Abstract
Purpose
To evaluate factors associated with visual field (VF) progression, using all available follow-up through nine years after treatment initiation, in the Collaborative Initial Glaucoma Treatment Study (CIGTS).
Design
Longitudinal follow-up of participants enrolled in a randomized clinical trial.
Participants
607 newly diagnosed glaucoma patients.
Methods
In a randomized clinical trial, 607 subjects with newly diagnosed open-angle glaucoma were initially treated with either medication or trabeculectomy. After treatment initiation and early follow-up, subjects were evaluated clinically at 6-month intervals. Study participants in both arms of the CIGTS were treated aggressively in an effort to reduce intraocular pressure (IOP) to a level at or below a predetermined, eye-specific target pressure. VF progression was analyzed using repeated measures models.
Main outcome measures
VF progression, measured by Humphrey 24-2 full threshold testing and assessed by the change in the mean deviation (MD), and an indicator of substantial worsening of the VF (MD decrease of ≥3 dB from baseline), assessed at each follow-up visit.
Results
Follow-up indicates minimal change from baseline in each initial treatment group’s average MD. However, at the eight year follow-up examination, substantial worsening (≥3 dB) of MD from baseline was found in 21.3% and 25.5% of the initial surgery and initial medicine groups, respectively. The effect of initial treatment on subsequent VF loss was modified by time (P<0.0001), baseline MD (P=0.03), and diabetes (P=0.01). Initial surgery led to less VF progression than initial medicine in subjects with advanced VF loss at baseline, whereas subjects with diabetes had more VF loss over time if treated initially with surgery.
Conclusions
The CIGTS intervention protocol led to a lowering of IOP that persisted over time in both treatment groups. Progression in VF loss was seen in a subset increasing to over 20% of the subjects. Our findings regarding initial surgery being beneficial for subjects who present at diagnosis with more advanced VF loss, but detrimental for patients with diabetes, are noteworthy and warrant independent confirmation.
Introduction
Much has changed in the understanding of open-angle glaucoma treatment in the thirty-five years since Smith,1 in a randomized clinical trial comparing initial treatment with filtering surgery to initial treatment with medications, found that even though the surgical patients achieved lower intraocular pressure (IOP), the visual acuity (VA) and visual field (VF) results were about the same. However, Smith observed that 50% of the medically treated group required surgery within four years of the start of the study. In 1981, Watson and Grierson2 reviewed 424 trabeculectomies performed over a 10-year period and recommended earlier surgery due to its effective control of IOP and VF loss. Jay and Murray3 noted that 32% of medically treated patients needed filtering surgery within one year and that, by four years, 53% needed it. In 1994, Migdal, Gregory, and Hitchings4 published the final report of a clinical trial in which patients with newly diagnosed open-angle glaucoma were randomized to medications, argon laser trabeculoplasty (ALT), or trabeculectomy. Criteria for successful treatment included an IOP of <22 mmHg and, after 5 years of follow-up, this was achieved in 98% of the surgical eyes, in 83% of the medically treated eyes, and in 68% of the ALT-treated eyes.
The studies cited above1-4 supported filtering surgery as a reasonable and possibly preferred initial treatment for newly diagnosed open-angle glaucoma. It was assumed from these earlier studies that the lower IOP resulting from filtering surgery compared to medical treatment was the most important factor for the better preservation of VFs in the surgically treated eyes. However, the number and variety of pharmaceutical agents to treat glaucoma were considerably fewer when these studies were performed than are available today. These studies also did not establish a specific goal for IOP reduction for each eye. Instead, in nearly all cases, a single IOP value (e.g., <22 mmHg) was chosen to represent success of treatment. More recently, the Advanced Glaucoma Intervention Study (AGIS) has demonstrated that when IOP reduction from baseline is substantial, progression of VF loss can be prevented.5 Eyes with IOP <18 mmHg at every time point during at least 6 years of follow-up had essentially no further VF loss. Yet, in eyes where the IOP was <18 mmHg at fewer than half of the visits, there was a statistically significant worsening of the VF.
A recent Cochrane Library Report from the Cochrane Eyes and Vision Group6 reviewed four clinical trials that reported on this subject and noted that three of the four had concluded that initial surgery was more effective than initial medical treatment in reducing VF loss from glaucoma. The fourth trial, the Collaborative Initial Glaucoma Treatment Study (CIGTS),7 reported in its interim findings, after completion of four years of follow-up, no difference between initial treatments (topical medications or trabeculectomy) in terms of subsequent VF loss.8 One important difference, however, between CIGTS and the other three trials is that the CIGTS cohort overall had milder glaucoma at baseline than did the subjects in the other three clinical trials. Likewise, the CIGTS outcomes were termed “interim” in nature, with more definitive findings awaiting longer follow-up. The purpose of this study is to use the longer term follow-up now available on CIGTS participants to evaluate the association of initial treatment and other factors that were measured at baseline with VF loss up to nine years after treatment initiation.
Methods
The eligibility criteria, randomization procedure, and surgical and medical treatments to which patients were assigned in the CIGTS have been described.7 In brief, upon obtaining written informed consent, 607 newly diagnosed subjects with open-angle glaucoma were enrolled at 14 clinical centers from October 1993 through April 1997. All study centers obtained institutional review board approval for the study. Enrollees were randomized to receive initial treatment with topical medications (n=307) or trabeculectomy (n=300). If initial treatment failed to control IOP or if progression in VF loss was documented, argon laser trabeculoplasty (ALT) was administered. Subsequent failure to control IOP or progression in VF loss led to treatment crossover (surgery for those randomized to initial medicine, and vice versa).
The primary study outcome was progressive VF loss, which was assessed at each follow-up visit using the Humphrey full threshold 24-2 testing strategy. The measure of VF loss reported herein is the mean deviation (MD), which is the average depression (or elevation) of the measured VF compared to a normal reference VF. Its continuous nature and favorable test-retest variability were reported in Gillespie et al.9 Substantial VF loss, defined as a MD decrease of ≥3 dB compared to baseline, was also considered. The CIGTS protocol required all enrollees to have two baseline VF tests, conducted within 30 days of each other, in order to document eligibility and establish a reference for comparison to follow-up VF tests.7 This report includes only data on the “study eye” – identified prior to randomization as the first eye to be treated for each patient.
Mixed linear regression10 was used to investigate the effect of treatment and other baseline covariates on the MD outcome over follow-up from two through nine years after randomization. During the first two years of follow-up, different MD patterns by treatment group would have required complex time by treatment interaction terms. To avoid this problem, and because the primary focus of this study was on long term outcomes, we modeled MD starting at year two. Repeated measures logistic regression, using generalized estimating equations,11 was used to assess treatment differences in rates of substantial VF loss over the available follow-up after randomization. Analysis included follow-up data for years two through nine, as in the mixed linear regression model. When time-specific crude (not model-based) estimates of means and percentages are presented in the results, we limited these estimates to eight years of follow-up, when the estimates were most stable.
Given the impact that cataract has on VF and its association with trabeculectomy,12 cataract was accounted for in the model as a time dependent variable indicating a visit within one year prior to cataract surgery. While the results address variables that were significant contributors to the models, other variables that were also assessed and found un-related included clinical center, gender, education, current smoking status, alcohol intake, family history of glaucoma, type of glaucoma, pupil response, iris color, optic disc hemorrhage, corneal thickness, and systemic hypertension. In addition, we evaluated models that included VF loss at baseline within a number of specific areas (e.g., quadrants, hemifields, peripheral, and central) to determine if location of VF loss was predictive of subsequent VF progression. Since no area-specific measures of baseline VF loss approached the predictive strength of the overall baseline MD (results not shown), the overall MD was used to represent baseline VF loss in the final models. All two-way interactions with initial treatment, time, and quadratic time were also evaluated (except for center, due to the large number of resulting interactions).
To model the correlation among the repeated measurements on a person, a heterogeneous toeplitz structure was used in the mixed linear regression analysis, and a heterogenous compound symmetry structure was used in the logistic model. The repeated measures analyses were performed using SAS Proc Mixed and SAS Proc Genmod (SAS Institute, Cary, NC).
Results
Enrollees were newly diagnosed with open-angle glaucoma (OAG). Most of the diagnoses were primary OAG (550/607, 91%), with the remainder distributed almost equally between pseudoexfoliative (n=29) and pigmentary (n=28) glaucoma. Study participants’ average age was 57.5 years (range, 28 to 75 years). Whites made up the majority (337/607, 56%) and blacks were a substantial subset (231/607, 38%) of enrollees. The average IOP at the baseline examination was 27 mmHg. The average MD from VF testing was -5.5 dB, and there was evidence of glaucomatous cupping of the optic disc (cup:disc ratios in the 0.6 to 0.7 range, on average).
Follow-up over the nine year period is displayed in Table 1. Discounting deaths, study follow-up was active for 90% (524/583) of participants five years after treatment initiation. By nine years after treatment initiation, that percentage had diminished to 27% (152/565). Statistical analyses were based on outcome data obtained through nine years. A comparison of subjects who lacked follow-up versus those with follow-up at 7 or 9 years found no evidence of differential drop-out by initial treatment, age, race, sex, or baseline severity of VF loss.
Table 1.
Participant Availability
| Time | Medicine (n=307) | Surgery (n=300) | ||||
|---|---|---|---|---|---|---|
| Active (% Active*) | Cumulative Inactive | Cumulative Died | Active (% Active*) | Cumulative Inactive | Cumulative Died | |
| 1 Year | 301 (98.0 %) | 6 | 0 | 294 (98.0 %) | 6 | 0 |
| 2 Years | 293 (95.8 %) | 13 | 1 | 285 (96.0 %) | 12 | 3 |
| 3 Years | 285 (94.1 %) | 18 | 4 | 272 (92.5 %) | 22 | 6 |
| 4 Years | 276 (91.7 %) | 25 | 6 | 266 (91.7 %) | 24 | 10 |
| 5 Years | 269 (90.6 %) | 28 | 10 | 255 (89.2 %) | 31 | 14 |
| 6 Years | 255 (86.4 %) | 40 | 12 | 231 (82.2 %) | 50 | 19 |
| 7 Years | 218 (74.1 %) | 76 | 13 | 198 (71.7 %) | 78 | 24 |
| 8 Years | 153 (52.2 %) | 140 | 14 | 142 (51.8 %) | 132 | 26 |
| 9 Years | 77 (26.5 %) | 214 | 16 | 75 (27.4 %) | 199 | 26 |
Percent active is defined as the number of active patients divided by the sum of the active and inactive patients. Deaths are excluded.
Time Trends by Initial Treatment
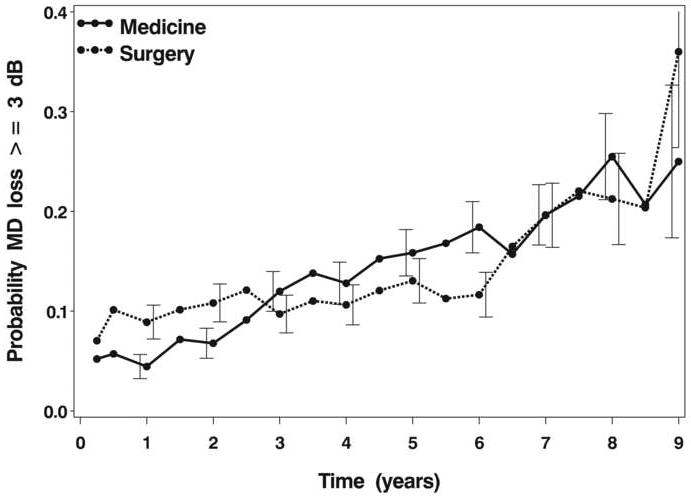
An inspection of the average MD over nine years since treatment initiation shows several trends (Figure 1A). The surgically treated group had an average MD at baseline of -5.7 dB [standard error (SE), 0.2 dB]. Five years after treatment initiation, this group’s average MD had improved minimally (mean = -5.3 dB), and at eight years it had worsened slightly to -6.3 dB. The medically treated group had an average baseline MD of -5.2 dB (SE, 0.2 dB), which worsened to -5.5 dB at five years and to -6.1 dB at eight years. Throughout the follow-up period, change from baseline in both groups’ average MD was minimal. Figure 1B shows the percentages in each group of substantial worsening from baseline (-3 dB or more) in MD over time. At five years of follow-up, 13.0% and 15.9% of surgery and medicine group subjects, respectively, showed this degree of VF loss; by eight years, these percentages were 21.3% (surgery) and 25.5% (medicine). Substantial improvement from baseline (+3 dB or more) in MD was documented in 16.1% (surgery) and 11.8% (medicine) at five years and in 12.5% (surgery) and 17.7% (medicine) after eight years of follow-up.
Figure 1.
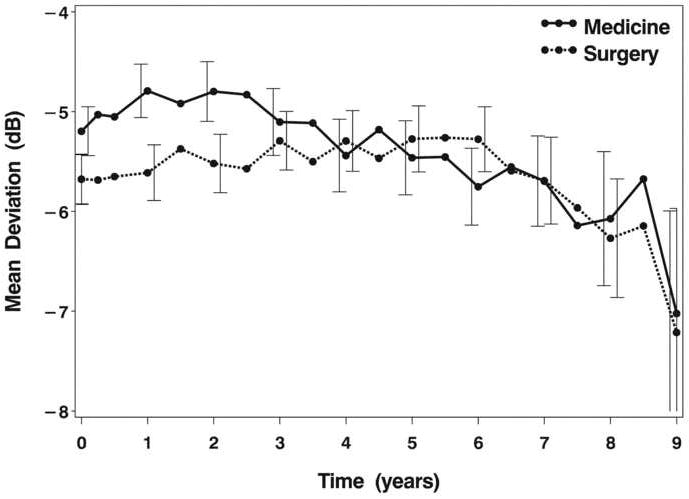
A: Mean deviation by time and treatment group. Error bars are +/- 1 standard error and are slightly offset to show the bar for each treatment more clearly.
B: Percentage of participants with a decrease (worsening) of mean deviation from baseline of 3 dB or more, by time and treatment group. Error bars are +/- 1 standard error and are slightly offset to show the bar for each treatment more clearly.
Two analytical approaches, both described in the Methods section, were used to identify associations of factors measured at baseline with VF outcomes assessed over time. The results of a mixed linear regression model are shown in Table 2. This model includes MD as the dependent variable and independent variables that were either associated with MD alone or in interaction with other variables. Results of a complementary analysis, using repeated measures logistic regression, are shown in Table 3. This analysis addressed the proportion of patients at each follow-up interval that experienced clinically substantial VF loss (a worsening of the MD from baseline of 3 or more dB). It is important to distinguish this outcome from permanent visual field loss. Visual field fluctuation of 3 dB or more at a single time point could occur due to intra-patient variability, measurement error, or temporary ophthalmic conditions, e.g., cataract development and extraction. The findings of these two analyses follow.
Table 2.
Mixed model estimates of baseline factors associated with 2-9 year mean deviation.*
| Variable | Estimate | Standard Error | P-Value |
|---|---|---|---|
| Range of 6 Baseline IOP Measures | 0.0184 | ||
| Range ≥ 8.5 mmHg vs < 8.5 mmHg | -0.55 | 0.23 | |
| Race * Cataract | 0.0011 | ||
| White & Other / No Cataract | (reference) | ||
| White & Other / Cataract | -1.92 | 0.24 | <0.0001 |
| Black / No Cataract | -0.31 | 0.23 | 0.1869 |
| Black / Cataract | -3.65 | 0.40 | <0.0001 |
| Diabetes * Treatment * Time | 0.0110 | ||
| Medicine / Diabetes, MD at 9 years vs 2 years | -1.89 | 0.50 | 0.0002 |
| Medicine / No Diabetes, MD at 9 years vs 2 years | -1.87 | 0.25 | <0.0001 |
| Surgery / Diabetes, MD at 9 years vs 2 years | -2.65 | 0.62 | <0.0001 |
| Surgery / No Diabetes, MD at 9 years vs 2 years | -0.53 | 0.26 | 0.0392 |
| Age * Time | <0.0001 | ||
| 40 years at baseline, MD at 9 years vs 2 years | -0.35 | 0.31 | 0.2518 |
| 55 years at baseline, MD at 9 years vs 2 years | -1.26 | 0.18 | <0.0001 |
| 70 years at baseline, MD at 9 years vs 2 years | -2.17 | 0.25 | <0.0001 |
| Baseline MD * Treatment | 0.0263 | ||
| MD0 = -2 / Surgery vs Medicine @ 2yrs | -0.64 | 0.24 | 0.0085 |
| MD0 = -2 / Surgery vs Medicine @ 3yrs | -0.50 | 0.23 | 0.0320 |
| MD0 = -2 / Surgery vs Medicine @ 5yrs | -0.22 | 0.24 | 0.3561 |
| MD0 = -2 / Surgery vs Medicine @ 7yrs | 0.06 | 0.27 | 0.8195 |
| MD0 = -2 / Surgery vs. Medicine @ 9yrs | 0.34 | 0.33 | 0.2954 |
| MD0 = -10 / Surgery vs Medicine @ 2yrs | 0.32 | 0.40 | 0.4149 |
| MD0 = -10 / Surgery vs Medicine @ 3yrs | 0.46 | 0.39 | 0.2317 |
| MD0 = -10 / Surgery vs Medicine @ 5yrs | 0.74 | 0.38 | 0.0531 |
| MD0 = -10 / Surgery vs Medicine @ 7yrs | 1.03 | 0.40 | 0.0108 |
| MD0 = -10 / Surgery vs Medicine @ 9yrs | 1.31 | 0.44 | 0.0028 |
| Baseline MD * Cataract | <0.0001 | ||
| MD0 = -2 /Cataract vs No Cataract | -3.18 | 0.23 | <0.0001 |
| MD0 = -10 /Cataract vs No Cataract | -1.26 | 0.34 | 0.0002 |
MD = Mean Deviation; MD0 = Baseline Mean Deviation
Mixed model for MD included the following covariates: time, quadratic time, MD0, quadratic MD0, treatment, age, race, cataract, diabetes, range of baseline IOP measures, and the following interactions: treatment by time, age by time, MD0 by treatment, diabetes by time, diabetes by quadratic time, diabetes by treatment, diabetes by treatment by time, cataract by MD0, and cataract by race.
Table 3.
Generalized estimating equation (GEE) model estimates of baseline factors associated with the probability of 3+ dB loss of MD in the 2 to 9 year period.*
| Variable | OR | Confidence Interval | P-Value |
|---|---|---|---|
| Follow-up time (per year) | 1.20 | (1.15, 1.25) | <0.0001 |
| Age (per 10 years) | 1.35 | (1.14, 1.60) | 0.0006 |
| Range of 6 Baseline IOP Measures | 0.0006 | ||
| Range ≥ 8.5 mmHg vs < 8.5 mmHg | 1.96 | (1.34, 2.88) | |
| Baseline MD * Treatment | 0.1170 | ||
| MD0 = -2 / Surgery vs Medicine | 1.09 | (0.69, 1.71) | 0.7205 |
| MD0 = -10 / Surgery vs Medicine | 0.65 | (0.39, 1.08) | 0.0970 |
| Baseline MD * Cataract | 0.0075 | ||
| MD0 = -2 /Cataract vs No Cataract | 4.54 | (2.99, 6.90) | <0.0001 |
| MD0 = -10 /Cataract vs No Cataract | 1.98 | (1.22, 3.22) | 0.0057 |
| Diabetes * Treatment | 0.0470 | ||
| Diabetic/Surgery vs Medicine | 1.86 | (0.83, 4.16) | 0.1310 |
| Non-Diabetic/Surgery vs Medicine | 0.74 | (0.50, 1.11) | 0.1465 |
| Surgery/ Diabetic vs Non-Diabetic | 2.12 | (1.10, 4.10) | 0.0245 |
| Medicine/Diabetic vs Non-Diabetic | 0.85 | (0.46, 1.58) | 0.6067 |
MD = Mean Deviation; MD0 = Baseline Mean Deviation
GEE model for MD included the following covariates: time, MD0, treatment, age, cataract, diabetes, range of baseline IOP measures, and the following interactions: MD0 by treatment, MD0 by cataract, and diabetes by treatment.
Effect of Initial Treatment
The association of initial treatment assignment with subsequent VF loss was not explained by a main effect of initial treatment, but rather by significant interactions of initial treatment with baseline MD (P=0.0263) and diabetes (P=0.0011) in the mixed model, and only with diabetes (P=0.0470) in the logistic model.
An association between treatment and VF loss at baseline was found in the mixed model. Subjects who presented with more substantial VF loss showed less VF loss over time when treated surgically than medically, as demonstrated for a baseline MD of -10 dB (Table 2 & Figure 2A). No such difference was seen for patients who presented with more minimal loss at baseline (illustrated for a MD of -2 dB in Figure 2A). For example, at 7 years post-treatment initiation in patients who had a -10 dB MD at baseline, on average those treated surgically had a 1.03 dB (SE, 0.40 dB) better MD than those treated medically (P=0.0108).
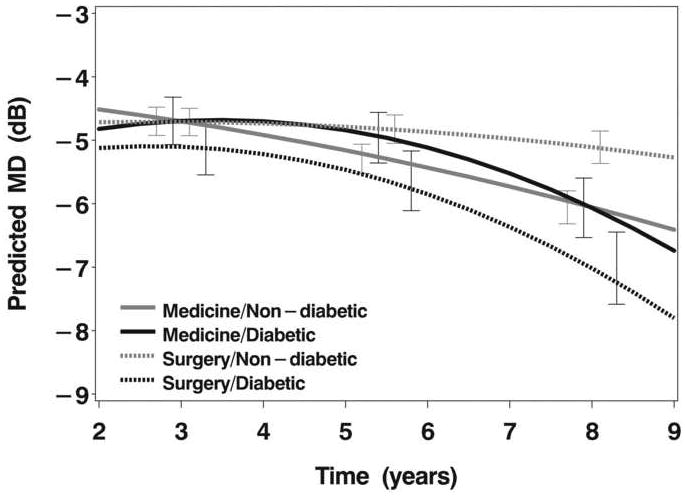
The association of diabetes with initial treatment is also potentially important, since diabetics (n=102, or 17% of enrolled subjects) were significantly better off in terms of VF loss over extended follow-up if their initial treatment was medical (Figure 2B). This finding can be demonstrated quantitatively by evaluating how much worse the MD was at year 9 vs. year 2 in diabetics treated medically (1.89 dB worse) vs. diabetics treated surgically (2.65 dB worse). Both groups showed worsening of MD from year 2 to year 9, but the amount of worsening was greater in diabetics treated surgically.
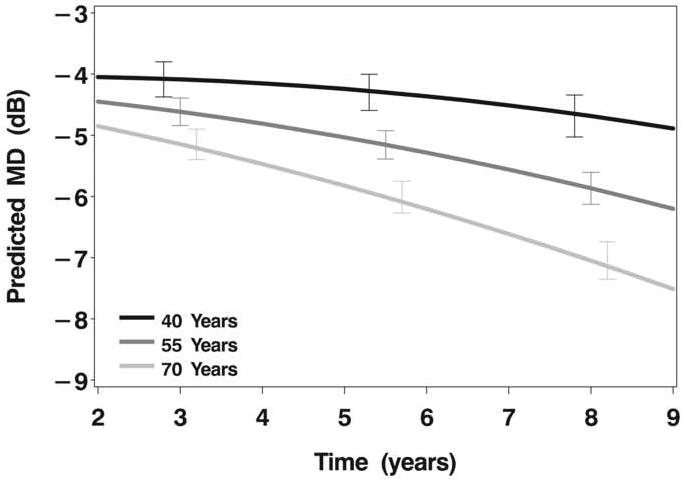
Figure 2.
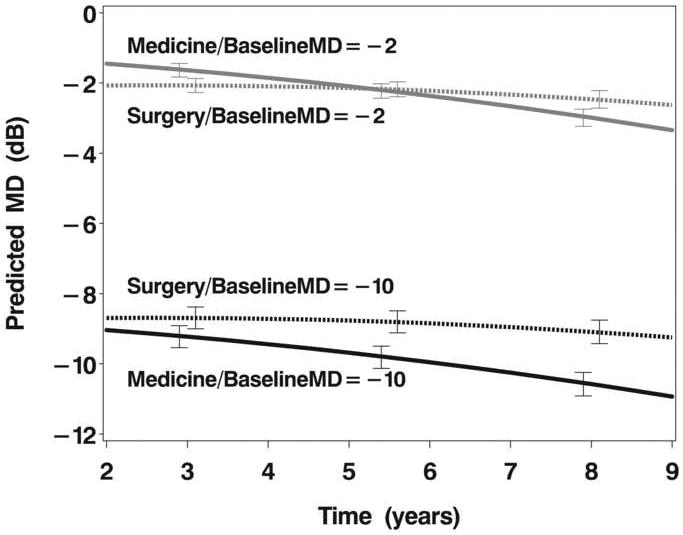
A: Model estimates of mean deviation (MD) over follow-up time as a function of initial treatment (medicine or surgery) and baseline MD (-2 dB or -10 dB).
B: Model estimates of mean deviation over follow-up time as a function of initial treatment (medicine or surgery) and diabetes status (diabetic or non-diabetic).
C: Model estimates of mean deviation over follow-up time as a function of age at enrollment (40, 55, or 70 years).
Effect of Initial IOP
The level of initial IOP, which was required by inclusion criteria to be at least 20 mmHg, was not significantly associated with subsequent VF loss in either model. However, the range (maximum IOP – minimum IOP) of the six IOP measurements taken at baseline, which varied from 0.5 to 25 mmHg with a median of 6.5 mmHg, did relate to subsequent VF loss. In the mixed model (Table 2), as the range of the six baseline IOPs increased, subsequent VF loss increased (P=0.0184). This association was also evident for substantial VF loss (Table 3). The impact of baseline IOP range was most evident for those whose range was 8.5 mmHg or greater. Those whose range was 8.5 mmHg or greater had a 96% greater odds of substantial VF loss (P=0.0006) than those with a lesser IOP range at baseline (Table 3).
Effect of Age
Older age at baseline was associated with more VF loss over time. In the mixed model, the age effect was modified by time (P<0.0001), such that the effect of age on VF loss increased over time (Figure 2C). In the logistic model, the effect of age was independent of time. For every 10 year increase in age, the risk of a 3 dB worsening of MD increased 35% (P=0.0006).
Other Associations
The impact of race was evident only in the mixed model, in which the effects of race and cataract were associated (P=0.0011). Blacks who had cataract extraction during follow-up had more VF loss over time than whites who had cataract extraction. No race effect on VF loss was observed in whites and blacks who were free of cataract. Cataract also interacted with baseline severity of VF loss in both models. The VF impact of having a cataract develop that required extraction during follow-up was more evident in patients whose baseline VF loss was mild (MD = -2 dB) than in those with more baseline VF loss (MD = -10 dB).
Discussion
We found that VF progression in the CIGTS participants was influenced by several factors that were measured before they received initial treatment: age, VF loss, diabetes mellitus, and the extent of IOP fluctuation when assessed multiple times before treatment. The impact of two of these pre-treatment factors – VF loss and diabetes - differed by which initial treatment the subjects received.
Older age was reported to be associated with an increased risk of VF loss in both the AGIS13 and the Early Manifest Glaucoma Trial (EMGT),14,15 but not in the Collaborative Normal-tension Glaucoma Study.16 In the AGIS, each 5 year increment in age increased by 30% the odds of a 1.0 dB or greater worsening of VF. In the EMGT,15 subjects who were 68 years or older were at a 51% increased risk of progressive VF loss (relative to subjects <68 years old). Our estimate of the age association was intermixed with follow-up time, such that the effect of age became more pronounced as follow-up continued. Although VF progression was measured in different ways in these clinical trials, the AGIS, EMGT, and CIGTS all show more VF progression with increasing age.
In the EMGT,15 the amount of VF loss present at baseline was associated with subsequent risk of progression. A MD value of -4 dB or worse at baseline was associated with a 38% increased risk of progressive VF loss (relative to those with a MD better than -4 dB). We found an interaction between treatment and baseline VF loss, such that subjects who presented with more advanced VF loss (-10 dB or worse) showed better VF control over time when treated with initial surgery vs. initial medications. No such effect was seen in subjects who presented with mild VF loss (-2 dB or better). These findings relate to Jay and Murray’s 1988 report3 of patients randomized to initial treatment with conventional medications, in whom the rate of operation three years after treatment began was higher in patients who had dense scotomas and higher IOP at baseline.
Patients who had more advanced VF damage and received initial surgery had less subsequent VF loss than those who received medications. While we are not aware of any previous studies that inspected this interaction, it could relate to our IOP findings. Patients with advanced VF loss may benefit from lower mean IOP during the course of follow-up, as happened in the CIGTS surgical vs. medical arms. In 1977, Werner et al.17 reported lack of VF progression after trabeculectomy in eyes with better quality of IOP control. Subsequent studies18-21 that contrasted IOP information from patients whose glaucoma was stable vs. progressed over extended follow-up noted that stable patients had better indices of IOP control – lower mean, standard deviation, and range of IOP. The AGIS study, in a report by Nouri-Mahdavi and colleagues,13 found that IOP variability was a critical factor associated with progressive VF loss.
Better control of diurnal variation by surgery might contribute to this finding. In a prospective observational study of 60 patients—30 treated with maximum tolerated medication alone and 30 treated by initial trabeculectomy—Konstas et al.22 found that the trabeculectomy group had lower mean, maximum, and range of IOP during a 24-hour IOP test wherein the IOP was measured every four hours. We did not measure diurnal IOP in the CIGTS and so we cannot do more than speculate that our findings would be consonant with a scenario in which surgery controlled diurnal variation better than medications, and the impact of this better control is limited to those with more advanced VF loss at baseline.
The issue of medication compliance deserves to be considered, as it could affect outcomes of patients in the medically-treated group. If compliance was a problem in medically treated patients, and noncompliance contributed to progression in VF loss more in those with substantial VF loss than in those with mild VF loss at baseline, then surgical treatment would be advantageous (as indicated by the interaction). Our data on medication compliance behavior in the CIGTS was not adequate to address its role in any findings. Evidence in the literature for the impact of medication compliance on glaucoma treatment is mixed. Kass et al.23 found no significant difference in compliance to pilocarpine therapy between subjects with high (>25 mmHg) and low (≤16 mmHg) IOP. This same group went on to report a higher rate of compliance with timolol treatment in patients treated with two or more drugs, and suggested that patients who believe their disease is more serious may comply better with medical treatment.24
Diabetes as a risk factor for progressive VF loss was assessed in the AGIS13 and found to not differ between those who progressed (21.2% were diabetic) and those who did not progress (21.5% were diabetic), but treatment-specific findings were not reported. In the EMGT,14,15 diabetes was not listed among the factors evaluated for VF progression, which likely relates to the fact that only nine (3.5%) of the 255 enrollees reported a history of diabetes. In an observational study of trabeculectomy success, Edmunds et al.25 found that diabetes was significantly associated with a poorer outcome, as did several earlier studies of trabeculectomy outcome.26,27 We found that diabetics treated with initial surgery showed worse VF outcomes than diabetics treated medically. Diabetes was a self-reported diagnosis in the CIGTS baseline interview, and included separate coding for “managed by diet,” “managed by oral medication,” and “managed by insulin.” In sensitivity analyses (results not shown), our findings for diabetes were consistent whether we defined diabetes as any of the three management options, or confined it to those who reported being managed by oral medications or insulin. While black subjects were over-represented among diabetics in the CIGTS (60%, 61/102, of diabetics were black vs. 37% black overall), diabetes was more predictive of VF loss than race in models with each variable included separately or combined. If diabetics are more prone to scarring after trabeculectomy, as proposed previously,26 this finding may have a biological rationale.
The fact that more variation in multiple baseline IOP measurements is predictive of worsening in VF loss over long follow-up is a unique and surprising outcome. This finding was present in both the mean and substantial VF loss models after adjustment for other factors. The six baseline IOPs, from which the range was derived, were obtained during two baseline visits. Within each visit the three IOP measurements were not standardized in terms of the time of day they were obtained, but they were separated in time by at least one hour. Perhaps these six IOP measurements identify subjects whose IOP inherently varies more than others, as others have reported that more variation in IOP during postoperative follow-up is associated with an increased risk of VF progression.13,18,20,21,28
Among other associations we found of baseline factors with VF loss, the impact of race was only evident in those subjects who required cataract extraction (CE) during the course of follow-up. Blacks who underwent CE had more VF loss than whites undergoing CE. This may relate to differing thresholds for surgery or differing responses to that procedure. There is some evidence for a threshold difference. In the 99 subjects who underwent CE, blacks (n=34) did have more VF loss (MD, -11.1 dB) at the visit prior to CE than whites (n=65; MD, -8.8 dB; P=0.0074). We previously reported that a subject’s race was not significantly associated with the risk or timing of CE.12
Because we began to collect central corneal thickness (CCT) measures near the end of our study’s active phase, and did so in a voluntary manner, we were limited in our ability to assess CCT as a risk factor. Based on CCT measurements of 272 participants (45%), its association with progressive VF loss was not significant (p=0.42) in a model that adjusted for other factors. This result differs from that reported by Leske et al. in the recent EMGT report,15 wherein thinner CCT was associated with an increased risk of VF progression among those with higher baseline IOP. We could not assess unmeasured factors like lower ocular systolic perfusion pressure and lower systolic blood pressure,15 migraine16 and IOP rise during pupillary dilatation,29 which have been reported to be associated with an increased risk of VF progression. Several measures of IOP control during treatment have been related to the risk of glaucomatous progression.13,15 Given this study’s focus on associations with factors measured at baseline, we intend to report on the role of IOP control during treatment in a separate manuscript.
Our results rely upon follow-up of glaucoma patients that diminishes over time, such that by nine years after treatment initiation, a subset of the original 607 patients remained under observation. While our models relied on available follow-up data, the possibility of differential dropout warrants consideration. After investigating numerous baseline characteristics (age, sex, race, MD, treatment, diabetes status, or range of IOP), we found no significant difference between those with and without follow-up information 7 or 9 years after treatment initiation.
Due to the lack of standardization of how VF progression is defined, comparing our findings with those of other major clinical trials (AGIS & EMGT) as well as observational studies of glaucoma patients is challenging. In AGIS and EMGT, VF progression was defined uniquely to each study, and then treated as a binary outcome in assessing risk factors. In CIGTS, our definition of progression relied on the mean deviation, rather than threshold sensitivity change at two or more specific points (AGIS) or pattern deviation change at three or more points (EMGT). We also did not view VF progression, once it occurred, as a static finding, because the assessment of VF progression is affected by within subject variability and measurement error. Our analytical approaches encompassed longitudinal change in VF, whether that change was worsening beyond the 3 dB threshold or subsequent improvement.
A possible bias could relate to treatment crossovers. In CIGTS, a target pressure was set and used to determine the need for additional therapy. Thus, if the initial treatment received by the CIGTS subjects—medications or trabeculectomy—failed to achieve the target IOP, then argon laser trabeculoplasty was performed. If that treatment still did not achieve the target IOP goal, then subjects were crossed over to surgery (in the initial medication patients) or to medications (in the initial trabeculectomy group). In the analyses reported herein, subjects were always analyzed within the group to which they were initially assigned. Unbalanced treatment crossover could affect the visual field outcome. However, the frequency of crossovers from medication to trabeculectomy and from trabeculectomy to medication was essentially identical in the two groups.
The incidence of ocular disease that might impact on visual field assessment and was recorded – retinal vein occlusion, optic neuritis or neuropathy, and retinal detachment – was very infrequent (n=21 participants experienced at least one of these events), and so we do not expect this to influence the findings considerably. Of course, there are many other ocular diseases that could affect visual field, such as macular degeneration, but the recording of these diseases was imprecise regarding their impact on visual function. Since we were especially interested to know if these events differed between the surgery and medication group, we performed a Kaplan-Meier analysis (results not shown) to evaluate the time to first occurrence of any of the following events that may interfere with VF assessment: retinal vein occlusion, retinal artery occlusion, ischemic optic neuritis, optic neuritis, and retinal detachment. Stratification by treatment showed no difference in time to these events between the medicine and surgery groups (log-rank p-value = 0.9453)
Among the implications of our results to clinical practice, perhaps of most importance is the limited amount of VF loss that occurred over extended follow-up. We attribute this to the attention paid in the CIGTS to IOP control, which likely was much stricter than what occurs in the community. Our VF outcomes for those with more advanced VF loss at baseline, in whom surgery was advantageous, and our outcomes for diabetics, in whom surgery was disadvantageous, are important. Likewise, observing how much IOP varies upon repeated measurements when a patient is diagnosed with OAG may identify patients who are prone to further VF loss even while under efficacious treatment. While further confirmation from independent studies would strengthen these findings, they warrant consideration in treating subjects with newly diagnosed glaucoma.
Acknowledgments
This study was supported by the National Institutes of Health (NIH), National Eye Institute (NEI), Grant number EY015860.
The CIGTS was funded from 1993 through 2004 by the NIH, NEI, Grant numbers EY09100, EY09140, EY09141, EY09142, EY09143, EY09144, EY09145, EY09148, EY09149, EY09150, and EY09639, and from 2004 to 2006 by an unrestricted grant from Allergan, Inc.
Appendix
The CIGTS Investigators, by Center
Clinical Centers
Albany, NY: The Center for Sight - Steven T. Simmons, MD
Baltimore, MD: Wilmer Ophthalmological Institute, Johns Hopkins University - Henry D. Jampel, MD, MHS
Cleveland, OH: Division of Ophthalmology, The Cleveland Clinic Foundation - Edward J. Rockwood, MD
Gainesville, FL: Department of Ophthalmology, University of Florida - Mark B. Sherwood, MD
Houston, TX: Cullen Eye Institute, Baylor College of Medicine - Ronald L. Gross, MD
Los Angeles, CA: Doheny Eye Institute, University of Southern California - Dale K. Heuer, MD (1993-97); Rohit Varma, MD, MPH (1997-)
Lynbrook, NY: Long Island Ophthalmic Surgery Consultants, PC - Stanley J. Berke, MD
Minneapolis, MN: Department of Ophthalmology, University of Minnesota - Martha M. Wright, MD
New York, NY: New York Eye and Ear Infirmary - Robert Ritch, MD
Oklahoma City, OK: Dean A. McGee Eye Institute, University of Oklahoma - Gregory L. Skuta, MD
Philadelphia, PA: Scheie Eye Institute, University of Pennsylvania - Jody R. Piltz-Seymour, MD
Philadelphia, PA: Wills Eye Hospital, Jefferson Medical College - George L. Spaeth, MD
Seattle, WA: Department of Ophthalmology, University of Washington - Richard P. Mills, MD, MPH (1993-99); Philip Chen, MD (1999-); Howard Barnebey, MD (Satellite center)
Winston-Salem, NC: Wake Forest University Eye Center - L. Frank Cashwell, MD & J. Brent Bond, MD
Resource Centers
Administrative Center: University of Michigan, Ann Arbor, MI: Paul R. Lichter, MD (Study Chairman). University of Washington, Seattle, WA: Richard P. Mills, M.D. (Associate Chairman)
Coordinating Center: University of Michigan, Ann Arbor, MI: David C. Musch, PhD, MPH (Director); Keneth E. Guire, MS (Deputy Director); Brenda W. Gillespie, PhD (Biostatistician); Leslie M. Niziol, MS (Biostatistician); Carol L. Standardi, RN, CRNO (Protocol Monitor)
Interviewing Center: University of Michigan, Ann Arbor, MI: Nancy K. Janz, PhD (Director); Patricia A. Wren, PhD, MPH (Deputy Director)
Project Office
National Eye Institute, Bethesda, MD: Donald F. Everett, MA (NEI Representative)
Data and Safety Monitoring Committee
Voting members: Sheryl F. Kelsey, PhD (Chair); Anne M. Damiano, ScD; Frederick L. Ferris, MD; Paul Palmberg, MD, PhD; Kenneth W. Phifer, PhD; Alfred W. Rademaker, PhD; Angela Vela-Thomas, MD
Ex officio members: Donald F. Everett, MA; Nancy K. Janz, PhD; Paul R. Lichter, MD; David C. Musch, PhD, MPH
Footnotes
The authors have no conflicts of interest that pertain to the topic of the manuscript.
This article contains online-only material. The following appears online-only: Appendix 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith RJH. Medical versus surgical therapy in glaucoma simplex. Br J Ophthalmol. 1972;56:277–83. doi: 10.1136/bjo.56.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson PG, Grierson I. The place of trabeculectomy in the treatment of glaucoma. Ophthalmology. 1981;88:175–96. doi: 10.1016/s0161-6420(81)35051-9. [DOI] [PubMed] [Google Scholar]
- 3.Jay JL, Murray SB. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol. 1988;72:881–9. doi: 10.1136/bjo.72.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651–7. doi: 10.1016/s0161-6420(94)31120-1. [DOI] [PubMed] [Google Scholar]
- 5.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005;18 doi: 10.1002/14651858.CD004399.pub2. CD004399. [DOI] [PubMed] [Google Scholar]
- 7.Musch DC, Lichter PR, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study (CIGTS): study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–62. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 8.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in The Collaborative Initial Glaucoma Treatment Study (CIGTS) comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie BW, Musch DC, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: baseline visual field and test-retest variability. Invest Ophthalmol Vis Sci. 2003;44:2613–20. doi: 10.1167/iovs.02-0543. [DOI] [PubMed] [Google Scholar]
- 10.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 11.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 12.Musch DC, Gillespie BW, Niziol LM, et al. Cataract extraction in the Collaborative Initial Glaucoma Treatment Study: incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality of life outcomes. Arch Ophthalmol. 2006;124:1694–1700. doi: 10.1001/archopht.124.12.1694. [DOI] [PubMed] [Google Scholar]
- 13.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment. The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 17.Werner EB, Drance SM, Schulzer M. Trabeculectomy and the progression of glaucomatous visual field loss. Arch Ophthalmol. 1977;95:1374–7. doi: 10.1001/archopht.1977.04450080084008. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien C, Schwartz B, Takamoto T, Wu DC. Intraocular pressure and the rate of visual field loss in chronic open-angle glaucoma. Am J Ophthalmol. 1991;111:491–500. doi: 10.1016/s0002-9394(14)72386-4. [DOI] [PubMed] [Google Scholar]
- 19.Vogel R, Crick RP, Newson RB, et al. Association between intraocular pressure and loss of visual field in chronic simple glaucoma. Br J Ophthalmol. 1990;74:3–6. doi: 10.1136/bjo.74.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart WC, Chorak RP, Hunt HH, Sethuraman G. Factors associated with visual loss in patients with advanced changes in the optic nerve head. Am J Ophthalmol. 1993;116:176–81. doi: 10.1016/s0002-9394(14)71282-6. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WC, Kolker AE, Sharpe ED, et al. Factors associated with long-term progression or stability in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:274–9. doi: 10.1016/s0002-9394(00)00487-6. [DOI] [PubMed] [Google Scholar]
- 22.Konstas AGP, Topouzis F, Leliopoulou O, et al. 24-hour intraocular pressure control with maximal medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology. 2006;113:761–5. doi: 10.1016/j.ophtha.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Kass MA, Gordon M, Meltzer DW. Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol. 1986;101:524–30. doi: 10.1016/0002-9394(86)90940-2. [DOI] [PubMed] [Google Scholar]
- 24.Kass MA, Gordon M, Morley RE, Jr, Meltzer DW, Goldberg JJ. Compliance with topical timolol treatment. Am J Ophthalmol. 1987;103:188–93. doi: 10.1016/s0002-9394(14)74225-4. [DOI] [PubMed] [Google Scholar]
- 25.Edmunds B, Bunce CV, Thompson JR, et al. Factors associated with success in first-time trabeculectomy for patients at low risk of failure with chronic open-angle glaucoma. Ophthalmology. 2004;111:97–103. doi: 10.1016/j.ophtha.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Hugkulstone CE, Smith LF, Vernon SA. Trabeculectomy in diabetic patients with glaucoma. Eye. 1993;7:502–6. doi: 10.1038/eye.1993.109. [DOI] [PubMed] [Google Scholar]
- 27.Shin DH, Hughes BA, Song MS, et al. Primary glaucoma triple procedure with or without adjunctive mitomycin. Prognostic factors for filtration failure. Ophthalmology. 1996;103:1925–33. doi: 10.1016/s0161-6420(96)30406-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee PP, Walt JW, Rosenblatt LC, et al. Association between intraocular pressure variation and glaucoma progression: data from a United States chart review. Am J Ophthalmol. 2007;144:901–7. doi: 10.1016/j.ajo.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 29.Siam GA, de Barros DSM, Gheith ME, et al. The amount of intraocular pressure rise during pharmacological pupillary dilatation is an indicator of the likelihood of future progression of glaucoma. Br J Ophthalmol. 2007;91:1170–2. doi: 10.1136/bjo.2007.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]


