Abstract
Candida glabrata, an opportunistic fungal pathogen, adheres to mammalian epithelial cells; adherence is mediated primarily by the Epa1 adhesin. EPA1 is a member of a large gene family of ∼23 paralogues, which encode putative adhesins. In this study, we address how EPA1 transcription is regulated. Our data show that EPA1 expression is subject to two distinct negative regulatory mechanisms. EPA1 transcription is repressed by subtelomeric silencing: the Sir complex (Sir2–Sir4), Rap1, Rif1, yKu70, and yKu80 are required for full repression. Activation of EPA1 occurs immediately after dilution of stationary phase (SP) cells into fresh media; however, transcription is rapidly repressed again, limiting expression to lag phase, just as the cells exit stationary phase. This repression following lag phase requires a cis-acting regulatory negative element (NE) located in the EPA1 3′-intergenic region and is independent of telomere proximity. Bioinformatic analysis shows that there are 10 copies of the NE-like sequence in the C. glabrata genome associated with other EPA genes as well as non-EPA genes.
CANDIDA glabrata, an opportunistic fungal pathogen normally present in the mucosal flora, can cause severe disseminated infections. C. glabrata is the second most common agent of candidiasis, accounting for 15–20% of Candida bloodstream infections worldwide (Pfaller and Diekema 2010). Some traits of C. glabrata that allow it to cause disease have been described (Kaur et al. 2005; Roetzer et al. 2011). These include resistance to oxidative stress (Cuellar-Cruz et al. 2008, 2009) and adherence to epithelial cells (Cormack et al. 1999; Castaño et al. 2005).
Adherence to host cells has been proposed to be an important initial step for virulence. Additionally, the ability to adhere to abiotic substrates and the adherence between microbial cells are essential attributes for biofilm formation in many pathogens. Adherence in pathogenic fungi has been shown to be mediated primarily by glycosylphosphatidylinositol-anchored cell wall proteins (GPI-CWPs) which are found broadly in different fungal species including Saccharomyces cerevisiae, C. glabrata, and C. albicans (Castaño et al. 2006). In S. cerevisiae, the FLO family of genes (FLO1, FLO5, and FLO9–FLO11) encode a group of (GPI-CWPs) that are required for flocculation, pseudohyphal growth, and biofilm formation on abiotic substrates (Kobayashi et al. 1998; Guo et al. 2000). C. albicans encodes ∼104 putative GPI-CWPs including the Als family, the Hwp family, Hyr1, and Eap1 (De Groot et al. 2003a,b; Li and Palecek 2003), many of which are thought to mediate adhesion to host epithelial and endothelial cells as well as to extracellular matrix proteins (Hoyer 2001; Li and Palecek 2003; Sheppard et al. 2004; Klotz et al. 2004; Hoyer et al. 2008). C. glabrata can adhere to epithelial cells and also to inert surfaces. EPA1 encodes the major epithelial adhesin in the BG2 strain, binding to N-acetyl lactosamine-containing glycoconjugates (Cormack et al. 1999; Zupancic et al. 2008). EPA1 belongs to a large gene family (EPA family) of ∼23 paralogues, of which EPA6 and EPA7 have also been shown to mediate adherence to epithelial cells in vitro (De Las Peñas et al. 2003; Castaño et al. 2005; De Groot et al. 2008).
Proper regulation of the expression of the EPA adhesin genes is thought to be of importance for survival and proliferation in the host environment. One layer of EPA gene transcriptional regulation is related to the fact that most EPA genes are encoded in subtelomeric loci, where they are subject to chromatin-based silencing mediated by the Sir complex (Sir2, Sir3, and Sir4), yKu70, yKu80, Rif1, and Rap1 (De Las Peñas et al. 2003; Castaño et al. 2005). Deletion of genes encoding the silencing factors results in the cells becoming hyperadherent, due to overexpression of some EPA genes, including EPA6 and EPA7 (Castaño et al. 2005). Other subtelomeric EPAs are not expressed even in sir mutant backgrounds, indicating additional gene-specific regulation for individual EPA genes (De Las Peñas et al. 2003; Castaño et al. 2005; Domergue et al. 2005).
In this article, we focus on the detailed regulation of EPA1 transcription. EPA1 resides 20.7 kb upstream from the right telomere in chromosome E and forms a cluster with two other EPA genes (EPA1–EPA2–EPA3 telomere, Figure 1A) (De Las Peñas et al. 2003). Our data show that EPA1 expression is tightly controlled negatively and positively. EPA1 transcription is repressed by the Sir complex (Sir2, Sir3, and Sir4) and by Rap1, Rif1, yKu70, and yKu80. Transcription of EPA1 is induced immediately after dilution of stationary phase (SP) cells into fresh media and concomitantly, the cells become adherent. Interestingly, EPA1 expression is limited to lag phase, and is tightly repressed in long-term log phase (LP) cultures as well as in SP. We show that a cis-acting regulatory negative element (NE) localized at the intergenic region between EPA1 and EPA2, 300 bp downstream of EPA1 stop codon (TAA), plays a major role in transcriptional repression of EPA1.
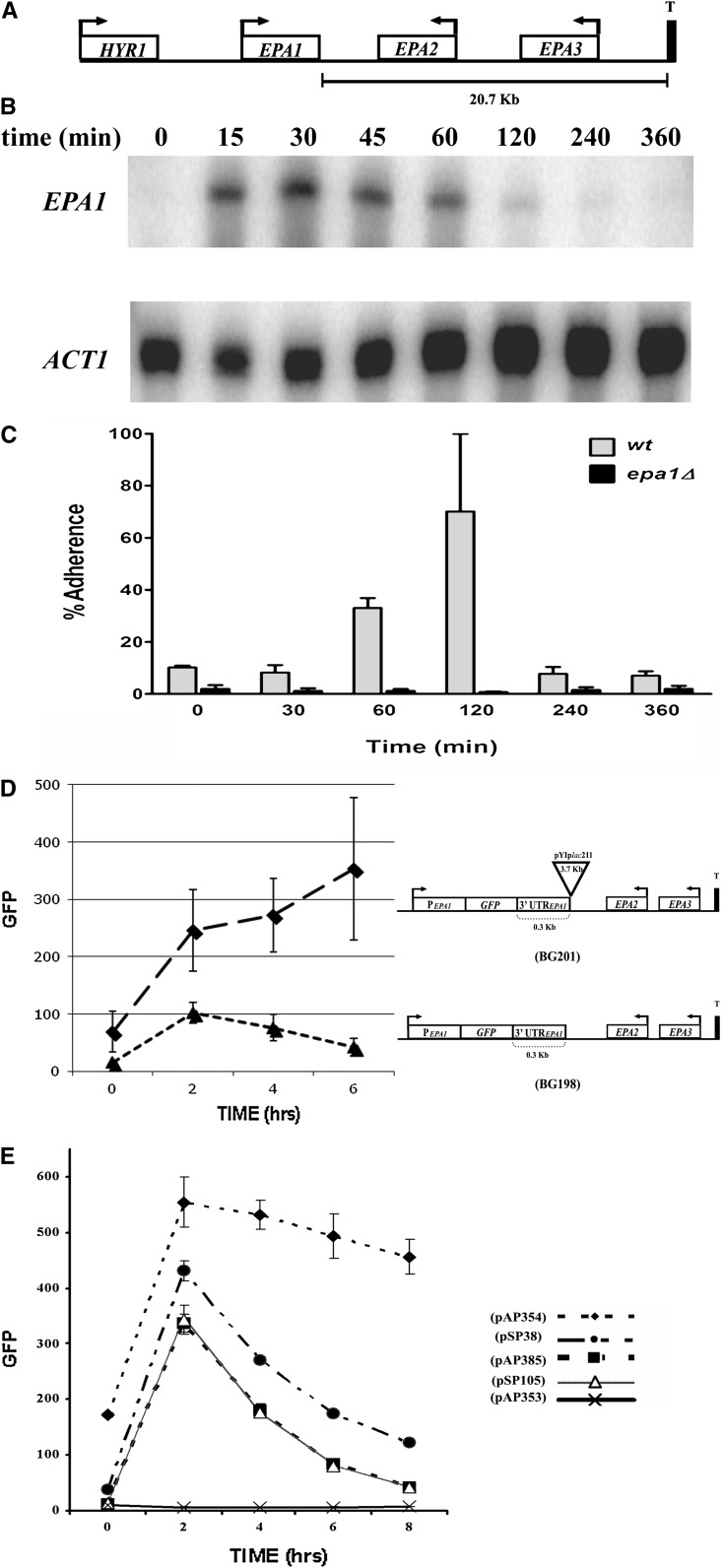
Figure 1 .
Regulation of the expression of EPA1. (A) Schematic representation of the EPA1 genomic locus. (B) EPA1 transcript levels measured by S1 nuclease protection. BG14 (WT) cells were grown for 48 hr and 30° in YPD media. Cells were diluted into fresh media and samples were taken at different time points (see Materials and Methods). Time 0 is undiluted stationary phase (SP) cells. (C) Adherence of C. glabrata cells to HeLa cells. C. glabrata wild-type strain BG14 (WT) and strain BG64 (epa1Δ) were grown for 48 hr at 30° in YPD media. Cells were diluted into fresh media and samples were taken at different time points. Cells were adjusted to OD600 of 1.0 in HBSS supplemented with 5 mM CaCl2. Cell suspensions were diluted serially in sterile water and appropriate dilutions were made and plated on YPD plates to determine input colony forming units (CFU) (see Materials and Methods). Each experiment was made in triplicate. (D) EPA1 promoter activity measured by FACS. Strains BG198 (PEPA1::GFP) and BG201 (PEPA1::GFP::pYIplac211) were grown for 48 hr at 30° in YPD media. Cells were diluted into fresh media and samples were taken every 2 hr. Yeast cells were washed and resuspended in 1 ml PBS and fluorescence was assessed by FACS analysis using a BD FACSCalibur flow cytometer (see Materials and Methods). EPA1 promoter GFP fusion is at the chromosomal EPA1 locus. GFP was used as reporter of the activity of the EPA1 promoter. (E) EPA1 promoter activity measured by FACS as in D, but cells were grown in SC −Ura media and all constructs are borne in plasmids. Strain BG14 (WT) carrying plasmids pAP353 (promoterless control, GFP::3′UTRHIS3), pAP354 (PEPA1::GFP::3′UTRHIS3), pAP385 (PEPA1::GFP::3′UTREPA1NE(3.1Kb)), pSP38 (PEPA1::GFP::3′UTRHIS3:: NE(200bp)), and pSP105 (NE200bp::PEPA1::GFP::3′UTREPA1).
Materials and Methods
Strains
All strains used in the study are described in Table 1.
Table 1 . Strains used in this study.
| Strain | Parent | Genotype | Reference |
|---|---|---|---|
| Escherichia coli strain | |||
| DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara,leu)7697galU galK λ− rpsL nupG | Calvin and Hanawalt (1988) | |
| Candida glabrata strains | |||
| BG2 | Clinical isolate (strain B) | Fidel et al. (1996) | |
| BG14 | BG2 | ura3Δ::Tn903 G418R | Cormack and Falkow (1999) |
| BG64 | BG14 | epa1Δ ura3Δ::Tn903 G418R | Cormack et al. (1999) |
| BG198 | BG14 | ura3Δ::Tn903 G418R epa1Δ::GFP. GFP under the control of the EPA1 promoter (Figure 1C) | De Las Peñas et al. (2003) |
| BG201 | BG14 | ura3Δ::Tn903 G418R epa1Δ::GFP pYIplac211 integrated 300bp from TAA of EPA1. GFP under the control of the EPA1 promoter (Figure 1C) | This work |
| BG509 | BG14 | ura3Δ::Tn903 G418R rif1Δ::hph HygR | Castaño et al. (2005) |
| BG592 | BG14 | ura3Δ::Tn903 G418R rap1-21 | De Las Peñas et al. (2003) |
| BG646 | BG14 | ura3Δ::Tn903 G418R Tn7 at intergenic region between EPA1 and EPA2 | De Las Peñas et al. (2003) |
| BG676 | BG14 | ura3Δ::Tn903 G418R sir3Δ::hph HygR | De Las Peñas et al. (2003) |
| BG1048 | BG14 | ura3Δ::Tn903 G418R sir2Δ::hph HygR | Domergue et al. (2005) |
| BG1050 | BG14 | ura3Δ::Tn903 G418R sir4Δ::hph HygR | Rosas-Hernandez et al. (2008) |
| BG1080 | BG14 | ura3Δ::Tn903 G418R hdf1Δ::hph HygR | Rosas-Hernandez et al. (2008) |
| BG1081 | BG14 | ura3Δ::Tn903 G418R hdf2Δ::hph HygR | Rosas-Hernandez et al. (2008) |
| BG1124 | BG1212 | ura3Δ::Tn903 G418R epa1Δ::URA3. EPA1 replaced by URA3. URA3 under the control of the EPA1 promoter | This work |
| BG1132 | BG14 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat. EPA1 replaced by URA3 and NE (negative element) replaced by the bacterial cat gene, chloramphenicol acetyl transferase from pACYC184 URA3 under the control of the EPA1 promoter | This work |
| sir2Δ | |||
| CGM172 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 sir2Δ::hph HygR | This work |
| CGM174 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat sir2Δ::hph HygR | This work |
| sir3Δ | |||
| CGM283 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 sir3Δ::hph HygR | This work |
| CGM285 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat sir3Δ::hph HygR | This work |
| sir4Δ | |||
| CGM188 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 sir4Δ::hph HygR | This work |
| CGM190 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat sir4Δ::hph HygR | This work |
| hdf1Δ | |||
| CGM198 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 hdf1Δ::hph HygR | This work |
| CGM184 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat hdf1Δ::hph HygR | This work |
| hdf2Δ | |||
| CGM187 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 hdf2Δ::hph HygR | This work |
| CGM185 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat hdf2Δ::hph HygR | This work |
| rif1Δ | |||
| CGM210 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 rif1Δ::hph HygR | This work |
| CGM212 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat rif1Δ::hph HygR | This work |
| hst1Δ | |||
| CGM213 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3 hst1Δ::hph HygR | This work |
| CGM214 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cat hst1Δ::hph HygR | This work |
| hst2Δ | |||
| CGM208 | BG1124 | ura3Δ::Tn903 G418R epa1Δ::URA3hst2Δ::hph HygR | This work |
| CGM217 | BG1132 | ura3Δ::Tn903 G418R epa1Δ::URA3 neΔ::cathst2Δ::hph HygR | This work |
| rap1-21 | |||
| CGM349 | BG592 | ura3Δ::Tn903 G418R rap1-21 epa1Δ::URA3 | This work |
| CGM374 | BG592 | ura3Δ::Tn903 G418R rap1-21 epa1Δ::URA3 neΔ::cat | This work |
Plasmids
All plasmids used in this study are described in Table 2.
Table 2 . Plasmids used in this study.
| Plasmid | Relevant genotype | Reference |
|---|---|---|
| pCYC184 | Cloning vector CmR TcR | Chang and Cohen (1978) |
| pYIplac211 | Cloning vector, integrative vector URA3 ApR | Gietz and Sugino (1988) |
| pMB11 | Cloning vector, sacB counterselection CmR ori p15A | Lab collection |
| pGRB2.0 | Cloning vector, pRS406 URA3 C.g.CEN ARS ApR | De Las Peñas et al. (2003) |
| pRS306 | Cloning vector, integrative vector URA3 ApR | Sikorski and Hieter (1989) |
| pAP599 | Cloning vector for construction of knockout mutants with two FRT direct repeats flanking the hygromycin cassette to remove the selection marker for construction of multiple mutants in C. glabrata. [FRT-PPGK::hph::3′UTRHIS3-FRT] URA3 HygR ApR | Domergue et al. (2005) |
| pAP640 | Cloning vector, pRS306 cut with NdeI/NaeI blunt ended with Klenow and religated. ura3Δ ApR | This work |
| pAP353 | A 0.738-kb EcoRI/SalI PCR fragment carrying GFP (no BstXI site) and a 0.397-kb XhoI/KpnI PCR fragment carrying HIS3 3′UTR were cloned into pGRB2.0. For GFP promoter fusions. URA3 ApR. (Figure 1D) | This work |
| pAP354 | A 2.5-kb BstXI PCR fragment (primers 666/667) carrying the promoter region of EPA1 was cloned into pAP353. URA3 ApR PEPA1::GFP::3′UTRHIS3 (Figure 1D) | This work |
| pAP385 | A 3.1-kb XhoI PCR fragment (primers 723/724) carrying the 3′UTREPA1 fragment cloned at XhoI site of pAP354. URA3 ApREPA1Promoter::GFP::3′UTREPA1-3.1Kb (Figure 1D) | This work |
| pAP407 | Similar to pAP354 but 3′-UTR from EPA1.URA3 ApR PEPA1::GFP::3′UTREPA1-0.300Kb | Domergue et al. (2005) |
| pSP7 | A 0.100-kb XhoI PCR fragment (primers 780/795) carrying the 3′ intergenic region of EPA1cloned into pAP407 digested with XhoI. URA3 ApR (+400 bp; Figure 2, line 4) | This work |
| pSP19 | A 0.50-kb XhoI PCR fragment (primers 780/841) carrying the 3′ EPA1 (NE mapped) was cloned into pAP407 digested with XhoI. URA3 ApR (+350 bp; Figure 2, line 5) | This work |
| pSP20 | A 0.150-kb XhoI PCR fragment (primers 780/842) carrying the 3′ intergenic region of EPA1cloned into pAP407 digested with XhoI. URA3 ApR (+450 bp; Figure 2, line 3) | This work |
| pSP21 | A 0.200-kb XhoI PCR fragment (primers 780/843) carrying the 3′ intergenic region of EPA1cloned into pAP407 digested with XhoI. URA3 ApR (+500 bp; Figure 2, line 1) | This work |
| pSP26 | A 0.200-kb XhoI/SalI PCR fragment (primers 776/839) carrying the 3′ UTR of EPA1cloned into pAP407 digested with XhoI/SalI. URA3 ApR (+200 bp; Figure 2, line 2) | This work |
| pSP31 | A 0.250-kb XhoI PCR fragment (primers 843/913) carrying the 3′ intergenic region of EPA1cloned into pSP26 digested with XhoI. URA3 ApR (−50 bp; Figure 2, line 7) | This work |
| pSP32 | A 0.200-kb XhoI PCR fragment (primers 780/843) carrying the 3′ intergenic region of EPA1cloned into pSP26 digested with XhoI. URA3 ApR (−100 bp; Figure 2, line 8) | This work |
| pSP33 | A 0.150-kb XhoI PCR fragment (primers 914/843) carrying the 3′ intergenic region of EPA1cloned into pSP26 digested with XhoI. URA3 ApR (−150 bp; Figure 2, line 9) | This work |
| pSP34 | A 0.100-kb XhoI PCR fragment (primers 915/843) carrying the 3′ intergenic region of EPA1cloned into pSP26 digested with XhoI. URA3 ApR (−200 bp; Figure 2, line 10) | This work |
| pSP35 | A 0.50-kb XhoI PCR fragment (primers 916/843) carrying the 3′ intergenic region of EPA1cloned into pSP26 digested with XhoI. URA3 ApR (−250 bp; Figure 2, line 11) | This work |
| pSP38 | Similar to pAP354 with the NE cloned immediately after the HIS3 3′-UTR. URA3 ApR PEPA1::GFP::3′UTRHIS3::NE+. | This work |
| pSP105 | A 0.300-kb fragment (primers 1135/1136) carrying the NE cloned 5′ the EPA1 promoter in pSP26. URA3 ApR (Figure 1D) | This work |
Primers
All primers used for cloning are summarized in Table 3.
Table 3 . Oligonucleotides used in this study.
| Primer | Sequence | Sites |
|---|---|---|
| 666 | TCGTTAAGCCATTGTGTTGGATCACTTTCAACACCAAATG | BstXI |
| 667 | AAAATCATCCATTGTGTTGGGTTAATTGCAAAGACTAAAT | BstXI |
| 723 | TGGTACTTCTCGAGTCCCACCAGTTGG | XhoI |
| 724 | CATAATAGTGATGAACATAGGGACCTAAAACCAGAAAAT | XhoI |
| 776 | CATAGGGGTCGACAACCAGAAAATATAATAAC | SalI |
| 777 | AAAGTTCTCGAGTCTGGGAGAATAGAAAAGGCA | XhoI |
| 795 | ATTGACCTCTCGAGGAAGTTTAATTCGAGATT | XhoI |
| 796 | ACCTGAAACTCGAGAATAGTCCGTTACCTACC | XhoI |
| 780 | CTCCCAGACTCGAGAACTTTTGAGCAGGGACCA | XhoI |
| 839 | TAATATCTCTCGAGTCAAGTGTGACCAGGAAT | XhoI |
| 841 | GTGATTTGCTCGAGCTTTCTCTTGCTTTTGAA | XhoI |
| 842 | ATGATCATCTCGAGTTAGAATAATAAGTTGTT | XhoI |
| 843 | CCTTGCACCTCGAGCGTATAAACTCTCATATT | XhoI |
| 913 | TTCAGGGTCTCGAGTTTACATACGAAGCCTAA | XhoI |
| 914 | AAAGTTAGCTCGAGTCACGAAAATCCAGAAGA | XhoI |
| 915 | TAAACTTCCTCGAGAGGTCAATTGTCAAAAAA | XhoI |
| 916 | TATTCTAACTCGAGATGATCATATGAACATAC | XhoI |
| 1135 | CTCCCAGAGGATCCAACTTTTGAGCAGGGACCA | BamHI |
| 1136 | CCTTGCACGGATCCCGTATAAACTCTCATATT | BamHI |
| ACT1 | CTCAAAATAGCGTGTGGCAAAGAGAAACCGGCGTAAATTGGAACAACGTGGGTAACACCGTCACCAGAGTCCTTTTG | Probe for S1 |
| EPA1 | GCCAGTTCTAGGGTAATTGGGATCTAAATATGCTGCATCCCAACATGGGTACGAACCCTTCTTCCGAAAATCTATCC | Probe for S1 |
Media and growth conditions
All cell cultures were grown for 48 hr at 30°. SP cells are cells grown for 48 hr. LP cells are dividing cells. Lag phase is considered when SP cells are diluted into fresh media and cells are preparing for cell division. Yeast media were prepared as described (Sherman et al. 1986), and 2% agar was added to plates. YPD media contains yeast extract 10 g/liter, peptone 20 g/liter, supplemented with 2% glucose. When needed, YPD plates were supplemented with 400 µg/ml of hygromycin (A. G. Scientific). Synthetic complete media (SC) contains YNB without amino acids and nitrogen source (1.7 g/liter), NH2SO4 (5 g/liter), supplemented with 0.6% of casamino acids and 2% glucose and, when needed, supplemented with 50 mg/liter uracil and 0.9 g/liter 5-fluoroorotic acid (5-FOA, Toronto Research Chemicals) for 5-FOA plates. Bacterial media were prepared as described (Ausubel et al. 2001), and 1.5% agar was used for plates. Luria-Bertani (LB) media contained yeast extract at 5 g/liter, bactopeptone at 10 g/liter, and NaCl at 10 g/liter. When needed, LB plates were supplemented with 100 μg/ml of carbenicillin (Cb100, Invitrogen). Phosphate buffer saline (PBS) was 8 g/liter NaCl, 0.2 g/liter KCl, 1.65 g/liter Na2HPO4 · 7H2O, and 0.2 g/liter KH2PO4.
Yeast transformation
Yeast transformation was performed using the lithium acetate protocol as described previously (Castaño et al. 2003).
Construction of deletion strains
To construct deletion strains in this study, we used the one-step gene replacement procedure. Briefly, we cloned two fragments (the promoter region and the 3′-UTR, flanking the gene to be deleted) at each side of the hygromycin resistance cassette in the integrative URA3 plasmid, pAP599 (see Table 1). The plasmid was digested with restriction enzymes that cut within the two cloned fragments generating homologous ends. After inactivation of the enzymes, C. glabrata was transformed with the digestion mix and transformants were selected on YPD −hygromycin or SC −Ura plates. PCR analysis was done to confirm the structure of the deletion. The absence of each deleted gene was also verified by the inability to PCR amplify an internal fragment of the gene. Strains constructed in this way are described in Table 1.
S1 nuclease protection assay
BG14 cells were grown for 48 hr at 30°. RNA was extracted as previously described (De Las Peñas et al. 2003). The ACT1 and the EPA1 probes (Table 3) were end labeled using [γ-32P]-ATP with T4 polynucleotide kinase. A total of 30 µg of RNA was hybridized with each end-labeled probe at 55° overnight. The mix was digested at room temperature with 150 units of S1 nuclease (Invitrogen) for 30 min. The samples were then extracted with phenol, precipitated, and resuspended in 17 µl of 1× loading buffer. A total of 5µl of each sample was separated by electrophoresis on a 10% acrylamide gel and the signal detected using a phosphorimager.
EPA1 promoter::URA3 plate assay
Cells were grown for 48 hr at 30° in YPD and the cultures were adjusted to 0.5 OD600nm. Ten-fold serial dilutions were spotted on YPD, SC −Ura, and SC +5-FOA plates and incubated at 30° for 48 hr. Ura+ cells die on SC +5-FOA plates. Only cells with the URA3 gene transcriptionally repressed can grow on SC +5-FOA.
In vitro adherence assays
Cervical carcinoma cells (HeLa) were cultured in 24-well plates in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 80 units ml−1 of penicillin G, 50 μg ml−1 of streptomycin sulfate, and 7% calf serum (HyClone Laboratories). The cells were maintained at 37° in 5% CO2 until confluent growth. Each well was washed once with sterile water and PBS. Cells were fixed with 4% of paraformaldehyde. Plates were stored at 4° containing 1 ml of PBS supplemented with Pen/Strep (penicillin 100 units ml−1 and streptomycin 100 µg ml−1). For adherence assay, C. glabrata cells were grown for 48 hr at 30° and, where indicated, diluted into fresh media. SP and LP cultures were adjusted to an OD600 of 1.0 with Hanks balanced salt solution (HBSS) supplemented with 5 mM CaCl2. A total of 100 µl of cells was added to a 24-well plate containing fixed HeLa cells in 900 µl of PBS without antibiotics. The plates were incubated at room temperature for 1 hr. Nonadherent yeast cells were washed three times with HBSS with CaCl2 and once with PBS. Adherent cells were recovered from the epithelial cells by scraping off the plate in 500 µl of PBS containing 0.1% Triton and 0.5% SDS. The cells were plated and viable accounts recorded.
FACS analysis of GFP expression
Strains were grown for 48 hr at 30° in SC −Ura media. Cells were diluted into fresh media to induce EPA1 expression. GFP was used as reporter gene to measure the activity of the EPA1 promoter. Yeast cells were washed with PBS and resuspended in 1 ml PBS and fluorescence was assessed by FACS analysis using a BD FACSCalibur flow cytometer with Cell Quest Pro software.
Results
EPA1 is induced in lag phase
C. glabrata strain BG14 SP cells do not adhere to cultured epithelial cells. However, upon dilution and growth in fresh media, BG14 cells become adherent. This in vitro adherence is mediated by the Epa1 adhesin. epa1Δ mutant cells are virtually nonadherent in any growth condition (Cormack et al. 1999). To begin to dissect the transcriptional regulation of EPA1, we measured transcript levels of EPA1 in SP cells and in cells following dilution into fresh media. Little or no EPA1 transcript is observed in C. glabrata SP cells, but dilution into fresh media results in significant EPA1 transcription, which reaches maximal levels at 30 min following dilution (Figure 1B). However, 120 min after dilution, the EPA1 transcript level has fallen significantly, and at 240 min postdilution, it returns to background level (Figure 1B). The cells become adherent at 60 min with maximal adherence at 120 min postdilution, but as cells grow in prolonged LP, they become nonadherent, since new EPA1 transcription is repressed and preexisting Epa1 protein is diluted upon cell division (Figure 1C). These data indicate that EPA1 expression is tightly regulated and is expressed neither in SP nor in long-term LP cultures, but rather is limited to lag phase, as cells exit SP.
EPA1 expression is controlled by positive and negative regulation
To analyze the regulation of EPA1, we replaced the EPA1 ORF in its chromosomal location with the reporter gene GFP (BG198, PEPA1::GFP). GFP fluorescence is measured in cells in SP and upon dilution into fresh media by fluorescence activated cell sorting (FACS). The expression profile of PEPA1::GFP parallels that of EPA1 transcript shown in Figure 1B. In SP cells, the PEPA1::GFP reporter shows background levels of fluorescence; expression is highly induced immediately after dilution into fresh media (Figure 1D). We noticed that correct regulation of EPA1 was lost in a mutant strain carrying an insertion of 3.797 kb (pYiplac211 plasmid, strain BG201) 300 bp downstream of the PEPA1::GFP reporter stop codon: higher basal levels of expression and dilution of SP cells into fresh medium resulted in strong GFP induction; however, GFP reporter activity remained induced as cells divided, remaining elevated through LP and even in SP (Figure 1D). These data strongly suggest that normally, EPA1 expression is repressed in LP cells and that in the insertion mutant strain, such repression is relieved (Figure 1D). The lack of repression of the reporter gene in the insertional mutant (BG201) could in principle be caused by disruption of a trans-acting repressor gene encoded downstream of EPA1. However, sequence analysis of the 3.1-kb intergenic region shows no evidence of any ORF sequences within the 3′-intergenic region; moreover, complementation experiments in which the entire 3.1-kb intergenic region was cloned on a plasmid and introduced into BG201 did not suppress the high GFP-expression phenotype (data not shown).
Next, we tested whether the transcriptional regulation of the EPA1 locus can be reconstituted on a plasmid. We cloned in a URA3 CEN-ARS plasmid (pGRB2.0) the PEPA1::GFP fusion followed by the 3.1-kb EPA1–EPA2 intergenic region (PEPA1::GFP::3.1-kb intergenic region, pAP385). When this plasmid was transformed into C. glabrata, the GFP expression profile mirrored that observed from the chromosomal EPA1 locus (BG198): activation of the EPA1 promoter upon dilution into fresh media, followed by immediate repression (compare Figure 1, D and E, BG198 vs. pAP385). This suggests that the EPA1 regulatory regions in pAP385 contain the cis-acting elements required for the positive as well as negative regulation in response to the growth state of cells, and that both regulatory mechanisms can function independently of the chromosomal location. When the 3.1-kb intergenic region was replaced with the HIS3 3′-UTR (PEPA1::GFP::3′UTRHIS3, pAP354), the resulting GFP expression profile mimics that derived from the insertional mutation in the chromosome (BG201): higher basal levels of expression, normal induction of EPA1 transcription upon dilution, and loss of LP transcriptional repression (compare Figure 1, D and E, BG201 vs. pAP354). These results further support the important role for the 3.1-kb intergenic region in the transcriptional repression of EPA1 locus, and suggest the presence in this 3′ region of a cis-acting element (NE) responsible for the repression of the expression of EPA1.
Mapping of the NE in the intergenic region between EPA1 and EPA2
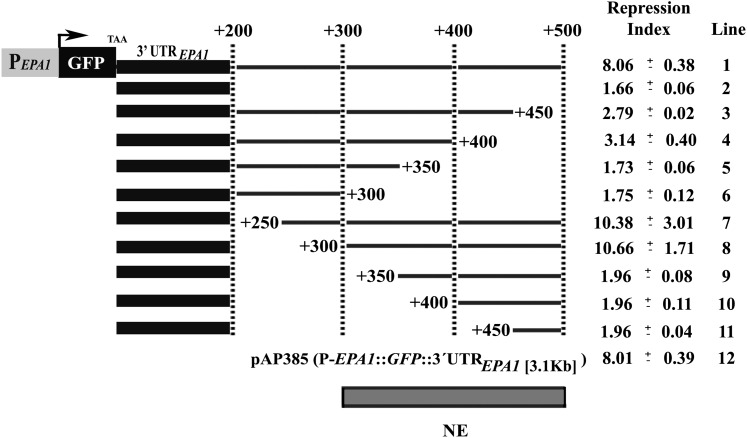
To map the NE present in the intergenic region between EPA1 and EPA2, we constructed a series of deletions in the 3.1-kb intergenic region carried on pAP385 (PEPA1::GFP::3.1-kb intergenic region), transformed the resulting plasmids into strain BG14 and screened for loss of repression by monitoring GFP expression by FACS. As described above, when GFP is controlled by EPA1 regulatory regions on the chromosome or in a plasmid, the GFP activity reaches the maximum at 2 hr after diluting SP C. glabrata cells into fresh media, then dropping to background levels by 6 hr postdilution. In Figure 2, the extent of EPA1 transcriptional repression is indicated by the ratio (repression index, RI) of GFP reporter activity at 2 hr vs. 8 hr postdilution. First, we mapped the 3′-UTR of EPA1 from +1 to +200 bp. This construct (pSP26, PEPA1::GFP::+200 bp) is induced but has a RI of only 1.66 (compare with parental plasmid carrying the 3.1-kb intergenic region, RI of 8.01) (Figure 2, line 12). A construct with only 500 bp of the 3′-UTR, however, is fully induced and repressed, with a RI of 8.06 (Figure 2, line 1) at the same level as the parental plasmid carrying the 3.1-kb intergenic region (pAP385; Figure 2, line 12) or the PEPA1::GFP in the chromosome (BG198) (Figure 1D). These data suggest that the NE may reside in the region between 200 to 500 bp downstream of EPA1 ORF. Within this defined region, we further generated constructs containing deletions in 50-bp increments from either the 5′ or 3′ (Figure 2, line 3–11). These deletion series showed that the NE is contained in this +500-bp region. The first 50-bp deletion (from +450 to +500, relative to the stop codon) completely eliminate repression (RI of 2.79; Figure 2, line 3). Further 50-bp deletions from +300 to +450 had no additional impact (Figure 2, lines 3–6). The 5′ to 3′ deletion series revealed that the NE begins at +300 bp since deletion of +200 to +300 had no impact on the RI (Figure 2, lines 7 and 8), but deletion of +300 to +350 completely eliminated repression (RI of 1.96; Figure 2, line 9). These experiments indicate that the minimal region (NE), which confers the LP-specific repression of EPA1, is 200 bp long and is localized between +300 and +500 bp downstream of EPA1 ORF, indicating a complex cis-acting element.
Figure 2 .
Mapping of the negative element (NE). The activity of the NE was assayed by measuring EPA1 promoter activity by FACS analysis of the GFP reporter fused to the EPA1 promoter. BG14 (WT) strain carrying a collection of plasmids containing serially deleted fragments (5′ to 3′ and 3′ to 5′) of the intergenic region between EPA1 and EPA2, were grown in SC −Ura media and assayed as described in Figure 1D legend. Fold of repression is the maximal expression of EPA1 at 2 hr divided by the expression at 8 hr. Line 1 is pSP21, line 2 is pSP26, line 3 is pSP20, line 4 is pSP7, line 5 is pSP19, line 6 is pAP407, line 7 is pSP31, line 8 is pSP32, line 9 is pSP33, line 10 is pSP34, line 11 is pSP35, and line 12 is pAP385 (Table 2). Experiments were done in triplicate and SDs are shown.
Characterization of the NE
We asked whether function of the NE depends on native polyadenylation sequences. To do this, we replaced the 200 bp EPA1 3′-UTR sequence immediately after the stop codon of GFP with the 200 bp from the 3′-UTR of C. glabrata HIS3. Following the HIS3 3′-UTR, we placed the 300-bp NE region (+200 to +500, pSP38). In this construct, pSP38, EPA1 promoter is induced upon dilution into fresh media, followed by immediate repression; however, repression of this construct does not follow that of the EPA1 promoter in pSP385 (PEPA1::GFP::3.1-kb intergenic region) or pSP105 (see below). This result indicates that the EPA1 polyadenylation sequences contribute partially to the overall negative regulation of EPA1 (Figure 1E). The next question we asked was whether the LP-specific repression mediated via the NE depends on its location relative to the ORF. To test this, we placed the 300-bp negative element region (from +200 to +500 bp downstream of EPA1 ORF) immediately in front of the EPA1 promoter fused to GFP (NE::PEPA1::GFP::3′UTREPA1, pSP105). The GFP expression profile is nearly identical to the parental construct (Figure 1E, compare pSP105 vs. pAP385) in which the EPA1 regulatory elements are in the original order. This shows that the regulation exerted by the NE can be location independent. These experiments confirm that the NE itself is a cis-acting regulatory element mediating the LP-specific repression. While the proximity of the NE to the 3′-UTR raised the possibility that the NE functions to regulate transcript stability, the fact that the NE can function upstream of the ORF suggests rather that it acts at the level of transcription initiation.
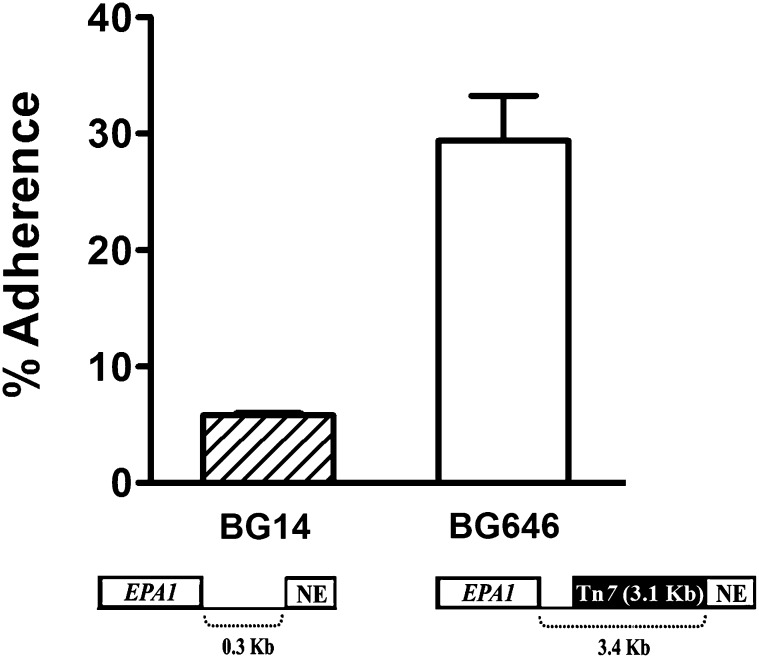
The effect of the NE on transcription of the EPA1 locus has a substantial impact on adherence, as would be expected. We compared adherence of a wild-type strain BG14 with strain BG646. Both strains contain the functional EPA1 ORF at the normal chromosomal locus, except that the latter carries a Tn7 insertion localized at 300 bp downstream of EPA1 ORF which separates the EPA1 ORF from the NE by 3.4 kb. As expected, the parental strain BG14 in SP does not express EPA1 and showed little adherence to HeLa cells (Figure 3) but SP cells of the mutant strain BG646 were hyperadherent (Figure 3). Consistent with this experiment, RT–PCR analysis of strain BG646 showed an increased transcript level of EPA1 (data not shown). This experiment indicates that the regulation mediated through the NE influences the adhesion phenotype of C. glabrata cells.
Figure 3 .
NE effect on adherence. C. glabrata wild-type strain BG14 (WT) (hatched bar) and strain BG646 containing a Tn7 insertion between EPA1 and the NE (open bar) were grown for 48 hr at 30° in YPD media. Cells were diluted to OD600 of 1.0 in HBSS supplemented with 5 mM CaCl2. The adherence assays were done as described in the Figure 1C legend. Adherent cells were recovered from the epithelial cells and were plated for viable accounts. See Materials and Methods.
EPA1 is controlled by the Sir complex and by yKu70, yKu80, Rap1, and Rif1
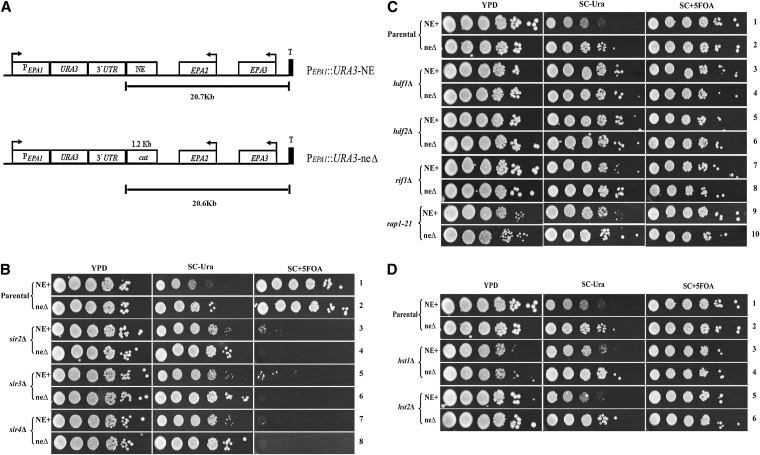
We have previously shown that the EPA1-3 loci (Figure 1A) is under the control of subtelomeric silencing. In those experiments, a URA3 reporter gene inserted at 20.7 kb from the telomere (300 bp from the TAA of EPA1), was largely not silenced, suggesting that the telomere position effect (TPE) ends before the EPA1 locus (De Las Peñas et al. 2003; Rosas-Hernandez et al. 2008). However, in contrast to those data, mutations in SIR2–SIR4 in fact increase the expression of EPA1 (Castaño et al. 2005; Domergue et al. 2005). We therefore revisited whether the EPA1 locus itself is controlled by silencing, by assessing the role of the Sir complex, yKu70, yKu80, Rap1, and Rif1 as well as the sirtuins Hst1 and Hst2. To monitor silencing of the EPA1 locus we constructed a sensitive silencing reporter strain in which the EPA1 ORF in its chromosomal location was replaced with the URA3 ORF. Separately, we replaced a 1.0-kb fragment (carrying the NE) from the EPA1/EPA2 intergenic region with a 1.2-kb fragment containing the cat gene (chloramphenicol acetyl transferase gene) from the bacterial plasmid pACYC184 to maintain the same distance from the telomeric repeats (Figure 4A). This pair of constructs permits assessment of silencing of the EPA1 locus as well as the impact of the NE on that silencing.
Figure 4 .
Silencing effect on the expression of EPA1. Schematic representation of the reporter strains. (A) EPA1 was replaced by the URA3 gene and the NE was replaced by the bacterial cat gene and recombined in the chromosome. URA3 reports the activity of the EPA1 promoter. (B) The parental strains NE+ and neΔ and the strains carrying null mutations in SIR2–SIR4 (sir2Δ–sir4Δ), (C) HDF1 (yKu70), HDF2 (yKu80), RIF1, and rap1-21 (hdf1Δ, hdf2Δ, and rap1-21), and (D) HST1 and HST2 (hst1Δ and hst2Δ) were grown for 48 hr in YPD. Strains were diluted to OD600nm 0.5 with distilled water and 10-fold serial dilutions were spotted onto YPD, SC −Ura and SC +5-FOA plates. Plates were incubated at 30°. Ura+ cells die on SC +5-FOA plates. Only cells with the URA3 gene transcriptionally repressed can grow on SC +5-FOA. See Materials and Methods.
The two strains (PEPA1::URA3::NE+, BG1124, and PEPA1::URA3::neΔ, BG1132) were grown in YPD media and spotted onto YPD (for viable counts), SC −Ura (to assess activity of the EPA1 promoter) and 5-FOA plates (to assess silencing of the EPA1::URA3 locus, since the synthesis of Ura3 is toxic to the cell in the presence of 5-FOA). The NE+ strain (PEPA1::URA3::NE+) grew poorly on SC −Ura medium (Figure 4B, line 1), whereas the neΔ strain (PEPA1::URA3::neΔ) grew better but not to the same extent as the viable count on YPD (Figure 4B, line 2). This is consistent with the repressor role of the NE on the expression of EPA1. Both parental strains (PEPA1::URA3::NE+ and PEPA1::URA3::neΔ) grew on 5-FOA plates at almost the same extent as the viable count on YPD, suggesting that EPA1 is subject to silencing independent of the NE (Figure 4B, lines 1 and 2). Consistent with this, even the neΔ strain (PEPA1::URA3::neΔ) did not grow as well on the SC −Ura plates as on the YPD or 5-FOA plates (Figure 4B, line 2), suggesting NE-independent repression of EPA1. These results suggest two levels of negative regulation of EPA1. One depends on the NE; in addition, a NE-independent repression is indicated by the growth of both strains (NE+ and neΔ) on 5-FOA plates and by the reduced growth of the neΔ strain (PEPA1::URA3::neΔ) on SC −Ura (Figure 4B, line 2).
To assess the impact of known silencing genes on repression of the EPA1 locus, we deleted the SIR2–SIR4, HDF1 (yKu70), HDF2 (yKu80), RIF1, HST1, and HST2 genes in the strains carrying the PEPA1::URA3::NE and PEPA1::URA3::neΔ constructs on the chromosome. In addition, in both strains, we replaced the essential RAP1 gene with the silencing defective rap1-21 allele. Strains were grown and spotted onto YPD, SC −Ura and 5-FOA plates. sir2Δ–sir4Δ mutant strains did not grow on 5-FOA plates, indicating that silencing is completely relieved independently of the presence of the NE (Figure 4B, lines 3–8). Consistent with this, growth of the sir2Δ–sir4Δ mutant strains on SC −Ura plates was better than the corresponding parental strains. Notably, growth of sir2Δ, sir3Δ, or sir4Δ NE+ strains on SC −Ura plates was consistently worse than the corresponding sir2Δ, sir3Δ, or sir4Δ neΔ strains (Figure 4B, lines 3, 5, and 7 and lines 4, 6, and 8 on SC −Ura plates). This suggests that the function of the NE is independent of the Sir proteins.
We analyzed the effect of yKu70, yKu80, Rif1, and Rap1 on the expression of EPA1. Loss of HDF1 (yKu70), HDF2 (yKu80), RIF1, and RAP1 in the presence (NE+) or absence (neΔ) of the NE did not substantially affect silencing of EPA1 since the vast majority of hdf1Δ, hdf2Δ, rif1Δ, or rap1-21 mutant strains grow on 5-FOA plates (Figure 4C, compare lines 1 and 2 with lines 3–10). The hdf1Δ, hdf2Δ, rif1Δ, and rap1-21 NE+ strains grew better than the parental NE+ strain on SC −Ura plates (Figure 4C, compare line 1 with lines 3, 5, 7, and 9), suggesting a derepression of EPA1 transcription in these backgrounds, even though the effect on silencing was minimal. Notably, for the hdf1Δ and hdf2Δ mutants, growth of the NE+ and neΔ strains on SC −Ura plates were the same, suggesting that HDF1 (yKu70) and HDF2 (yKu80) are required for function of the NE (Figure 4C, lines 3–6). The sirtuins Hst1 and Hst2 do not participate in the regulation of EPA1 since hst1Δ and hst2Δ strains in the presence (NE+) or absence (neΔ) of the NE behave the same as their corresponding parental strains (Figure 4D).
To assess the role of Sir2–Sir4, yKu70, yKu80, Rap1, and Rif1, on NE function independently of the telomere, we assayed the repression index of PEPA1::GFP::+500 bp (pSP21; Figure 2, line 1) in each mutant background. Consistent with no effect of Sir2–Sir4, Rif1, and Rap1 on NE function, the RI in these backgrounds was no different than wild-type strain BG14. By contrast, for yKu70 and yKu80, the RI was decreased to 8.74 and 8.66, respectively, consistent with a role for yKu70 and yKu80 in NE-mediated repression (Table 4).
Table 4 . Role of silencing proteins on NE function independently of telomere.
| Strain | Genotype | Plasmid | Repression index |
|---|---|---|---|
| BG14 | WT | pSP21 | 16.26 ± 2.00 |
| BG509 | rif1Δ | pSP21 | 15.06 ± 1.83 |
| BG592 | rap1-21 | pSP21 | 13.04 ± 1.87 |
| BG676 | sir3Δ | pSP21 | 16.71 ± 2.10 |
| BG1048 | sir2Δ | pSP21 | 14.62 ± 1.78 |
| BG1050 | sir4Δ | pSP21 | 15.78 ± 0.78 |
| BG1080 | hdf1Δ | pSP21 | 8.74 ± 1.54 |
| BG1081 | hdf2Δ | pSP21 | 8.66 ± 2.10 |
| BG14 | WT | pSP26 | 2.54 ± 0.40 |
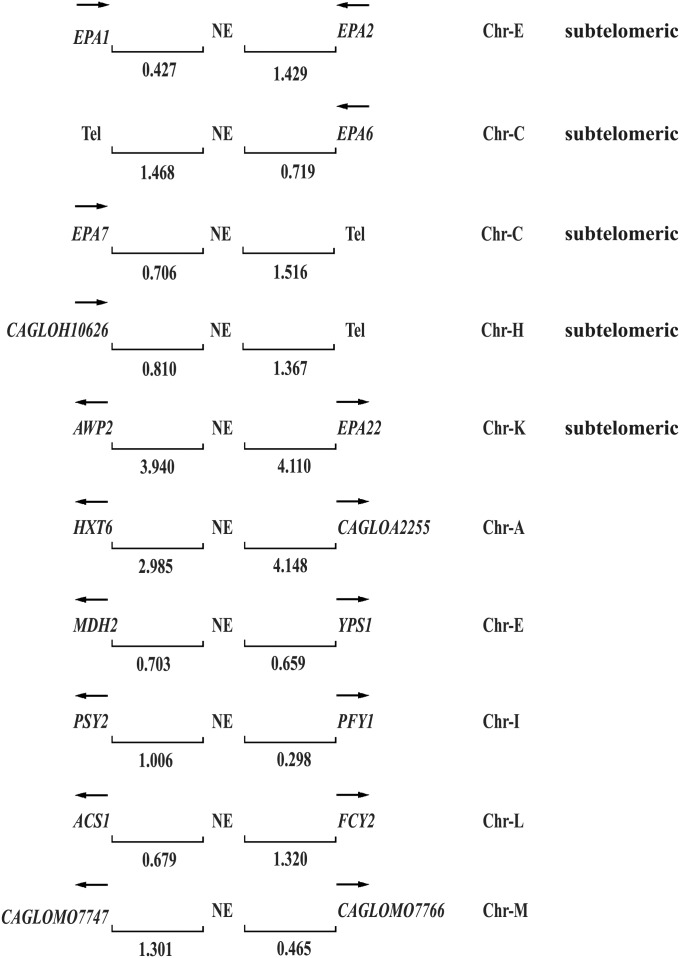
The NE is present 10 times in the C. glabrata genome
Finally, we asked whether additional copies of the NE were present in the C. glabrata genome and associated to other EPAs. We carried out a Blast search with the 200-bp sequence of the NE and found that the last 60 bp are present 10 times in the C. glabrata genome. This sequence is associated both with EPA genes as well as other genes (Figure 5).
Figure 5 .
Blast analysis of the NE in the C. glabrata genome. The last 60 bp of the NE is associated with other EPAs and non-EPA genes. EPA1, EPA2, EPA6, EPA7, AWP2, and CAGLOH10626 (15) encode cell wall proteins, all are subtelomeric and the NE associated is localized at their 3′ ends, except for AWP2 and EPA22, which is localized between these two divergently transcribed genes. The NE associated to non-EPA genes is located at the 5′ regions (promoters) of these divergently expressed genes. HXT6 (high-affinity glucose transporter of the major facilitator superfamily), MDH2 (cytoplasmic malate dehydrogenase), YPS1 (GPI-anchored aspartyl protease), PSY2 (putative subunit of an evolutionarily conserved protein phosphatase complex containing the catalytic subunit Pph3p and the regulatory subunit Psy4p), PFY1 (profilin, binds actin involved in cytoskeleton organization), ACS1 (acetyl-coA synthetase isoform), FCY2 (purine-cytosine permease), and CAGLOA2255, CAGL0M07747, and CAGL0M07766 are of unknown function (Saccharomyces Genome Database http://www.yeastgenome.org/ and C. glabrata Genome Database http://www.genolevures.org/cagl.html). Arrows indicate direction of transcription; numbers show the distance in kilobases between the negative element (NE) and the genes or the telomere (Tel); and Chr-(letter) denotes chromosome notation.
Discussion
Adherence to specific cell tissues is important for pathogens. Their capacity to adhere to cells, tissues, abiotic surfaces, as well as their ability to form biofilms is often tied to expression of families of cell surface proteins whose transcription is tightly controlled. In C. glabrata, the EPA genes encode GPI-anchored cell wall proteins of which EPA1, EPA6, and EPA7 have been shown to mediate adherence to epithelial cells in vitro (Cormack et al. 1999; De Las Peñas et al. 2003; Castaño et al. 2005; Domergue et al. 2005). Interestingly, many of these EPA genes are subject to chromatin-based subtelomeric silencing. This epigenetic regulation of adhesins is advantageous, since pathogens need not commit all cells in the population to express a particular adhesin, allowing a balance between adherence, colonization, and dissemination (De Las Peñas et al. 2003; Halme et al. 2004; Domergue et al. 2005).
A 3′ cis-acting element (NE) negatively regulates the expression of EPA1
In this study, we showed that EPA1 expression is negatively regulated by two independent mechanisms: subtelomeric silencing and a telomere-independent, novel mode of negative regulation, dependent on a cis-acting NE contained in a 200-bp fragment required for full activity, located 300 bp downstream from the stop codon of EPA1 in the intergenic region between EPA1 and EPA2 (Figure 2). The NE can still repress transcription independent of the 3′-UTR used and if placed upstream of the EPA1 promoter (Figure 1E), underscoring that the NE is a transcriptional, rather than a post-transcriptional regulatory element. Furthermore, the EPA1 3′-UTR has a partial contribution on the expression of EPA1.
To our knowledge, cis-acting elements located outside promoters, or 3′ of the ORF affecting the expression of promoters have not previously been reported in yeast for Pol II promoters, though similar cis-acting elements have been described in yeast for Pol III transcribed genes and in other organisms, some localized inside introns (Errede et al. 1987; Martin et al. 2001; Stark et al. 2001; Calderwood et al. 2003; Delaloy et al. 2008). In yeast, activators or repressors generally function when their corresponding cis-acting elements are located at distances no greater than 700 bp (Guarente and Hoar 1984; Struhl 1984; Keegan et al. 1986) upstream of the start site of transcription. Notably, cis-acting elements (UAS or operator) heterologously positioned at distances >700 bp on the 3′ end of a gene, can activate/repress if the reporter gene and the cis-element are localized near a telomere (de Bruin et al. 2001; Zaman et al. 2002). One explanation is that yeast telomeres can fold back and form a higher order structure or loop, allowing the cis-acting element with a tethered trans-acting factor to interact with the promoter and in this way activate or repress transcription of that gene. A similar mechanism could occur between the NE and some element in the EPA1 promoter, in which looping of the DNA would establish an interaction leading to repression of EPA1. In support of this hypothesis, the NE does not function to repress transcription of five other promoters (HHT2, MET3, PGK1, PDC1, and EGD1) when carried on a CEN ARS plasmid (data not shown).
Interestingly, there have been no reports in other Candida species of transcriptional regulation dependent on a cis-element 3′ to the ORF. However, in an example with clear parallels to EPA1 regulation, C. albicans, ALS1 and ALS7, which, like EPA1, encode cell surface adhesions, are also induced in lag phase (Green et al. 2005). Whether this regulation depends on a 3′ element has not, to our knowledge, been tested.
Repression of EPA1 expression by the NE depends on HDF1 (yKu70) and HDF2 (yKu80) but is independent of the telomere
EPA1 repression after induction analyzed in the context of the chromosome, can be recapitulated in a plasmid (BG198 vs. pAP385, Figure 1, D and E), indicating that all regulatory elements for proper regulation of EPA1 are contained in the plasmid (pAP385) and that NE-mediated repression of EPA1 transcription is telomere independent. This telomere-independent regulation of EPA1 expression must coordinate with telomere-dependent silencing.
Surprisingly, our genetic data show that the NE-mediated repression depends on HDF1(yKu70) and HDF2 (yKu80) (Figure 4C, lines 3–6). yKu70 and yKu80 are essential to repair double strand breaks by nonhomologous end joining (Rosas-Hernandez et al. 2008), prevent native chromosome ends from degradation and fusion, and initiate silencing by recruiting Sir4 to the telomere (Tham and Zakian 2002). Interestingly, C. glabrata yKu70/yKu80 are not required for subtelomeric silencing at the EPA1 telomere (chromosome E Right) (Rosas-Hernandez et al. 2008). How might yKu70 and yKu80 repress transcription of EPA1 through the NE? Given that it has been shown that the Ku complex also associates with subtelomeric regions (Martin et al. 1999) and can nucleate silencing when tethered (Tham and Zakian 2002; Rusche et al. 2003), a possible model is that Ku associates directly with the NE ultimately leading to repression. We suggest that the repression mechanism might result from Ku-mediated interactions between the NE and elements in the EPA1 promoter that form a transcriptionally repressed chromatin loop.
EPA1 expression is silenced
We have shown previously that EPA1 expression is regulated by the silencing machinery (Castaño et al. 2005; Domergue et al. 2005). However, the analysis of URA3 reporter genes inserted in the EPA1–EPA3 region suggested that subtelomeric silencing ends in the intergenic region between EPA1 and EPA2 (De Las Peñas et al. 2003). We revisited this in the current study, and our genetic experiments confirm that Sir2–Sir4 silence the expression of EPA1. We suggest that differences in the reporter constructs used affect the silencing assay. The URA3 reporter silencing experiments were done with two different promoters—the EPA1 promoter fused to the URA3 gene (shown in this article) and the URA3 gene driven by its own promoter in the previous analysis (De Las Peñas et al. 2003; Castaño et al. 2005; Rosas-Hernandez et al. 2008). We suggest that the EPA1–URA3 construct more faithfully reported silencing than the heterologous URA3 gene either because the EPA1 promoter is weaker and more sensitive to silencing or potentially because the URA3 reporter inherently can be an imperfect measure of silencing (Stillman et al. 2011).
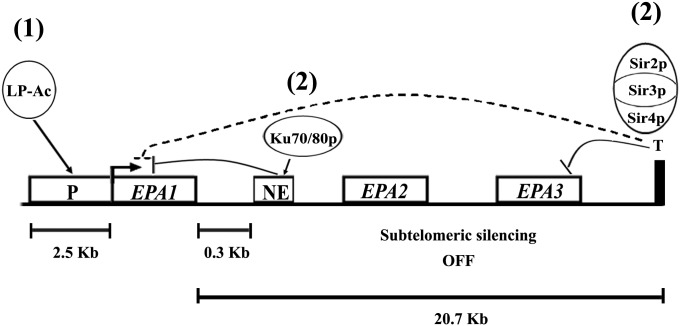
Our data suggest, therefore, that there are two distinct mechanisms that maintain the expression of EPA1 tightly controlled: negative regulation by silencing, which depends on the Sir complex and telomere proximity, and a telomere-independent, Sir-independent repression by a NE at the 3′ end of the gene that depends on yKu70 and yKu80. These mechanisms operate independently of one another but in conjunction tightly control expression of EPA1. In our current model of EPA1 transcriptional regulation, upon dilution into fresh media, a log-phase–specific transcriptional activator (LP-Ac, Figure 6) induces EPA1 expression (Figure 1B). After one cell division, both silencing (Sir complex dependent) and the NE with yKu70/80 cooperate to repress transcription. These two regulatory mechanisms counteract the putative LP-Ac that is present and active throughout LP, keeping the expression of EPA1 repressed. This mode of regulation keeps the expression of EPA1 tightly repressed, but poised to be transiently induced in the appropriate environment. We do not know the identity of the activator; however, the transcription factors Flo8 and Mss1 are candidates since both transcription factors are normally required for the expression of EPA6 under inducing environmental conditions (Mundy and Cormack 2009).
Figure 6 .
Model of EPA1 regulation. In stationary phase (SP), EPA1 is not expressed and cells are nonadherent. Upon dilution into fresh media, (1) a log-phase specific transcriptional activator (LP-Ac) induces expression of EPA1 and cells become adherent. This transcriptional activation is counteracted in log phase (LP) by the concerted action of silencing and the NE (2). The Sir complex silences the expression of EPA1 and yKu70/yKu80 repress EPA1 expression through the NE. These two regulatory mechanisms assure that EPA1 is not expressed even in the presence of the LP-Ac, which is active throughout LP.
It is worth pointing out that traditional gene expression analyses in yeast do not usually use cognate 3′-UTR/3′-intergenic regions or assess their potential impact on regulation. Our data suggest that it might be worth doing so.
Acknowledgments
We thank B. Ma for careful reading of the manuscript. This work was funded by a Consejo Nacional de Ciencia y Tecnología (CONACYT) fellowship to V.G.-G. (143037), J.J.-C. (48549), M.B.-M.-C. (209276) and V.M.-J. (205619). This work was funded by CONACYT grant no. CB-2005-48279 to A.D.L.P and by National Institutes of Health grant 5R01AI046223 to B.C. We will always remember Meg.
Footnotes
Communicating editor: F. Winston
Literature Cited
- Ausubel, F., R. Brent, R. E. Kingston, D. Moore, J. G. Seidman et al., 2001 Current Protocols in Molecular Biology. John Wiley & Sons, New York.
- Calderwood M. S., Gannoun-Zaki L., Wellems T. E., Deitsch K. W., 2003. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J. Biol. Chem. 278: 34125–34132 [DOI] [PubMed] [Google Scholar]
- Calvin N. M., Hanawalt P. C., 1988. High-efficiency transformation of bacterial cells by electroporation. J. Bacteriol. 170: 2796–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño I., Kaur R., Pan S., Cregg R., De Las Peñas A., et al. , 2003. Tn7-based genome-wide random insertional mutagenesis of Candida glabrata. Genome Res. 13: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño I., Pan S. J., Zupancic M., Hennequin C., Dujon B., et al. , 2005. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 55: 1246–1258 [DOI] [PubMed] [Google Scholar]
- Castaño I., De Las Peñas A., Cormack B., 2006. Function and regulation of adhesin gene families in Saccharomyces cerevisiae, Candida albicans, and Candida glabrata, p. 684 Molecular Principles of Fungal Pathogenesis, edited by Heitman J., Edwards J. E., Filler S. G., Mitchell A. P. ASM Press, New York [Google Scholar]
- Chang A. C., Cohen S. N., 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134: 1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack B. P., Falkow S., 1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack B. P., Ghori N., Falkow S., 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285: 578–582 [DOI] [PubMed] [Google Scholar]
- Cuellar-Cruz M., Briones-Martin-del-Campo M., Canas-Villamar I., Montalvo-Arredondo J., Riego-Ruiz L., et al. , 2008. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot. Cell 7: 814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Cruz M., Castaño I., Arroyo-Helguera O., De Las Peñas A., 2009. Oxidative stress response to menadione and cumene hydroperoxide in the opportunistic fungal pathogen Candida glabrata. Mem. Inst. Oswaldo Cruz 104: 649–654 [DOI] [PubMed] [Google Scholar]
- de Bruin D., Zaman Z., Liberatore R. A., Ptashne M., 2001. Telomere looping permits gene activation by a downstream UAS in yeast. Nature 409: 109–113 [DOI] [PubMed] [Google Scholar]
- De Groot P. W. J., Hellingwerf K. J., Klis F. M., 2003a. Genome-wide identification of fungal GPI proteins. Yeast 20: 781–796 [DOI] [PubMed] [Google Scholar]
- De Groot P. W. J., Dekker H. L., De Boer A. D., Hellingwerf K. J., De Koster C. G., et al. , 2003b. Identification of cell wall proteins of the fungal pathogen Candida albicans and other fungi using mass-spectrometric and genome-wide computational approaches. Yeast 20: S57–S57 [Google Scholar]
- De Groot P. W. J., Kraneveld E. A., Yin Q. Y., Dekker H. L., Gross U., et al. , 2008. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 7: 1951–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Peñas A., Pan S. J., Castaño I., Alder J., Cregg R., et al. , 2003. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 17: 2245–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloy C., Elvira-Matelot E., Clemessy M., Zhou X. O., Imbert-Teboul M., et al. , 2008. Deletion of WNK1 First Intron Results in Misregulation of Both Isoforms in Renal and Extrarenal Tissues. Hypertension 52: 1149–1154 [DOI] [PubMed] [Google Scholar]
- Domergue R., Castaño I., De Las Peñas A., Zupancic M., Lockatell V., et al. , 2005. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 308: 866–870 [DOI] [PubMed] [Google Scholar]
- Errede B., Company M., Hutchison C. A., 3rd, 1987. Ty1 sequence with enhancer and mating-type-dependent regulatory activities. Mol. Cell. Biol. 7: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Cutright J. L., Tait L., Sobel J. D., 1996. A murine model of Candida glabrata vaginitis. J. Infect. Dis. 173: 425–431 [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A., 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534 [DOI] [PubMed] [Google Scholar]
- Green C. B., Zhao X., Yeater K. M., Hoyer L. L., 2005. Construction and real-time RT-PCR validation of Candida albicans PALS-GFP reporter strains and their use in flow cytometry analysis of ALS gene expression in budding and filamenting cells. Microbiology 151: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Guarente L., Hoar E., 1984. Upstream activation sites of the CYC1 gene of Saccharomyces cerevisiae are active when inverted but not when placed downstream of the “TATA box”. Proc. Natl. Acad. Sci. USA 81: 7860–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Styles C. A., Feng Q. H., Fink G. R., 2000. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97: 12158–12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A., Bumgarner S., Styles C., Fink G. R., 2004. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell 116: 405–415 [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., 2001. The ALS gene family of Candida albicans. Trends Microbiol. 9: 176–180 [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., Green C. B., Oh S. H., Zhao X., 2008. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med. Mycol. 46: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Domergue R., Zupancic M. L., Cormack B. P., 2005. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 8: 378–384 [DOI] [PubMed] [Google Scholar]
- Keegan L., Gill G., Ptashne M., 1986. Separation of DNA binding from the transcription-activating function of a eukaryotic regulatory protein. Science 231: 699–704 [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Gaur N. K., Lake D. F., Chan V., Rauceo J., et al. , 2004. Degenerate peptide recognition by Candida albicans adhesins Als5p and Als1p. Infect. Immun. 72: 2029–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi O., Hayashi N., Kuroki R., Sone H., 1998. Region of Flo1 proteins responsible for sugar recognition. J. Bacteriol. 180: 6503–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Palecek S. P., 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell 2: 1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. P., Gerlach V. L., Brow D. A., 2001. A novel upstream RNA polymerase III promoter element becomes essential when the chromatin structure of the yeast U6 RNA gene is altered. Mol. Cell. Biol. 21: 6429–6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G., Laroche T., Suka N., Grunstein M., Gasser S. M., 1999. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97: 621–633 [DOI] [PubMed] [Google Scholar]
- Mundy R. D., Cormack B., 2009. Expression of Candida glabrata adhesins after exposure to chemical preservatives. J. Infect. Dis. 199: 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Diekema D. J., 2010. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36: 1–53 [DOI] [PubMed] [Google Scholar]
- Roetzer A., Gabaldon T., Schuller C., 2011. From Saccharomyces cerevisiae to Candida glabrata in a few easy steps: important adaptations for an opportunistic pathogen. FEMS Microbiol. Lett. 314: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Hernandez L. L., Juarez-Reyes A., Arroyo-Helguera O. E., De Las Peñas A., Pan S. J., et al. , 2008. yKu70/yKu80 and Rif1 regulate silencing differentially at telomeres in Candida glabrata. Eukaryot. Cell 7: 2168–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche L. N., Kirchmaier A. L., Rine J., 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481–516 [DOI] [PubMed] [Google Scholar]
- Sheppard D. C., Yeaman M. R., Welch W. H., Phan Q. T., Fu Y., et al. , 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279: 30480–30489 [DOI] [PubMed] [Google Scholar]
- Sherman, F., G. R. Fink, and J. B. Hicks, 1986 Methods in Yeast Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sikorski R. S., Hieter P., 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K., Kirk D. L., Schmitt R., 2001. Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carteri. Genes Dev. 15: 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Rossmann M. P., Luo W. J., Tsaponina O., Chabes A., 2011. A common telomeric gene silencing assay is affected by nucleotide metabolism. Mol. Cell 42: 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., 1984. Genetic properties and chromatin structure of the yeast gal regulatory element: an enhancer-like sequence. Proc. Natl. Acad. Sci. USA 81: 7865–7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham W. H., Zakian V. A., 2002. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21: 512–521 [DOI] [PubMed] [Google Scholar]
- Zaman Z., Heid C., Ptashne M., 2002. Telomere looping permits repression “at a distance” in yeast. Curr. Biol. 12: 930–933 [DOI] [PubMed] [Google Scholar]
- Zupancic M. L., Frieman M., Smith D., Alvarez R. A., Cummings R. D., et al. , 2008. Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol. Microbiol. 68: 547–559 [DOI] [PubMed] [Google Scholar]