defense against bacterial infection. The mice lacking the fMLP receptor not only had higher susceptibility to L. monocytogenes but also a higher mortality rate. From these data, we speculate that the fMLP pathway may be an important immunological response for the host to defend invasion of the CNS by L. monocytogenes.
IL-8 is known to be induced by human polymorphonuclear leukocytes when stimulated by the chemoattractant fMLP (Cassatella et al. 1992). During the bacterial infection, IL-8 served as a proinflammatory cytokine that attracted neutrophils and T-lymphocytes at the inflammatory site; therefore, it was regarded as essential for both early innate and adaptive T-cell immune responses (Conlan and North 1994). The reaction in HBMEC in our study concurs with the findings of Berche et al. (1987) who described an induction of T-cell-mediated immunity during intracellular growth of L. monocytogenes. The upregulation of the IL-8 gene appears to be a common event in other host cell responses to listerial infection (Cohen et al. 2000; Schmeck et al. 2006). Previous studies have also shown that IL-8 upregulates in HBMEC when stimulated by vascular endothelial growth factor to facilitate neutrophil transendothelial migration (Lee et al. 2002). Our microarray and RRT–PCR assays confirmed the induction of IL-8 by around 1.6-fold in HBMEC at 4 h postinfection. Similar results have been reported for human umbilical vein endothelial cells (Opitz et al. 2006; Schmeck et al. 2006), although IL-8 can elevate dramatically to 50-fold in human promyelocytic THP-1 cells (Cohen et al. 2000). This is the first report that confirmed the induction of IL-8 in HBMEC in response to L. monocytogenes infection.
Another cytokine, IL-15, increased notably. This proinflammatory cytokine plays an essential role in both host innate and adaptive immune systems by stimulating the proliferation of NK cells (French et al. 2006) as well as recruiting and stimulating growth of T-lymphocytes (Wilkinson and Liew 1995). Although the expression of IL-15 has been found in several types of cells, including unstimulated human umbilical vein endothelial cells (Nilsen et al. 1998; Stone et al. 2010), the elevation of IL-15 mRNA in stimulated human brain microvascular endothelial cells by L. monocytogenes has not been reported elsewhere. Previous research has shown that endogenous IL-15 helps to trigger NK cells and stimulate their adhesion to vascular endothelial inflammatory sites to kills cells infected with L. monocytogenes (Allavena et al. 1997). Our finding is similar to a study by Mitani et al. (1999) that reported that production of IL-15 can be found in stimulated intestinal intraepithelial lymphocytes by L. monocytogenes at an early stage of the infection.
G-proteins that contain an α-subunit and βγ-complex (including GNB1 and GNGT1) are known to couple with the human formyl peptide receptor (FPR) (Birnbaumer et al. 1990). FPR is a chemoattractant receptor in host cells (Lee et al. 2002). After bacteria activate the receptor, the fMLP pathway of host cells becomes activated. Numerous studies have noted that FPR plays a role not only in bacterial host defense but also in inflammatory reactions (Lee et al. 2002). During the activation of FPR, the βγ-complex dissociates from the α-subunit simultaneously. Previous studies have shown that after the dissociation, the βγ-complex activates phospholipase C-β and phosphoinositide 3-kinase-γ, which are crucial for downstream fMLP pathway function. The complex will reassemble and finish the G-protein cycle (Seifert and Wenzel-Seifert 2003). In our microarray study, the gene GNB1 did not show an upregulated pattern after the bacterial infection, which was confirmed by the RRT–PCR assay. This lack of upregulation may be due to the fact that GNB1 underwent dissociation–reassociation cycling without being overexpressed.
HBMEC are the first line of defense against CNS infection. This is the first report that confirmed upregulation of IL-8 and IL-15 in HBMEC in response to L. monocytogenes infection. Activating the fMLP pathway and generating certain proinflammatory cytokines, such as IL-8 and IL-15, in response to the bacterial intracellular invasion may be an important barrier for the endothelium. Since IL-8 and IL-15 activate and recruit neutrophils, NK cells, and T-cells, such responses suggest that HBMEC is capable of recruiting cells of innate and adaptive immune response during an early stage of L. monocytogenes infection.
Acknowledgement
This work was supported by grants from the United States Department of Agriculture – Agricultural Research Service (USDA/ARS) No. 58-6202-5-083 and the Mississippi Agriculture and Forestry Experiment Station Contribution No. J-11442. The authors are grateful to Jerald Ainsworth, Todd Parr, and Stephen Pruett for their review of the manuscript, and to Sang-Ryul Lee for technical assistance.
Contributor Information
Chinling Wang, Department of Basic Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA..
Chung-Hsi Chou, School of Veterinary Medicine, National Taiwan University, Taipei, Taiwan..
Charles Tseng, Department of Biological Sciences, Purdue University Calumet, Hammond, IN 46323-2094, USA..
Xijin Ge, Department of Mathematics and Statistics, South Dakota State University, Brookings, SD 57007, USA..
Lesya M. Pinchuk, Department of Basic Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA.
References
- Allavena P, Giardina G, Bianchi G, Mantovani A. IL-15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J. Leukoc. Biol. 1997;61(6):729–735. doi: 10.1002/jlb.61.6.729. PMID:9201264. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25(1):25–29. doi: 10.1038/75556. doi:10.1038/75556. PMID:10802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17(6):509–519. doi: 10.1093/bioinformatics/17.6.509. doi:10.1093/bioinformatics/17.6.509. PMID:11395427. [DOI] [PubMed] [Google Scholar]
- Baldwin DN, Vanchinathan V, Brown PO, Theriot JA. A gene-expression program reflecting the innate immune response of cultured intestinal epithelial cells to infection by Listeria monocytogenes. Genome Biol. 2002;4(1):R2.1–R2.14. doi: 10.1186/gb-2002-4-1-r2. doi:10.1186/gb-2002-4-1-r2. PMID:12537547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P, Gaillard JL, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J. Immunol. 1987;138(7):2266–2271. PMID:3104455. [PubMed] [Google Scholar]
- Betz AL. Epithelial properties of brain capillary endothelium. Fed. Proc. 1985;44(10):2614–2615. PMID:2989011. [PubMed] [Google Scholar]
- Birnbaumer L, Abramowitz J, Brown AM. Receptor–effector coupling by G proteins. Biochim. Biophys. Acta. 1990;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. PMID:2160274. [DOI] [PubMed] [Google Scholar]
- Cassatella MA, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorpho-nuclear leukocytes. The chemoattractant formyl-methionyl-leucylphenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J. Immunol. 1992;148(10):3216–3220. PMID:1578146. [PubMed] [Google Scholar]
- Cohen P, Bouaboula M, Bellis M, Baron V, Jbilo O, Poinot-Chazel C, et al. Monitoring cellular responses to Listeria monocytogenes with oligonucleotide arrays. J. Biol. Chem. 2000;275(15):11 181–11 190. doi: 10.1074/jbc.275.15.11181. doi:10.1074/jbc.275.15.11181. PMID:10753925. [DOI] [PubMed] [Google Scholar]
- Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 1994;179(1):259–268. doi: 10.1084/jem.179.1.259. doi:10.1084/jem.179.1.259. PMID:8270870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley LJ. Neutrophil activation by fMLP regulates FOXO (forkhead) transcription factors by multiple pathways, one of which includes the binding of FOXO to the survival factor Mcl-1. J. Leukoc. Biol. 2003;74(4):583–592. doi: 10.1189/jlb.0103020. doi:10.1189/jlb.0103020. PMID:12960271. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. doi:10.1186/gb-2003-4-5-p3. PMID:12734009. [PubMed] [Google Scholar]
- Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Invest. 2003;112(5):736–744. doi: 10.1172/JCI17335. PMID:12952922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets DA, Leenen PJ, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 2004;17(2):323–347. doi: 10.1128/CMR.17.2.323-347.2004. doi:10.1128/CMR.17.2.323-347.2004. PMID:15084504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35(5–6):229–234. doi: 10.1016/j.cyto.2006.08.006. doi:10.1016/j.cyto.2006.08.006. PMID:17052916. [DOI] [PubMed] [Google Scholar]
- Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 1999;189(4):657–662. doi: 10.1084/jem.189.4.657. doi:10.1084/jem.189.4.657. PMID:9989980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ML, Killinger AH. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 1966;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. PMID:4956900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiffenberg L, Goebel W, Kim KS, Weiglein I, Bubert A, Engelbrecht F, et al. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 1998;66(11):5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. PMID:9784531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 2002;159(1):91–102. doi: 10.1083/jcb.200202114. doi:10.1083/jcb.200202114. PMID:12370241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa JK, Norris A, Bangera MG, Geiss GK, van 't Wout AB, Bumgarner RE, Lory S. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. U.S.A. 2000;97(17):9659–9664. doi: 10.1073/pnas.160140297. doi:10.1073/pnas.160140297. PMID:10931941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J. Biol. Chem. 2002;277(12):10445–10451. doi: 10.1074/jbc.M107348200. doi:10.1074/jbc.M107348200. PMID:11784713. [DOI] [PubMed] [Google Scholar]
- Mitani A, Nishimura H, Hirose K, Washizu J, Kimura Y, Tanaka S, et al. Interleukin-15 production at the early stage after oral infection with Listeria monocytogenes in mice. Immunology. 1999;97(1):92–99. doi: 10.1046/j.1365-2567.1999.00752.x. doi:10.1046/j.1365-2567.1999.00752.x. PMID:10447719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42(5):635–642. doi: 10.1136/gut.42.5.635. doi:10.1136/gut.42.5.635. PMID:9659156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Puschel A, Beermann W, Hocke AC, Forster S, Schmeck B, et al. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J. Immunol. 2006;176(1):484–490. doi: 10.4049/jimmunol.176.1.484. PMID:16365441. [DOI] [PubMed] [Google Scholar]
- Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, et al. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect. Immun. 2001;69(2):897–905. doi: 10.1128/IAI.69.2.897-905.2001. doi:10.1128/IAI.69.2.897-905.2001. PMID:11159983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeck B, Beermann W, van Laak V, Opitz B, Hocke AC, Meixenberger K, et al. Listeria monocytogenes induced Rac1-dependent signal transduction in endothelial cells. Biochem. Pharmacol. 2006;72(11):1367–1374. doi: 10.1016/j.bcp.2006.06.033. doi:10.1016/j.bcp.2006.06.033. PMID:16884694. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. The human formyl peptide receptor as model system for constitutively active G-protein-coupled receptors. Life Sci. 2003;73(18):2263–2280. doi: 10.1016/s0024-3205(03)00654-4. doi:10.1016/S0024-3205(03)00654-4. PMID:12941430. [DOI] [PubMed] [Google Scholar]
- Stone KP, Kastin AJ, Hsuchou H, Yu C, Pan W. Rapid endocytosis of interleukin-15 by cerebral endothelia. J. Neurochem. 2010;116(4):544–553. doi: 10.1111/j.1471-4159.2010.07142.x. doi:10.1111/j.1471-4159.2010.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. doi:10.1073/pnas.0506580102. PMID:16199517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J. Exp. Med. 1995;181(3):1255–1259. doi: 10.1084/jem.181.3.1255. doi:10.1084/jem.181.3.1255. PMID:7869044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Dave N, Tseng GC, Richards T, Xing EP, Kaminski N. Comparison of normalization methods for CodeLink Bioarray data. BMC Bioinformatics. 2005;6(1):309–322. doi: 10.1186/1471-2105-6-309. doi:10.1186/1471-2105-6-309. PMID:16381608. [DOI] [PMC free article] [PubMed] [Google Scholar]