Abstract
The “point” centromere of budding yeast is genetically defined by an ∼125-bp sequence. Recent fluorescence measurements of kinetochore clusters have suggested that this sequence specifies multiple centromere histone 3 (CenH3) nucleosomes. However, high-resolution mapping demonstrates that there is only one CenH3 nucleosome per centromere, providing biochemical confirmation of the point centromere model.
THE centromere is the genetic locus that organizes the kinetochore, which attaches to spindle microtubules for regular segregation to the poles at mitosis and meiosis. In most eukaryotes, centromeres are “regional,” comprising arrays of centromere histone 3 (CenH3)-containing nucleosomes that mediate attachment to multiple spindle microtubules. In contrast, the “point” centromeres of Saccharomyces cerevisiae are specified by an ∼125-bp sequence that is occupied by a single centromeric nucleosome (Furuyama and Biggins 2007) and attaches to a single spindle microtubule.
Recently, two groups have used fluorescence microscopy to estimate the stoichimetry of kinetochore proteins, which led them to conclude that the budding yeast CenH3 (Cse4) is present at a much higher abundance at centromeres than can be explained by the presence of a single centromeric nucleosome (Coffman et al. 2011; Lawrimore et al. 2011). These reports contradict the conclusion of studies using chromatin immunoprecipitation (ChIP), that there is only a single Cse4 nucleosome per centromere (Meluh et al. 1998; Furuyama and Biggins 2007), and challenge the simple concept of a point centromere (Short 2011). To explain this apparent discrepancy, Lawrimore et al. (2011) reanalyzed the native ChIP data of Furuyama and Biggins (2007) and argued that their indirect labeling method was not sufficiently sensitive to detect low levels of centromeric nucleosomes that might randomly occupy centromere-flanking regions. They proposed that each S. cerevisiae centromere includes multiple Cse4 nucleosomes, which they likened to regional centromeres. Here we ask whether centromere-flanking Cse4 nucleosomes are detected by high-resolution ChIP-seq mapping, which is orders of magnitude more sensitive than the Southern blot-based indirect labeling method used by Furuyama and Biggins (2007).
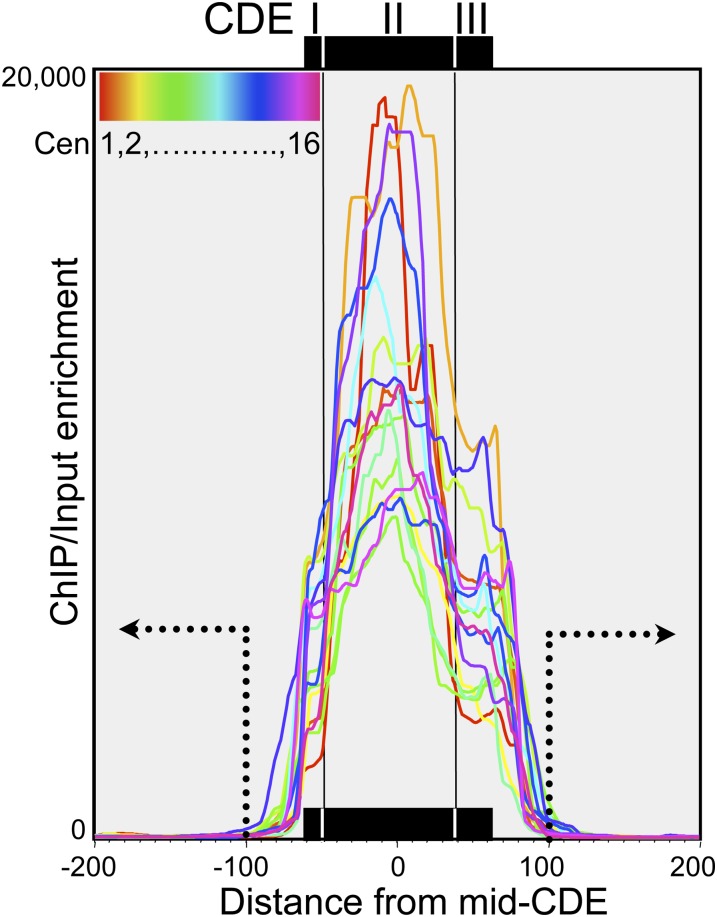
The functional S. cerevisiae centromere consists of three centromere DNA elements (CDEs): CDEI, CDEII, and CDEIII (Kamakaka and Biggins 2005). Recently, we performed native ChIP-seq using epitope-tagged Cse4 and histone H2A to determine the precise location and composition of each of the 16 yeast centromeres (Krassovsky et al. 2012). By obtaining near-quantitative recovery of soluble chromatin and applying a modified protocol for paired-end Solexa library preparation, we mapped individual fragments as small as ∼25 bp that were protected from micrococcal nuclease (MNase) digestion in both the soluble chromatin input and the Cse4 ChIP. Our single-base-pair resolution mapping revealed that the chromatin organization over all 16 centromeres precisely corresponds to the functional distinctions among CDE elements: Cse4 maps to the ∼80-bp CDEII element and is tightly flanked by distinct small particles over both CDEI and CDEIII (Krassovsky et al. 2012). Cse4 enrichment is confined to the CDEs of all 16 yeast centromeres (Figure 1).
Figure 1.
Single-base-pair resolution mapping of Cse4 nucleosomes. Native chromatin was extracted from nuclei of budding yeast cells grown in rich medium after MNase digestion over an eightfold range (Krassovsky et al. 2012). ChIP of FLAG-tagged Cse4 was performed on soluble chromatin (“Input”), and the resulting DNA fragments were used to prepare libraries as described for paired-end sequencing on an Illumina HiSeq 2000 instrument. Cse4 ChIP/input ratios of normalized counts over a 5-bp running average were plotted for the 400-bp interval centered over the mid-CDE. The cumulative ChIP/input data for four MNase time points (2.5, 5, 10, and 20 min) are superimposed for all 16 centromeres (colored lines). Dotted lines and arrows indicate the regions considered to be “flanks.” Paired-end reads for all eight samples used in this analysis were obtained from the eight lanes of a single flow cell with a total of 597.9 million clusters, yielding 371.4 million paired-end reads that were successfully mapped to the yeast genome.
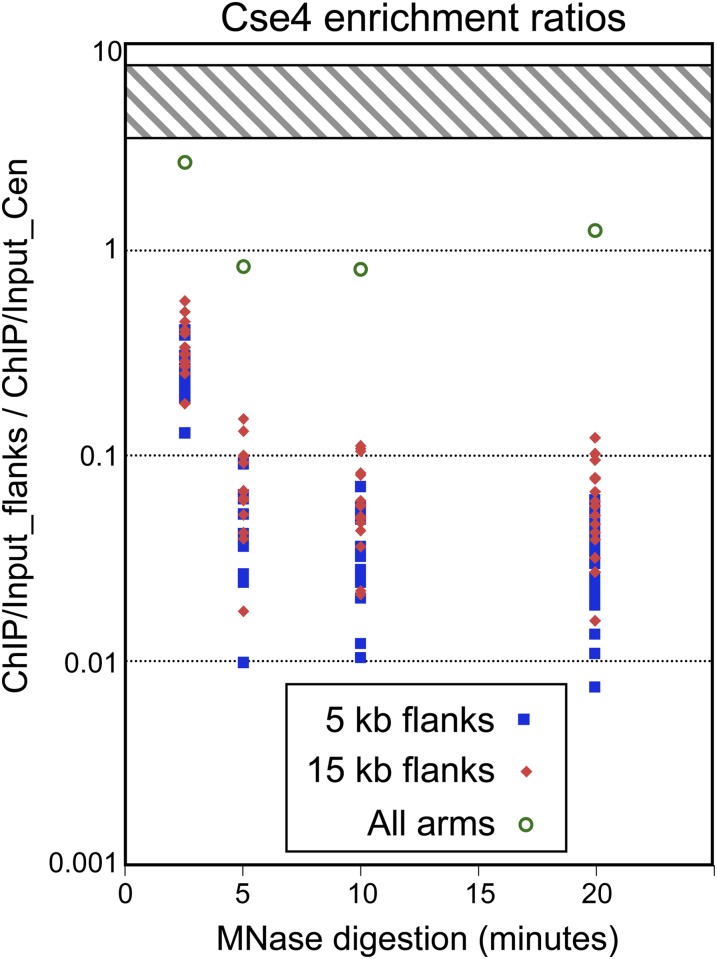
Cytologically, the 16 yeast kinetochores appear as a single near-diffraction-limited spot estimated to contain ∼5–10 kb of DNA (Lawrimore et al. 2011), and so we summed the Cse4/input ratios for each base pair out to 5 or 15 kb on both sides of each CDE. We found that, for all 16 centromeres, the cumulative enrichment for both flanks is only a small fraction of that over the CDE, varying from ∼1 to 10% CDE enrichment over a fourfold range of MNase digestion (Figure 2). We consider these to be conservative estimates, insofar as background was not subtracted, and so any Cse4 enrichment associated with nucleosome turnover as seen at active gene promoters in previous studies (Camahort et al. 2009; Lefrancois et al. 2009; Da Rosa et al. 2010) would have been measured as Cse4 incorporation into chromosome arms. Centromeres are so highly enriched in Cse4 relative to other regions of the genome that together they account for about half of the total Cse4 ChIP signal in the 5′–20′ MNase digestion data (flank/CDE = 0.8–1.2; Figure 2). It is highly unlikely that epitope masking of Cse4 had preferentially reduced its representation on chromosome arms because we had observed a low level of Cse4 incorporation at all nucleosome positions when Cse4 was overproduced five- to sixfold (Krassovsky et al. 2012). It is also unlikely that unstable Cse4 nucleosomes were missed because we did not observe any decrease in the flank/CDE ratio after 5′ MNase digestion, which was populated mostly by oligo- and di-nucleosomes; therefore, any such particles would be more susceptible to MNase cleavage than is linker DNA. We conclude that total ChIP enrichment outside of yeast centromeres is much too low for there to be even a single stable Cse4 particle in centromere-flanking regions, contrary to the assertions of Coffman et al. (2011) and Lawrimore et al. (2011) who estimated that several Cse4 particles occupy each of these regions.
Figure 2.
Low occupancy of Cse4 over centromere-flanking regions. For each centromere, Cse4 enrichment is the ChIP/input normalized count ratio summed over each base pair in the CDE. For each centromere flank, Cse4 enrichment is the ChIP/input ratio summed over each base pair, excluding the 200-bp interval centered over the mid-CDE (between the dotted lines in Figure 1). Flank/centromere ratios are shown for individual chromosomes, and include both flanks out to 5 kb (blue squares) or 15 kb (red diamonds). The ratio for all chromosome arms (green open circles) was obtained by dividing the total Cse4 ChIP/input ratio in the sample, excluding the CDE ±100 bp, by that for all 16 CDEs. A ratio of 1 implies that there is cumulative enrichment in both flanks that is equal to that in the CDE. The hatched region represents the predicted range of the ratio based on fluorescence (4–8 for Coffman et al. (2011) and 3.5–6 for Lawrimore et al. (2011).
It is possible that the fluorescence observations reflect the presence of a “cloud” of unincorporated Cse4 in the local vicinity of centromeres. Indeed, the Scm3 Cse4-specific chaperone binds DNA in vitro (Xiao et al. 2011) and maps by ChIP immediately adjacent to yeast centromeres (Camahort et al. 2009). From the structure of the Scm3–Cse4–H4 complex (Cho and Harrison 2011), it is evident that the intact complex cannot be incorporated into a nucleosome because Scm3 blocks surfaces of Cse4 that interact with DNA. As the Scm3 mapping study used formaldehyde cross-linking with sonication (Camahort et al. 2009), it is possible that any Scm3–Cse4–H4 that is loosely bound to chromatin in the vicinity of centromeres would be cross-linked and mapped (Krassovsky et al. 2012). In contrast, our native ChIP of stable Cse4 particles did not use cross-linking and so would likely have excluded any such loosely bound particles. Another possibility, not mutually exclusive, is that the GFP tag led to somewhat promiscuous incorporation of Cse4 in centromere-flanking regions. Variable misincorporation of Cse4-GFP might account for the consistent strain-to-strain differences in the estimated numbers of Cse4 nucleosomes, ranging from 3.5 to 8 per kinetochore in the two studies. Cse4-GFP misincorporation might also have contributed to the 31-fold increase in chromosome loss observed by Lawrimore et al. (2011) for Cse4-GFP.
Regardless of the explanation for the excess Cse4-GFP observed by fluorescence, our inability to detect any evidence for stable Cse4 particles in flanking regions, in contrast to the prominent signal observed over centromeres, rules out the proposals of both groups that yeast centromeres are regional. More generally, to the extent that in vivo fluorescence studies cannot distinguish between stably incorporated particles and unincorporated complexes, the issue of fluorescence calibration of kinetochore components addressed by these studies remains unresolved. External standards such as flagellar motors and viral particles might not be ideal for calibration of chromatin proteins as these defined sources of fluorophores are present in discrete units in a known context, whereas Cse4 is present in kinetochore clusters in both chromatin and chaperone-associated particles. Interestingly, Lawrimore et al. (2011) also found that components of the CBF3 complex, which recruits a single Cse4 nucleosome, are present in ∼1:1 stoichiometry with Cse4, consistent with a tetrameric “hemisome” structure proposed for centromeric nucleosomes (Dalal et al. 2007). Overrepresentation of the CBF3 complex is difficult to reconcile with its known specificity for a 26-bp consensus sequence within the functional yeast centromere (Lechner and Carbon 1991). Had Lawrimore et al. (2011) used their CBF3 subunit data for calibration, they might have concluded that there is only one Cse4-containing hemisome per kinetochore.
Acknowledgments
We thank Sue Biggins and Takehito Furuyama for helpful discussions and Paul Talbert for comments on the manuscript.
Footnotes
Communicating editor: E. Alani
Literature Cited
- Camahort R., Shivaraju M., Mattingly M., Li B., Nakanishi S., et al. , 2009. Cse4 is part of an octameric nucleosome in budding yeast. Mol. Cell 35: 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho U. S., Harrison S. C., 2011. Recognition of the centromere-specific histone Cse4 by the chaperone Scm3. Proc. Natl. Acad. Sci. USA 108: 9367–9371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman V. C., Wu P., Parthun M. R., Wu J. Q., 2011. CENP-A exceeds microtubule attachment sites in centromere clusters of both budding and fission yeast. J. Cell Biol. 195: 563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal Y., Wang H., Lindsay S., Henikoff S., 2007. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 5: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa J. L., Holik J., Green E. M., Rando O. J., Kaufman P. D., 2010. Overlapping regulation of CenH3 localization and histone H3 turnover by CAF-1 and HIR proteins in Saccharomyces cerevisiae. Genetics 187: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama S., Biggins S., 2007. Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc. Natl. Acad. Sci. USA 104: 14706–14711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakaka R. T., Biggins S., 2005. Histone variants: Deviants? Genes Dev. 19: 295–310 [DOI] [PubMed] [Google Scholar]
- Krassovsky K., Henikoff J. G., Henikoff S., 2012. Tripartite organization of centromeric chromatin in budding yeast. Proc. Natl. Acad. Sci. USA 109: 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrimore J., Bloom K. S., Salmon E. D., 2011. Point centromeres contain more than a single centromere-specific Cse4 (CENP-A) nucleosome. J. Cell Biol. 195: 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J., Carbon J., 1991. A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell 64: 717–725 [DOI] [PubMed] [Google Scholar]
- Lefrancois P., Euskirchen G. M., Auerbach R. K., Rozowsky J., Gibson T., et al. , 2009. Efficient yeast ChIP-Seq using multiplex short-read DNA sequencing. BMC Genomics 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh P. B., Yang P., Glowczewski L., Koshland D., Smith M. M., 1998. Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell 94: 607–613 [DOI] [PubMed] [Google Scholar]
- Short B., 2011. Setting a new standard for kinetochores. J. Cell Biol. 195: 539 [Google Scholar]
- Xiao H., Mizuguchi G., Wisniewski J., Huang Y., Wei D., et al. , 2011. Nonhistone Scm3 binds to AT-rich DNA to organize atypical centromeric nucleosome of budding yeast. Mol. Cell 43: 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]