Abstract
Molecules in the extracellular matrix (ECM) regulate cellular behavior in both development and pathology. Fibulin-1 is a conserved ECM protein. The Caenorhabditis elegans ortholog, FBL-1, regulates gonad-arm elongation and expansion by acting antagonistically to GON-1, an ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family protease. The elongation of gonad arms is directed by gonadal distal tip cells (DTCs). Here we report that a dominant mutation in the EMB-9/type IV collagen α1 subunit can compensate for loss of FBL-1 activity in gonadogenesis. A specific amino acid substitution in the noncollagenous 1 (NC1) domain of EMB-9 suppressed the fbl-1 null mutant. FBL-1 was required to maintain wild-type EMB-9 in the basement membrane (BM), whereas mutant EMB-9 was retained in the absence of FBL-1. EMB-9 (either wild type or mutant) localization in the BM enhanced PAT-3/β-integrin expression in DTCs. In addition, overexpression of PAT-3 partially rescued the DTC migration defects in fbl-1 mutants, suggesting that EMB-9 acts in part through PAT-3 to control DTC migration. In contrast to the suppression of fbl-1(tk45), mutant EMB-9 enhanced the gonadal defects of gon-1(e1254), suggesting that it gained a function similar to that of wild-type FBL-1, which promotes DTC migration by inhibiting GON-1. We propose that FBL-1 and GON-1 control EMB-9 accumulation in the BM and promote PAT-3 expression to control DTC migration.
COLLECTIVE cell migration is among the major strategies to shape tissues and organs (Bianco et al. 2007; Ewald et al. 2008; Lu and Werb 2008; Montell 2008; Friedl and Gilmour 2009; Rorth 2009). In this process, remodeling of the extracellular matrix (ECM) positively and negatively affects the interactions between basement membranes (BMs) and migrating cells or epithelia to yield the proper cell geometry and tissue architecture. However, how the ECM is remodeled and how migrating cells interact with changing ECM milieus during organogenesis is mostly unknown.
The development of Caenorhabditis elegans hermaphrodite gonads is regulated by migration of the gonadal leader cells, distal tip cells (DTCs), which promote the directional elongation of the gonad arms during the larval stages to form the U-shaped gonads found in adult animals (Kimble and Hirsh 1979; Hedgecock et al. 1987). Two secreted ADAMTS family metalloproteases, GON-1 and MIG-17, act cooperatively in this process; GON-1 is required for the motility of DTCs and gonadal expansion, whereas MIG-17 acts to control the direction of DTC movement but does not control DTC motility per se (Blelloch and Kimble 1999; Nishiwaki et al. 2000). GON-1 expressed in DTCs and that expressed in body wall muscles regulate gonadal elongation and expansion, respectively (Blelloch and Kimble 1999). GON-1 and MIG-17 are proposed to remodel the BM proteolytically to regulate the microenvironment that affects gonad shaping (Blelloch et al. 1999; Blelloch and Kimble 1999; Nishiwaki et al. 2000). GON-1 and FBL-1, a fibulin-1 ortholog in C. elegans, act antagonistically during gonadogenesis (Hesselson et al. 2004). GON-1 is homologous to mammalian ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) proteinases ADAMTS-9 and ADAMTS-20 (Llamazares et al. 2003; Somerville et al. 2003). ADAMTS-5, ADAMTS-9, and ADAMTS-20 cooperate with fibulin-1 to promote regression of the interdigital web by generating active versican fragments in the mouse (McCulloch et al. 2009).
To uncover new components involved in the antagonistic interaction between FBL-1 and GON-1 to control gonadogenesis, we conducted a suppressor analysis of fbl-1(tk45) null mutants. Two independently isolated suppressors were found to be identical mutations in the gene emb-9, which was previously identified by embryonic lethal mutations and encodes the type IV collagen α1 chain (Miwa et al. 1980; Guo et al. 1991). In contrast to the suppression of fbl-1, the emb-9 mutation enhanced the gonadal defects of gon-1. We also found that the reduced expression of PAT-3/β-integrin in DTCs of fbl-1 mutants was restored to the wild-type levels in the suppressor emb-9 mutant background. Our findings suggest that intermolecular interactions among FBL-1, EMB-9, and GON-1 regulate the type IV collagen-dependent extracellular microenvironment that involves the activation of integrin-mediated organogenesis.
Materials and Methods
Strains and genetic analysis
Culture, handling, and ethyl methanesulfonate (EMS) mutagenesis of C. elegans were conducted as described (Brenner 1974). Worms were grown at 20° unless otherwise noted. The following mutations and genetic balancers were used in this work: emb-9(g34, g23cg46, tk75), fbl-1(tk45), gon-1(e1254, q518), unc-119(e2498), hT2[bli-4(e937) let-?(q782) qIs48], and nT1[qIs51] (Gupta et al. 1997; Blelloch and Kimble 1999; Nishiwaki et al. 2000; Kubota et al. 2004; Hesselson and Kimble 2006). rhIs2[pat-3::GFP] is a genomic insertion of functional pat-3::GFP genes (Plenefisch et al. 2000). For suppressor screening, fbl-1(tk45); Ex[fbl-1(+), sur-5::GFP] hermaphrodites were used for EMS mutagenesis. The suppressor mutants tk75 and tk76 were isolated as described (Kubota et al. 2004) and backcrossed four times. The dominancy of the emb-9(tk75) mutation on fbl-1 phenotypes was examined as follows: unc-119(e2498); fbl-1(tk45)/nT1[qIs51] hermaphrodites were mated with emb-9(tk75); fbl-1(tk45) males, and gonadal phenotypes of non-uncoordinated hermaphroditic progeny without GFP were scored. Gene dosage effects of the emb-9 mutation on fbl-1 phenotypes were examined as follows: unc-119(e2498); fbl-1(tk45)/nT1[qIs51] hermaphrodites were mated with emb-9(g23cg46)/hT2[bli-4(e937) let-?(q782) qIs48]; fbl-1(tk45); Ex[fbl-1(+), sur5::GFP] males, and gonadal phenotypes of non-uncoordinated hermaphroditic progeny without GFP were scored. The dominancy of the emb-9(tk75) mutation on gon-1 phenotypes was examined as follows: unc-119(e2498); gon-1(e1254)/nT1[qIs51] hermaphrodites were mated with emb-9(tk75); gon-1(e1254)/nT1[qIs51] males, and gonadal phenotypes of non-uncoordinated hermaphroditic progeny without GFP were scored. Gene dosage effects of the emb-9 mutation on gon-1(e1254) phenotypes were examined as follows: unc-119(e2498); gon-1(e1254)/nT1[qIs51] hermaphrodites were mated with emb-9(g23cg46)/hT2[bli-4(e937) let-?(q782) qIs48]; gon-1(e1254); Ex[gon-1(+), sur-5::GFP] or emb-9(tk75); gon-1(e1254)/nT1[qIs51] males, and gonadal phenotypes of non-uncoordinated hermaphroditic progeny without GFP were scored.
Microscopy
Gonad migration phenotypes were scored using a Nomarski microscope (Axioplan 2; Zeiss). Analysis of gonadal phenotypes was performed at the young-adult stage as described (Nishiwaki 1999). A germline leakage phenotype was observed in 1-day-old adults. The localization of EMB-9, NID-1, and PAT-3–GFP was analyzed using a confocal laser scanning microscope (LSM5; Zeiss) equipped with a C-apochromat ×63 (water immersion; NA 1.2) lens and controlled by PASCAL version 3.2 SP2 software. Fluorescence microscopy was performed using the Axioplan 2 microscope. Images were captured with a Hamamatsu Photonics Chilled 3-CCD camera C5810 connected to a Windows computer (Dell).
Molecular cloning of suppressors
Single-nucleotide polymorphism (SNP) mapping (Wicks et al. 2001) and genomic sequence analysis of tk75 identified a point mutation from GGA to GAA (G1708E) in emb-9A. The PCR-amplified emb-9 fragment from emb-9(tk75); fbl-1(tk45) rescued the gonadal elongation defect and sterility of fbl-1(tk45) mutants. tk76 was identical to the emb-9(tk75) mutation.
Constructs
The wild-type emb-9, which corresponds to nucleotide positions 11,047–499 of the cosmid K04H4, was subcloned into PBSII KS(−). The emb-9(tk75) gene was constructed by introducing the tk75 mutation into the wild-type construct by site-directed mutagenesis.
Germline transformation
Germline transformation was carried out as described (Mello et al. 1991). Wild-type and mutant emb-9 plasmids were injected at 5 ng/µl with 55 ng/µl pBluescript KS(−) (carrier DNA), 20 ng/µl unc-119+ plasmid (pDP#MM016B) (Maduro and Pilgrim 1995), and 70 ng/µl marker plasmid (sur-5::GFP) (Yochem et al. 1998).
Antibody staining
Preparation of frozen sections and antibody staining were as described (Kubota et al. 2006). The primary antibodies were anti–EMB-9 (NW1910; 1:200) (Graham et al. 1997) and anti–NID-1 (5 µg/ml) (Kubota et al. 2008); Alexa Fluor 594-conjugated goat antirabbit IgG (1:500; Molecular Probes) was the secondary antibody.
Results
A dominant mutation in the type IV collagen α1 chain suppresses the gonadal defects in fbl-1(tk45) mutants
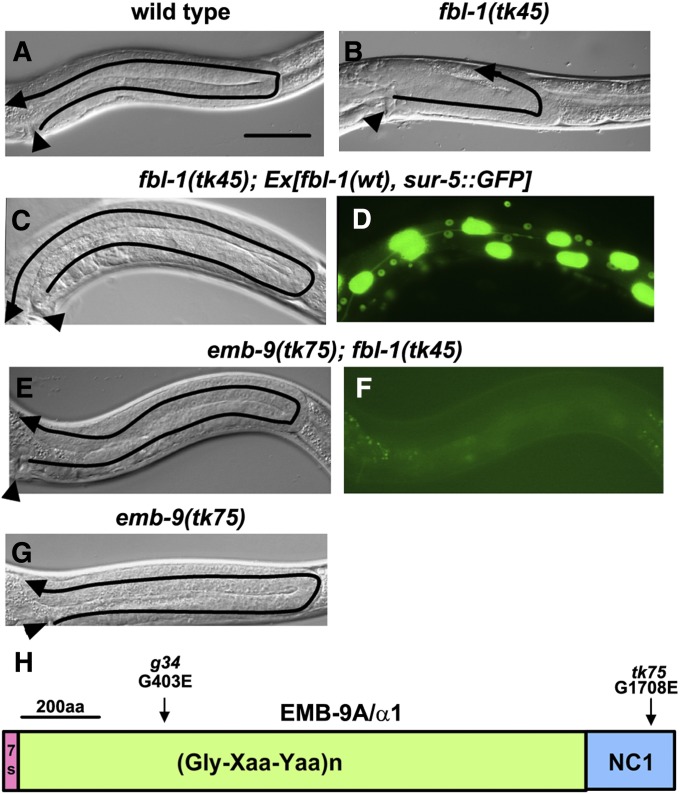
Fibulin family proteins are conserved ECM proteins (Argraves et al. 2003; Timpl et al. 2003). Loss-of-function mutations in fbl-1 result in gonads with short and swollen arms (Figure 1, A and B) (Hesselson et al. 2004; Kubota et al. 2004; Muriel et al. 2005). We used EMS mutagenesis to isolate suppressors of fbl-1(tk45), a null allele with a deletion in the fbl-1 coding region (Kubota et al. 2004; Hesselson and Kimble 2006). Although fbl-1(tk45) homozygotes are sterile, a transgenic strain fbl-1(tk45); Ex[fbl-1(+), sur-5::GFP] carrying an extrachromosomal array that consists of multiple copies of wild-type fbl-1 and sur-5::GFP can proliferate as a homozygote (Kubota et al. 2004). sur-5::GFP is a marker construct that expresses GFP in all somatic nuclei. In this strain, 37% of F1 animals (n = 142) inherited the GFP+ extrachromosomal array and showed wild-type U-shaped gonad arm morphology (Figure 1, C and D). We isolated GFP− animals with U-shaped gonad arms from the F2 or F3 generation of transgenic animals treated with EMS. Two independently isolated suppressor mutations, tk75 and tk76, were found to be identical mutations in emb-9, which encodes the α1 subunit of type IV collagen (Guo et al. 1991). The mutation was a G-to-A transition, resulting in a change from Gly-1708 to Glu in the C-terminal noncollagenous 1 (NC1) domain of EMB-9A (Figure 1H, Supporting Information, Figure S1). tk75 was dominant and strongly suppressed the short and swollen arm phenotype of fbl-1(tk45) mutants (Figures 1, E and F and 2A). The germ cells break out of the gonad and spread into the body cavity in older fbl-1(tk45) adults (Kubota et al. 2004). This phenotype was also suppressed by tk75 (Figure S2).
Figure 1 .
Gonadal phenotypes of the wild type and mutants. Nomarski (A–C, E, and G) and fluorescence (D and F) images of posterior gonads. Anterior is left, and dorsal is up. Posterior gonads of wild-type (A), fbl-1(tk45) (B), fbl-1(tk45); Ex[fbl-1(+), sur-5::GFP] (C and D), emb-9(tk75); fbl-1(tk45) (E and F), and emb-9(tk75) (G) hermaphrodites are shown. The predicted routes of DTC migration are shown by arrows. Arrowheads point to the vulvae. Bar, 50 µm. GFP is expressed in the somatic nuclei in D but not in F. (H) Domain structure of EMB-9 and the locations of mutations used in this study. Amino acid substitutions are shown. Amino acid positions in EMB-9 correspond to those of EMB-9A, one of the two alternatively spliced variants.
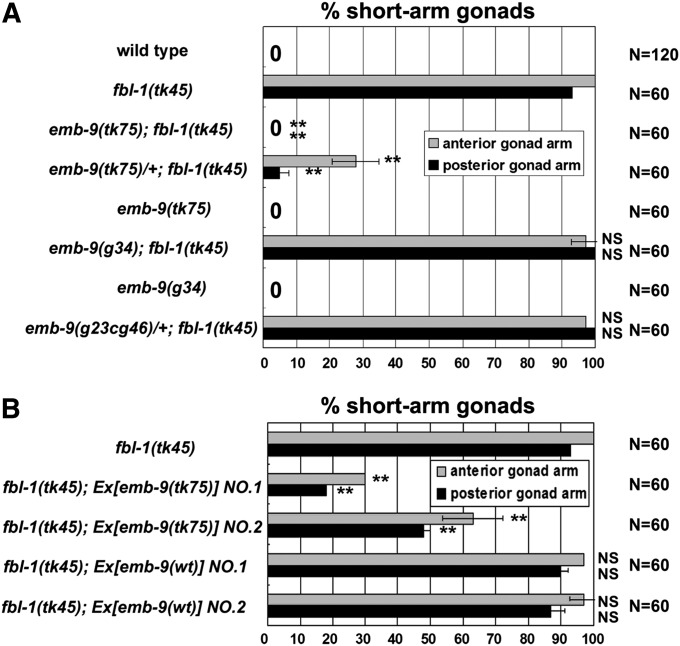
Figure 2 .
emb-9(tk75) suppresses the gonadal elongation defect of the fbl-1(tk45) mutant. (A) Quantification of gonadal elongation defects in fbl-1 and emb-9 mutants and in emb-9; fbl-1 double mutants. Data are shown as the mean ± SD. Results for Fisher’s exact test vs. fbl-1(tk45) are indicated; **P < 0.01; NS, not significant. (B) Effects of mutant and wild-type (wt) emb-9 transgenes on suppression. Two independent transgenic lines were examined for each transgene. The percentage of animals with anterior and posterior gonadal elongation defects is shown. Data are shown as the mean ± SD. Results for Fisher’s exact test vs. fbl-1(tk45) are indicated; **P < 0.01; NS, not significant.
Conventional emb-9 mutations are embryonic or larval lethal (Gupta et al. 1997). In contrast, tk75, the suppressor of the fbl-1 mutation, was homozygous viable and had no gonadal defects at 20° (Figures 1G and 2A). Only a small proportion of tk75 animals exhibited short and swollen arm defects at 25° (11% of anterior gonad arms, 5% of posterior gonad arms; n = 60). We also investigated the effects of a known emb-9 mutation on gonad morphogenesis in fbl-1(tk45) mutants. A temperature-sensitive (ts) embryonic lethal allele of emb-9, g34 (Gupta et al. 1997), did not exhibit gonadal elongation defects at 20°, an intermediate temperature that results in growth retardation: 50% of g34 larvae and 100% of wild-type larvae shifted from 16° to 20° at the first larval stage (L1) grew to be adults after 48 hr (n = 80). Shifting mutant L2 larvae to 25°, a restrictive temperature, resulted in severe gonadal defects (100% of both anterior and posterior gonad arms; n = 20) as reported (Kawano et al. 2009). g34 could not suppress the fbl-1(tk45) mutants at 20° (Figure 2A). Because of the dominant suppression effect, we investigated whether tk75 is a loss-of-function or a gain-of-function mutation. emb-9(g23cg46) (null)/+ exhibited no suppression activity. In addition, although the extrachromosomal transgenic arrays containing multiple wild-type copies of emb-9 failed to suppress fbl-1(tk45), the emb-9(tk75) mutant arrays significantly suppressed fbl-1 defects (Figure 2B).These results suggest that tk75 is a gain-of-function allele of emb-9 that suppresses fbl-1(tk45).
The suppressor EMB-9(tk75) localizes to BMs and suppresses loss of NID-1 from BMs in late larvae of fbl-1(tk45) mutants
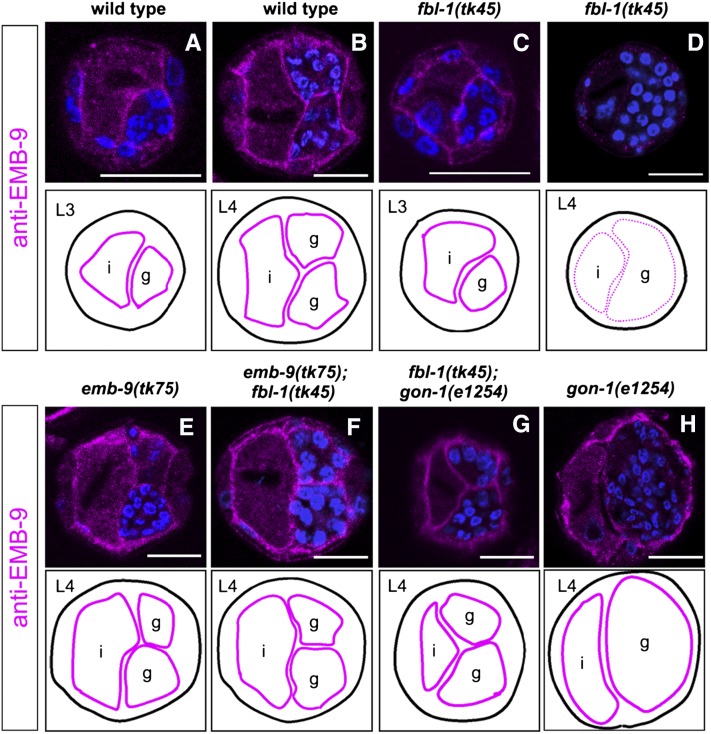
EMB-9 is secreted from the body wall muscle cells and localizes to the BM (Graham et al. 1997). An antibody against EMB-9 clearly stained gonadal and intestinal BMs in wild-type hermaphrodites at the third and fourth larval stages (L3 and L4, respectively) (Figure 3, A and B). EMB-9 accumulation in fbl-1(tk45) mutants was normal at L3 but was markedly reduced and punctate at L4 (Figure 3, C and D). These results suggest that FBL-1 is not required for gonadal accumulation of EMB-9 prior to L4, but for maintaining EMB-9 localization in the gonadal BM during the late larval stages.
Figure 3 .
emb-9(tk75) and gon-1(e1254) suppress the reduced gonadal BM localization of EMB-9 in fbl-1(tk45) mutants. Localization of wild-type EMB-9 in wild type (A and B) and in the mutants fbl-1(tk45) (C and D), fbl-1(tk45); gon-1(e1254) (G), and gon-1(e1254) (H). Localization of mutant EMB-9 in emb-9(tk75) (E) and emb-9(tk75); fbl-1(tk45) (F) animals. Cross-sections of L3 (A and C) and L4 (B and D–H) hermaphrodites were stained with anti–EMB-9 (magenta) and DAPI (blue) and analyzed with confocal microscopy. Borders of gonads and intestines are illustrated below the photos. Patterns of EMB-9 localization to the basement membrane are indicated by thick (normal) and dotted (weak and discontinuous) magenta lines. We observed similar results in more than three experimental sets (30–40 sections were observed for each genotype in an experiment). Bars, 25 µm. i, intestine; g, gonad.
We next examined the localization of the suppressor EMB-9(tk75). Interestingly, the EMB-9(tk75) mutant protein was not lost from the gonadal and intestinal BMs in either emb-9(tk75) or emb-9(tk75); fbl-1(tk45) mutants at L4 (Figure 3, E and F). These results suggest that EMB-9(tk75) suppresses the gonadal elongation defects of fbl-1(tk45) by remaining localized to the gonadal BM during the late larval development.
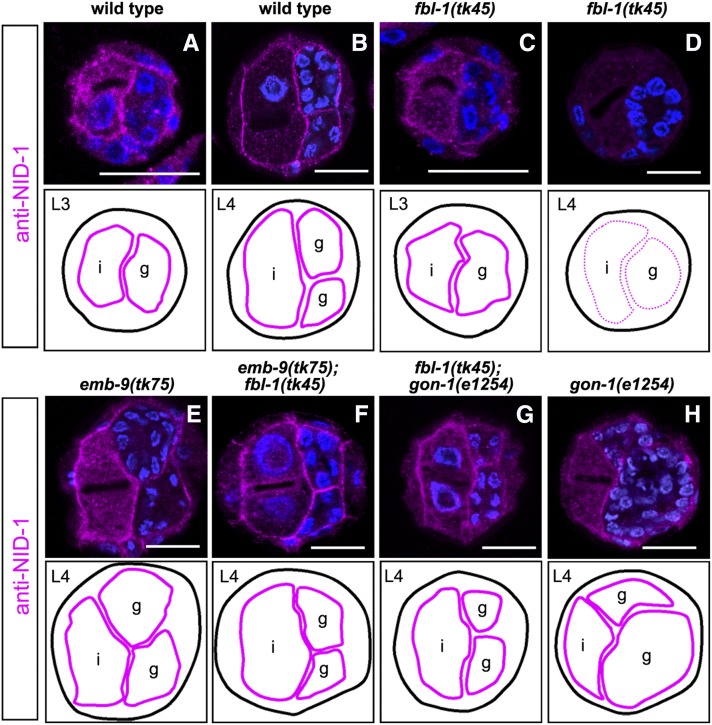
We previously showed that fbl-1 is required for BM localization of NID-1/nidogen, although nid-1 null mutants have little effect on gonadogenesis (Kubota et al. 2008). We examined whether defective localization of NID-1 is suppressed by emb-9(tk75). Anti–NID-1 detected a signal in the BM of the wild-type and fbl-1(tk45) L3 larvae, but the signal was substantially reduced in fbl-1(tk45) L4 larvae (Figure 4, A–D). As expected, the defective localization of NID-1 was suppressed by emb-9(tk75) (Figure 4, E and F), suggesting that the mutant EMB-9(tk75) protein acts to retain NID-1 in the BM in the late fbl-1(tk45) larvae.
Figure 4 .
emb-9(tk75) and gon-1(e1254) suppress the reduced gonadal BM localization of NID-1 in fbl-1(tk45) mutants. Localization of wild-type NID-1 in wild type (A and B) and in the mutants fbl-1(tk45) (C and D), fbl-1(tk45); gon-1(e1254) (G), and gon-1(e1254) (H). Localization of mutant NID-1 in emb-9(tk75) (E) and emb-9(tk75); fbl-1(tk45) (F) animals. Cross-sections of L3 (A and C) and L4 (B and D–H) hermaphrodites were stained with anti–NID-1 (magenta) and DAPI (blue) and analyzed with confocal microscopy. Borders of gonads and intestines are illustrated below the photos. Patterns of NID-1 localization in the BM are indicated by thick (normal) and dotted (weak and discontinuous) magenta lines. We observed similar results in more than three experimental sets as in Figure 3. Bars, 25 µm. i, intestine; g, gonad.
emb-9(tk75) suppresses the reduced PAT-3 expression in fbl-1(tk45) mutants
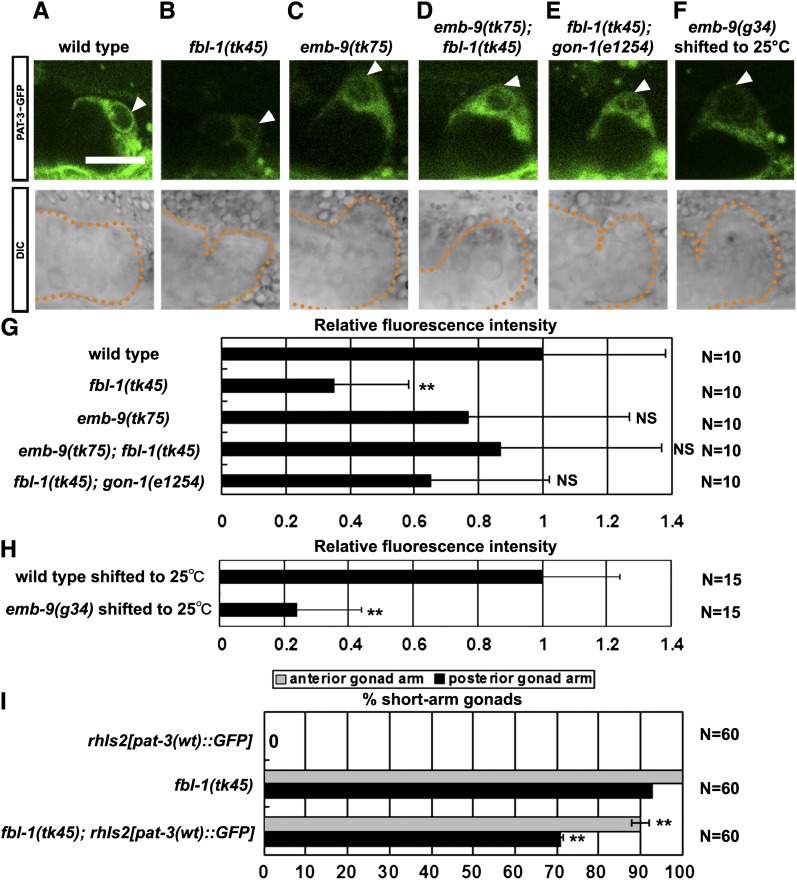
Because integrins are major receptors for ECM proteins, we examined the expression of PAT-3–GFP (Plenefisch et al. 2000), a functional GFP fusion of β-integrin, in the DTCs. The expression of PAT-3–GFP in fbl-1(tk45) was significantly weaker than that in the wild type (Figure 5, A, B, and G). PAT-3–GFP was expressed normally in emb-9(tk75) mutants (Figure 5C). The expression of PAT-3–GFP reduced in fbl-1(tk45) was restored in emb-9(tk75); fbl-1(tk45) double mutants (Figure 5, D, E, and G), indicating that emb-9(tk75) suppresses this phenotype of fbl-1(tk45). PAT-3–GFP expression in fbl-1(tk45) was reduced in gonadal sheath cells in addition to DTCs, and this reduction was also suppressed by emb-9(tk75) (Figure S3). Similarly, DTC expression of INA-1–GFP, a functional GFP fusion of α-integrin, was reduced in fbl-1(tk45), but it was restored in emb-9(tk75); fbl-1(tk45) animals (Figure S4).
Figure 5 .
PAT-3–GFP localization in DTCs. Confocal (upper) and Nomarski (lower) images of wild-type (A), fbl-1(tk45) (B), emb-9(tk75) (C), emb-9(tk75); fbl-1(tk45) (D), fbl-1(tk45); gon-1(e1254) (E), and emb-9(g34) (F) L3 hermaphrodites that expressed PAT-3–GFP. (F) emb-9(g34) mutants grown at 16° were shifted to 25° at the L2 larval stage. The arrowhead indicates PAT-3–GFP expression in DTCs. Scale bar, 20 µm. (G) Fluorescence intensity of PAT-3–GFP. All strains contained rhIs2[pat-3(wt)::GFP] in their background. The fluorescence intensity for each sample was normalized to that of the wild type. Ten confocal images of DTCs for each strain were used for quantification. The fluorescence intensity of a rectangular region (2.4 µm × 1.2 µm) at the tip of each DTC was quantified using the ImageJ program (National Institutes of Health), and values were averaged. Data are shown as the mean ± SD. Results for the Student’s t-test vs. the wild-type value are indicated; **P < 0.01; NS, not significant. All images were captured under the same conditions. (H) Fluorescence intensity of PAT-3–GFP in animals shifted to 25°. Fifteen animals for each strain were analyzed. The relative fluorescence intensity was determined as in G. (I) Effect of PAT-3–GFP overexpression in fbl-1(tk45) mutants as analyzed by quantification of gonadal elongation defects. Data are shown as the mean ± SD. Results for Fisher’s exact test vs. fbl-1(tk45) are indicated; **P < 0.01.
Because EMB-9 localization was reduced in fbl-1(tk45) animals, it is possible that the reduction of EMB-9 in the BM causes the decrease of PAT-3 in DTCs. Therefore, we analyzed PAT-3–GFP expression in wild-type and emb-9(g34) animals reared at 25°, a nonpermissive temperature for g34. The EMB-9(g34) mutant protein accumulates in the cytoplasm and is not secreted at nonpermissive temperatures (Gupta et al. 1997). We observed that PAT-3–GFP expression was significantly reduced in the DTCs of emb-9(g34) mutants (Figure 5, F and H), suggesting that EMB-9/type IV collagen localization in the BM is important for proper PAT-3 expression. Finally, we examined whether overexpression of PAT-3 can rescue the DTC migration defects of fbl-1 mutants. We found that PAT-3–GFP expression partially but significantly rescued the defects in fbl-1 mutants (Figure 5I), indicating that reduction of PAT-3 expression contributes to the migration defects of DTCs in the fbl-1 mutants.
A gon-1(e1254) allele also suppresses fbl-1(tk45)
Because an in-frame deletion mutation, fbl-1(q750) (a potential partial loss-of-function mutation) and a gon-1(q518) null mutation partially suppress the gonadal elongation defects resulting from each mutation, it has been proposed that wild-type FBL-1 and GON-1 have antagonistic roles in the control of gonadogenesis (Hesselson et al. 2004). Using the gon-1 weak allele e1254 (Blelloch et al. 1999; Blelloch and Kimble 1999), we investigated whether the combination of fbl-1(tk45) and gon-1(e1254) exhibits a similar genetic interaction. Although the suppression was partial, the gonadal phenotype of fbl-1(tk45); gon-1(e1254) double mutants was significantly weaker than that in either fbl-1(tk45) or gon-1(e1254) single mutants (Figure 6), indicating that they suppressed each other. EMB-9 and NID-1 accumulation decreased in fbl-1(tk45) L4 larvae (Figures 3D and 4D), but not gon-1(e1254) L4 larvae (Figures 3H and 4H). The reduced EMB-9 and NID-1 accumulation in fbl-1(tk45) animals was suppressed by gon-1(e1254) (Figures 3G and 4G). The reduced PAT-3-GFP expression in DTCs in fbl-1(tk45) animals was also suppressed by gon-1(e1254) (Figure 5, E and G).
Figure 6 .
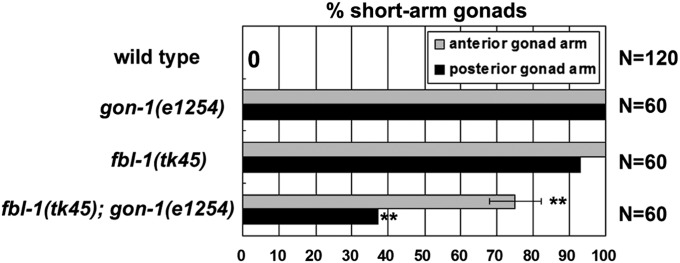
fbl-1(tk45) and gon-1(e1254) suppress each other with respect to the gonadal elongation defect. Quantification of gonadal elongation defects in gon-1(e1254), fbl-1(tk45) and the double mutants. Data are shown as the mean ± SD. Result for Fisher’s exact test vs. fbl-1(tk45) is indicated; **P < 0.01.
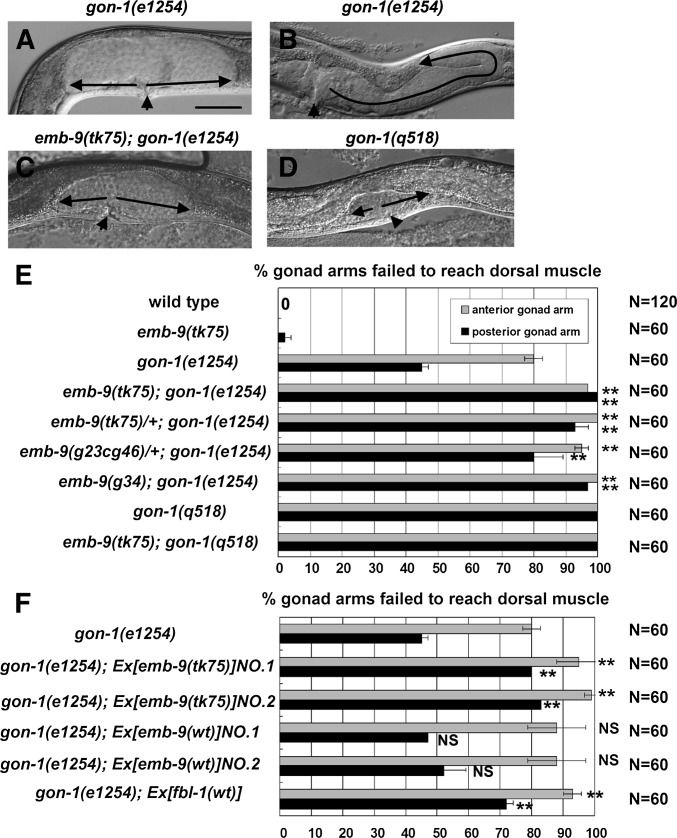
emb-9(tk75) enhances the gonadal elongation defects in gon-1(e1254) mutants
We tested the genetic interaction between emb-9 and gon-1. We used the gon-1 weak allele e1254 and null allele q518 (Blelloch et al. 1999; Blelloch and Kimble 1999). The emb-9(tk75); gon-1(e1254) double mutants showed strong gonad morphogenesis defects similar to those observed in either gon-1(q518) or emb-9(tk75); gon-1(q518) mutants (Figure 7, A–E). emb-9(tk75)/+ enhanced the gonadogenesis defects of the gon-1(e1254) mutant (Figure 7E). The emb-9(tk75); gon-1(e1254); fbl-1(tk45) triple mutants exhibited intermediate phenotypes between those of emb-9(tk75); fbl-1(tk45) and gon-1(e1254); fbl-1(tk45) double mutants (Figure S5). Because emb-9(tk75) was a dominant enhancer of gon-1(e1254), we examined whether it is a gain-of-function or a loss-of-function mutation. Interestingly, we found that emb-9(g23cg46)/+ enhanced the gonadal elongation defect of gon-1(e1254) mutants (Figure 7E), as was the case for emb-9(tk75)/+, suggesting that emb-9(tk75) acts as a loss-of-function mutation to enhance gon-1(e1254). This is supported by the fact that the ts loss-of-function allele emb-9(g34) enhanced the gonadal defects in gon-1(e1254) at 20° (Figure 7E). When we introduced multicopy mutant emb-9(tk75) arrays into gon-1(e1254) mutants, the gonadal elongation defects were significantly enhanced, although wild-type emb-9 arrays had no influence (Figure 7F). This could be because the wild-type EMB-9 was partially replaced by the overproduced mutant EMB-9(tk75) in the BM. The wild-type fbl-1 arrays also significantly enhanced the gonadal elongation defects of gon-1(e1254) mutants (Figure 7F). Taken together, these results suggest that EMB-9(tk75) may have gained a FBL-1–like activity to inhibit the GON-1 function that is required for elongation and expansion of the gonad arms. Alternatively, wild type EMB-9 may act in parallel with GON-1.
Figure 7 .
Genetic interaction between emb-9 and gon-1. Phenotypes of posterior gonads of gon-1(e1254) (A and B), emb-9 (tk75); gon-1(e1254) (C), and gon-1(q518) (D) hermaphrodites. Arrows and arrowheads are as in Figure 1. DTCs of posterior gonads in emb-9(tk75); gon-1(e1254) and gon-1(q518) animals were often arrested during migration on the ventral muscle. Most emb-9(tk75); gon-1(e1254) and gon-1(q518) mutants showed weak or no expansion of gonads. Bar, 50 µm. (E) Quantification of gonad arms that failed to reach the dorsal muscle. Data are shown as the mean ± SD. Results for Fisher’s exact test vs. gon-1(e1254) are indicated; **P < 0.01; NS, not significant. (F) Effects of extrachromosomal arrays containing mutant emb-9, wild-type (wt) emb-9, and wild-type fbl-1 transgenes. Quantification of gonad arms that failed to reach the dorsal muscle. Data are shown as the mean ± SD. Results for Fisher’s exact test vs. gon-1(e1254) are indicated; **P < 0.01; NS, not significant.
Discussion
EMB-9(tk75) may affect interactions between NC1 domains
EMB-9/type IV collagen α1 subunit is secreted from muscle cells and DTCs, probably as a trimer (two α1 and one α2 subunits) together with LET-2/type IV collagen α2 subunit and accumulates in the BM (Gupta et al. 1997). Unlike emb-9(g34) animals, which fail to secrete type IV collagen at nonpermissive temperatures (Gupta et al. 1997), emb-9(tk75); fbl-1(tk45) mutants exhibited mostly normal type IV collagen secretion, and the collagen clearly localized to the gonadal BM, implying that the strong suppression is probably due to a defect in the BM, which is induced by secreted mutant EMB-9(tk75). Gly-1708, which is substituted in the tk75 mutation, is evolutionarily conserved and is a component of the trimer–trimer interface region. This region is important for interactions between individual NC1 domains of collagen subunit trimers, which form the type IV collagen network (Figure S1) (Sundaramoorthy et al. 2002). Because the tk75 mutants are viable and fertile, the gonadal BM is presumably not as seriously impaired as in the lethal emb-9 or let-2 mutants (Gupta et al. 1997). The marked reduction of type IV collagen localization in the swollen gonad arms of fbl-1(tk45) mutants during late larval stages suggests that stage-specific maintenance of the type IV collagen network is required to generate gonads of the proper length and width. One possibility is that the trimer–trimer interactions between EMB-9(tk75) molecules may be strengthened during the late larval stages so that this protein is not lost from the BM in the absence of FBL-1. emb-9(tk75) also suppressed the defective maintenance of NID-1 in fbl-1(tk45) mutants, suggesting that EMB-9(tk75) acts to maintain NID-1. This is consistent with our previous observation that NID-1 localization is reduced in the ts let-2(b246) (α2 chain of type IV collagen) mutants reared at nonpermissive temperatures (Kubota et al. 2008). Because type IV collagen interacts with nidogen (Aumailley et al. 1989), it might be possible that EMB-9(tk75) acts as an anchor for NID-1.
Type IV collagen may be required for integrin-dependent gonadal elongation
Integrin family receptors consist of two subunits, α- and β-integrins. The regulated expression of two α-integrins, INA-1 and PAT-2, controls DTC migration; INA-1 is required for continuous migration, whereas PAT-2 is required for pathfinding in migration (Meighan and Schwarzbauer 2007). PAT-3 is the sole β-integrin in C. elegans. A dominant-negative PAT-3 disrupts DTC migration (Lee et al. 2001). We found that the expression of PAT-3–GFP in DTCs, as well as the BM localization of EMB-9, was reduced in fbl-1(tk45) mutants. A similar reduction in PAT-3–GFP expression was also observed in the emb-9(g34) ts mutant, in which the mutant EMB-9(g34) fails to be secreted. These results suggest that EMB-9 accumulation in the BM upregulates the expression of PAT-3 or stabilizes PAT-3 in DTCs. Consistent with this idea, the PAT-3–GFP reduction in fbl-1(tk45) mutants was suppressed in both emb-9(tk75); fbl-1(tk45) and fbl-1(tk45); gon-1(e1254) double mutants, in which EMB-9 accumulation was restored. A correlation between type IV collagen and the integrin expression level has been observed in pancreatic β cells. Type IV collagen promotes the α1β1 integrin-dependent migration on Matrigel of β cells in which integrin expression is upregulated (Kaido et al. 2004). Because integrins are major receptors for ECM proteins and regulate cytoskeletal activity (Bokel and Brown 2002; Wiesner et al. 2005; Berrier and Yamada 2007), accumulation of type IV collagen in BMs probably plays an important role in integrin-dependent cytoskeletal regulation. We observed that overexpression of PAT-3–GFP can partly suppress fbl-1 mutants. Thus, it is possible that EMB-9 may mediate FBL-1 function to promote integrin expression and cytoskeletal rearrangement in DTCs, which are required for gonadal elongation. In support of this, RNAi knockdown of ina-1 and ts let-2 and emb-9 mutants reared at nonpermissive temperatures exhibit the short-arm gonad phenotype (Cram et al. 2006; Meighan and Schwarzbauer 2007; Kawano et al. 2009) similar to that observed in fbl-1(tk45) mutants.
A similar reduction in PAT-3–GFP expression in fbl-1(tk45) animals and the resumption of its expression in emb-9(tk75); fbl-1(tk45) animals were observed in gonadal sheath cells, suggesting that type IV collagen regulation of β-integrin also occurs in sheath cells. The sheath cells modulate gonad morphogenesis by activating integrin signaling (Baum and Garriga 1997; Lee et al. 2005).
Interactions among FBL-1, EMB-9, and GON-1 in the regulation of gonadogenesis
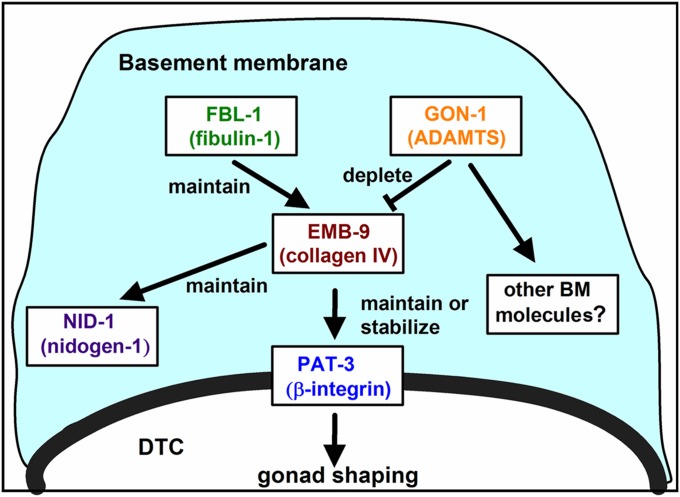
Because the fbl-1(tk45) null mutation is suppressed almost completely by emb-9(tk75), it is possible that the major function of FBL-1 is to maintain EMB-9, which leads to BM accumulation of NID-1 and expression of PAT-3 in the somatic gonad (Figure 8). Although its suppression is weaker than that of emb-9(tk75), gon-1(e1254) shares similar suppression characteristics with emb-9(tk75). Thus, it is possible that GON-1 interacts with the FBL-1 pathway. We speculate that EMB-9 may be located downstream of and regulated negatively by GON-1 (Figure 8). In this model, we postulated that the amount of BM-localized EMB-9 is maintained appropriately by a balance between EMB-9 accumulation (regulated by FBL-1) and its reduction by GON-1. This model can explain the dominant enhancing effect of EMB-9(tk75) if this mutation leads to its inappropriate maintenance in the BM despite the action of GON-1(e1254), which could have a much weaker effect than that of wild-type GON-1. This hypothesis does, however, raise a question as to why emb-9(g23cg46)/+ enhances gon-1(e1254). We speculate that reduction of GON-1 activity in gon-1(e1254) animals affects several BM proteins, including EMB-9, by modifying the localization or conformation of these proteins in the BM. When gon-1(e1254) is combined with emb-9(g23cg46)/+, which should disrupt the EMB-9–LET-2 stoichiometry in type IV collagen, this may further degrade the general physiology of the BM, resulting in phenotypic enhancement of gon-1(e1254). We could not determine whether EMB-9 localization was upregulated in the gon-1 mutants, because the immunohistochemical experiments performed in this study were not sensitive enough to assess subtle differences in protein localization. Further studies will be required to determine the precise mechanism of suppression underlying the action of EMB-9(tk75) and GON-1(e1254).
Figure 8 .
A model of the interactions among FBL-1, EMB-9, and GON-1 in gonad shaping. In the late larval stages, FBL-1 acts to maintain EMB-9, whereas GON-1 depletes EMB-9 to achieve appropriate levels of EMB-9 localization in the BM. The BM-localized EMB-9 maintains NID-1 in the BM and promotes expression or stabilization of PAT-3 in DTCs (and gonadal sheath cells). GON-1 may also affect other BM molecules.
The C. elegans gonad is a tubular structure that is covered with a BM (Hall et al. 1999). Type IV collagen is a major component of the BM and associates with other ECM molecules such as laminin, nidogen, and heparan sulfate proteoglycans in mammals (Fujiwara et al. 1984; Charonis et al. 1985; Aumailley et al. 1989). Mouse type IV collagen also interacts weakly with fibulin-1 in vitro (Pan et al. 1993). Therefore, EMB-9 and FBL-1 may bind directly or indirectly through other ECM molecules to form a supramolecular network in the BM to regulate gonadogenesis. Similar to the stage-specific function of FBL-1 in C. elegans, mouse fibulin-1 is required for maintenance of integrity of small vessels only after midgestation (Kostka et al. 2001), suggesting that fibulin-1 may have a conserved role in temporal regulation of structure and function of the BM. GON-1, which is homologous to mammalian ADAMTS-9 and ADAMTS-20 proteinases (Llamazares et al. 2003; Somerville et al. 2003), may act to degrade EMB-9. The interactions among these molecules probably affect the balance between gonadal elongation and expansion activities. Our findings provide the functional evidence for interactions among conserved molecules fibulin-1, type IV collagen, and an ADAMTS and therefore suggest an interesting possibility that a similar functional interaction may also occur in mammalian organogenesis.
Supplementary Material
Acknowledgments
We thank Jim Kramer for type IV collagen antibodies, John Plenefisch for a strain with an rhIs2 insertion, Alan Coulson for cosmid clones, and the Caenorhabditis Genetics Center for strains. We also thank Noriko Nakagawa and Asami Sumitani for technical assistance. We thank Masaki Kakehi for sharing unpublished results. This work was supported by a grant-in-aid for scientific research by the Ministry of Education, Culture, Sports, Science, and Technology to Y.K. (18570213) and Natural Science Scholarship by the Naito Foundation to K.N.
Footnotes
Communicating editor: D. I. Greenstein
Literature Cited
- Argraves W. S., Greene L. M., Cooley M. A., Gallagher W. M., 2003. Fibulins: physiological and disease perspectives. EMBO Rep. 4: 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M., Wiedemann H., Mann K., Timpl R., 1989. Binding of nidogen and the laminin-nidogen complex to basement membrane collagen type IV. Eur. J. Biochem. 184: 241–248 [DOI] [PubMed] [Google Scholar]
- Baum P. D., Garriga G., 1997. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19: 51–62 [DOI] [PubMed] [Google Scholar]
- Berrier A. L., Yamada K. M., 2007. Cell-matrix adhesion. J. Cell. Physiol. 213: 565–573 [DOI] [PubMed] [Google Scholar]
- Bianco A., Poukkula M., Cliffe A., Mathieu J., Luque C. M., et al. , 2007. Two distinct modes of guidance signalling during collective migration of border cells. Nature 448: 362–365 [DOI] [PubMed] [Google Scholar]
- Blelloch R., Kimble J., 1999. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature 399: 586–590 [DOI] [PubMed] [Google Scholar]
- Blelloch R., Anna-Arriola S. S., Gao D., Li Y., Hodgkin J., et al. , 1999. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev. Biol. 216: 382–393 [DOI] [PubMed] [Google Scholar]
- Bokel C., Brown N. H., 2002. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 3: 311–321 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary E. C., Yurchenco P. D., Furthmayr H., 1985. Binding of laminin to type IV collagen: a morphological study. J. Cell Biol. 100: 1848–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram E. J., Shang H., Schwarzbauer J. E., 2006. A systematic RNA interference screen reveals a cell migration gene network in C. elegans. J. Cell Sci. 119: 4811–4818 [DOI] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z., 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14: 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Gilmour D., 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10: 445–457 [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Wiedemann H., Timpl R., Lustig A., Engel J., 1984. Structure and interactions of heparan sulfate proteoglycans from a mouse tumor basement membrane. Eur. J. Biochem. 143: 145–157 [DOI] [PubMed] [Google Scholar]
- Graham P. L., Johnson J. J., Wang S., Sibley M. H., Gupta M. C., et al. , 1997. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J. Cell Biol. 137: 1171–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. D., Johnson J. J., Kramer J. M., 1991. Embryonic lethality caused by mutations in basement membrane collagen of C. elegans. Nature 349: 707–709 [DOI] [PubMed] [Google Scholar]
- Gupta M. C., Graham P. L., Kramer J. M., 1997. Characterization of α1(IV) collagen mutations in Caenorhabditis elegans and the effects of α1 and α2(IV) mutations on type IV collagen distribution. J. Cell Biol. 137: 1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Winfrey V. P., Blaeuer G., Hoffman L. H., Furuta T., et al. , 1999. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212: 101–123 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., Stern B. D., 1987. Genetics of cell and axon migrations in Caenorhabditis elegans. Development 100: 365–382 [DOI] [PubMed] [Google Scholar]
- Hesselson D., Kimble J., 2006. Growth control by EGF repeats of the C. elegans Fibulin-1C isoform. J. Cell Biol. 175: 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson D., Newman C., Kim K. W., Kimble J., 2004. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr. Biol. 14: 2005–2010 [DOI] [PubMed] [Google Scholar]
- Kaido T., Yebra M., Cirulli V., Montgomery A. M., 2004. Regulation of human β-cell adhesion, motility, and insulin secretion by collagen IV and its receptor α1β1. J. Biol. Chem. 279: 53762–53769 [DOI] [PubMed] [Google Scholar]
- Kawano T., Zheng H., Merz D. C., Kohara Y., Tamai K. K., et al. , 2009. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development 136: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Hirsh D., 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396–417 [DOI] [PubMed] [Google Scholar]
- Kostka G., Giltay R., Bloch W., Addicks K., Timpl R., et al. , 2001. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol. 21: 7025–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Kuroki R., Nishiwaki K., 2004. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr. Biol. 14: 2011–2018 [DOI] [PubMed] [Google Scholar]
- Kubota Y., Sano M., Goda S., Suzuki N., Nishiwaki K., 2006. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development 133: 263–273 [DOI] [PubMed] [Google Scholar]
- Kubota Y., Ohkura K., Tamai K. K., Nagata K., Nishiwaki K., 2008. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc. Natl. Acad. Sci. USA 105: 20804–20809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Cram E. J., Shen B., Schwarzbauer J. E., 2001. Roles for βpat-3 integrins in development and function of Caenorhabditis elegans muscles and gonads. J. Biol. Chem. 276: 36404–36410 [DOI] [PubMed] [Google Scholar]
- Lee M., Shen B., Schwarzbauer J. E., Ahn J., Kwon J., 2005. Connections between integrins and Rac GTPase pathways control gonad formation and function in C. elegans. Biochim. Biophys. Acta 1723: 248–255 [DOI] [PubMed] [Google Scholar]
- Llamazares M., Cal S., Quesada V., Lopez-Otin C., 2003. Identification and characterization of ADAMTS-20 defines a novel subfamily of metalloproteinases-disintegrins with multiple thrombospondin-1 repeats and a unique GON domain. J. Biol. Chem. 278: 13382–13389 [DOI] [PubMed] [Google Scholar]
- Lu P., Werb Z., 2008. Patterning mechanisms of branched organs. Science 322: 1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M., Pilgrim D., 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D. R., Nelson C. M., Dixon L. J., Silver D. L., Wylie J. D., et al. , 2009. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell 17: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan C. M., Schwarzbauer J. E., 2007. Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 21: 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J., Schierenberg E., Miwa S., von Ehrenstein G., 1980. Genetics and mode of expression of temperature-sensitive mutations arresting embryonic development in Caenorhabditis elegans. Dev. Biol. 76: 160–174 [DOI] [PubMed] [Google Scholar]
- Montell D. J., 2008. Morphogenetic cell movements: diversity from modular mechanical properties. Science 322: 1502–1505 [DOI] [PubMed] [Google Scholar]
- Muriel J. M., Dong C., Hutter H., Vogel B. E., 2005. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development 132: 4223–4234 [DOI] [PubMed] [Google Scholar]
- Nishiwaki K., 1999. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics 152: 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., Hisamoto N., Matsumoto K., 2000. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288: 2205–2208 [DOI] [PubMed] [Google Scholar]
- Pan T. C., Kluge M., Zhang R. Z., Mayer U., Timpl R., et al. , 1993. Sequence of extracellular mouse protein BM-90/fibulin and its calcium-dependent binding to other basement-membrane ligands. Eur. J. Biochem. 215: 733–740 [DOI] [PubMed] [Google Scholar]
- Plenefisch J. D., Zhu X., Hedgecock E. M., 2000. Fragile skeletal muscle attachments in dystrophic mutants of Caenorhabditis elegans: isolation and characterization of the mua genes. Development 127: 1197–1207 [DOI] [PubMed] [Google Scholar]
- Rorth P., 2009. Collective cell migration. Annu. Rev. Cell Dev. Biol. 25: 407–429 [DOI] [PubMed] [Google Scholar]
- Somerville R. P., Longpre J. M., Jungers K. A., Engle J. M., Ross M., et al. , 2003. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J. Biol. Chem. 278: 9503–9513 [DOI] [PubMed] [Google Scholar]
- Sundaramoorthy M., Meiyappan M., Todd P., Hudson B. G., 2002. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J. Biol. Chem. 277: 31142–31153 [DOI] [PubMed] [Google Scholar]
- Timpl R., Sasaki T., Kostka G., Chu M. L., 2003. Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 4: 479–489 [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164 [DOI] [PubMed] [Google Scholar]
- Wiesner S., Legate K. R., Fassler R., 2005. Integrin-actin interactions. Cell. Mol. Life Sci. 62: 1081–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Gu T., Han M., 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.