Abstract
It has been established beyond doubt that, as well as the liver, the small intestine is an important site of first-pass metabolism of numerous drugs, food components and toxic xenobiotics. However, there is not much information available about age-dependent changes of intestinal biotransformation pathways. In the present paper, we evaluated the relationships between intestinal cytochrome P450 complex activity and the age of animals. The study was carried out on male Sprague–Dawley rats (n = 5) from 5 age series: 0.5-, 2-, 4-, 20-, and 28 months old. Animals at every age series were divided into 4 groups: control and three groups of rats treated with the CYP450 specific inducers: phenobarbital, β-naphtoflavone and dexamethasone, respectively. RNA was isolated from intestinal mucosa, and then standard RT-PCR was used for the analysis of CYP1A1, CYP2B1/2 and CYP3A1 mRNA expression. Additionally, the activities of NADPH-cytochrome P450 and NADH-cytochrome b5 reductases in the microsomal fraction were biochemically estimated. The constitutive intestinal CYP1A1 mRNA expression changes during maturation and aging. Inducibility of CYP1A1 gene was evident in intestinal mucosa at 2-, 4- and 20-month-old rats. A similar pattern of changes was observed for CYP2B1/2 isoforms. CYP3A1 mRNA expression was not detected in small intestine of 2-week-old rats. In matured rats, constitutive intestinal CYP3A1 expression was low, although after induction, significant increases in CYP3A1 mRNA amount were noted in aged individuals. Intestinal activity of both analyzed reductases was lowest in immature rats and highest in 28-month-old animals. In conclusion, the activity of cytochrome P450 complex in rat small intestine was not decreased by the aging processes, so the high rate of oxidative metabolic reactions in intestinal mucosa can be maintained till the advanced life stage.
Keywords: Cytochrome P450, Intestine, Aging, Biotransformation, Xenobiotics
Introduction
Cytochrome P450-dependent metabolic pathways seem to play a crucial physiological role in all vertebrate species. The activity of this important system can be changed by inhibition or induction of the enzymes by the numerous drugs and other compounds that occur in the food, e.g., in fruits, herbs, vegetables as well as in either alcoholic or soft beverages (Ware 2009; Nekvindova and Anzenbacher 2007; Hidestrand et al. 2005; Fujita 2004). From the medical viewpoint, it is essential that these universally used substances do not cause failure of therapies causing serious health alterations. For example, potentially dangerous interactions of grapefruit or lemon juices, a potent cytochrome P450 inhibitors, with felodipine and cyclosporine, red wine with cyclosporine and St John’s wort with various medicines have been well demonstrated (Cuciureanu et al. 2010; Uesawa et al. 2010; Paine et al. 2008; Chaudhary and Willett 2006; Tsunoda et al. 2001; Baltes et al. 2001). Many potentially harmful food contaminants such as heterocyclic aromatic amines identified in fried meat, phenol complexes from the components of the Mediterranean diet and ethyl carbamate present in some kinds of brandy are able to induct some isoforms of cytochrome P450 in various organs (Hümmerich et al. 2004; Stupans et al. 2001; Kleman et al. 1995; Guengerich et al. 1994). Although, the biotransformation of xenobiotics takes place mostly in the liver, some extrahepatic cytochrome P450 complexes (e.g., gastrointestinal, renal and pulmonary) contribute substantially to the total metabolic potential of the living mammals. The small intestine is one of the organs involved in cytochrome P450-dependent metabolism of various food-derived substances, drugs and toxic agents (Martignoni et al. 2006). Metabolic processes performed by the gut mucosa may affect uptake, efflux and transport of orally delivered pharmaceuticals and dietary xenobiotics by cells, tissues and organs (Benet et al. 2004; Zhang and Benet 2001; Watkins 1997). The CYP3A4-dependent interaction by food-derived agents may be related to the high level expression of CYP3A4 in the small intestine, as well as its broad substrate specificity (Fujita 2004). It has been confirmed that role of intestinal metabolism is significantly greater than hepatic in the first-pass effect for some drugs, e.g., cyclosporine A and midazolam (Isin and Guengerich 2007; Kanazu et al. 2005; Kotegawa et al. 2002; Paine et al. 1996). Even more than half a percent of orally administered cyclosporine A is metabolized by enterocytes (Hebert 1997). The intestinal mucosa, therefore, is considered one of the most important sites of drug metabolism. Numerous recent studies have demonstrated that the intestinal epithelium contributes to the biotransformation of diltiazem and nifedipine (Mitschke et al. 2008; Iwao et al. 2004). On the other hand, local activation of procarcinogens in the small and large bowel via cytochrome P450-dependent microsomal monooxygenase metabolic pathway is considered to be an important factor in cancer etiology. The CYP1A1 isoform shows the highest level of expression in rat small intestine (Zhang et al. 1999). The other CYP450 isoforms were found in the epithelium of rat small intestine include CYP1A2 (Hakkak and Ronis 1993), CYP2B, CYP2C, CYP2E1, CYP3A, (Perloff et al. 2004; Takemoto et al. 2003), CYP4A1 (Catterall et al. 2003), CYP4F12 (Stark et al. 2004), CYP2J4 and CYP2J3 (Zhang et al. 1998). The extended exposure to the ritonavir and dexamethasone increased the expression of CYP3A isoforms in rat enteric mucosal cells (Perloff et al. 2004). In the human small intestine, mRNAs of CYP1A1, CYP1B1, CYP2C, CYP2D6, CYP2E1, CYP3A4 and CYP3A5 were identified (Ding and Kaminsky 2003), but only CYP3A4, CYP3A5 and, on minimal level, CYP1A1 (Zhang et al. 1999) were detected as proteins. Both omeprazol and acetylsalicylic acid act as potent inducers of CYP2D6, CYP2E1, CYP3A4 (Lindell et al. 2003).
It should be emphasized that in human intestinal mucosa, the members of CYP3A family play the leading role, especially CYP3A4 isoform (Emoto et al. 2000), which participates in metabolism of many xenobiotics and drugs and is generally responsible for the biotransformation of more than 50% of clinical pharmaceuticals. Moreover, CYP3A4 appears to be a key enzyme in food–drug interactions (Fujita 2004). The content of CY3A4 isoform is most abundant in the proximal portion and is decreased distally in the human and rodent small intestine (Zhang et al. 1999, De Waziers et al. 1990). The members of CYP1, CYP2 and CYP3 families are also expressed in human and animal colon (Bernauer et al. 2002).
Morphological and biochemical differentiation, which takes place in mammalian intestinal tract during maturation and aging, seems to be strictly related to the development of cytochrome P450 complex, which also includes the specific reductases as well as donors of electrons for CYP450-dependent catalytic cycle. Nevertheless, most experiments that referred to cytochrome P450-dependent intestinal metabolism concerned young, sexually mature animals. The main purpose of this study was to analyze the relationships between intestinal expression of selected cytochrome P450 mRNAs (CYP1A1, CYP2B1/2, CYP3A1) and the age of rats. A further aim of our experiment was the evaluation of age-related changes in the activities of NADPH-cytochrome P450 and NADH-cytochrome b5 reductases. In the present study, we focused on investigating the changes in the mRNA expression of the main intestinal cytochrome P450 isoforms as well as on evaluating the nonspecific activity of the reductases mentioned above. We have not studied either metabolic activity or protein expression corresponding to any CYP isoform. The first results of our experiment concerning age-dependent changes of selected cytochrome P450 proteins in rat small intestine were previously reported (Pałasz et al. 2003). The NADPH-cytochrome P450 and NADH-cytochrome b5 reductase activities presented in this article are not relating to CYP450 isoforms. Although the rate of biotransformation of the substrates to metabolic products was not measured, owing to that all the results reflects the CYPs activity merely indirectly, we suppose that the evaluation of age-related expression and inducibility of the intestinal CYP450 mRNAs and the activity of the reductases may also be helpful in the better understanding of intestinal metabolism. We appreciate the fact that our studies do not reflect the intestinal CYP450 activity in humans; nevertheless, any clinical equivalent of our experiment would be very difficult to perform.
Many carcinogenic metabolites are the products of CYP450-dependent microsomal activation of various food-derived substances such as aflatoxin B1 that is transformed to the genetically harmful exo-8,9-epoxide. Other food components affect the activity of selected CYP isoforms, e.g., flavones stimulate while naringenin, a major flavonoid of grapefruit juice, attenuates the rate of xenobiotic metabolism (Guengerich et al. 1994). Probably, the diet-related induction of the CYP genes in the small intestine of aged rats and humans could play an important role in cancer etiology within this organ. On account of that, the studies focusing on age-related changes of CYPs expression in the alimentary tract could also be interesting for scientists working in the field of nutrition and dietetics. Nevertheless, it should be clearly emphasized that the study reported herein may be value as an experimental paradigm to evaluate the cytochrome P450-dependent metabolism in animals. The clinical applicability of this model is extremely limited by numerous species differences in patterns of metabolic biotransformation as well as intestinal profile of CYP450 isoforms.
Materials and methods
Animals
The studies were carried out on 100 male Sprague–Dawley rats, and animals from 5 age series, 0.5-, 2-, 4-, 20-, and 28-month-old individuals, were divided into 4 experimental groups (n = 5): one control group and three groups of rats that were induced with selective CYP1A1, CYP2B1/2 and CYP3A1 inducers. The rats of all groups were fed on standard diet (Murigran, drinking water) and kept under the same conditions at 22°C with a regular 4-h day/night cycle. The induced groups of animals have received intraperitoneally two doses (for 2 consecutive days) of phenobarbital (50 mg/kg), β-naphtoflavone (80 mg/kg) or dexamethasone (30 mg/kg), respectively. It should be emphasized that the inducers used in this study are strictly appropriate to studied rat CYP450 isoforms, and they do not correspond to inducers of human enzymes. All the agents were administered at the same time when the rats were active, at approximately 7.00 AM to avoid any circadian changes. On the third day, the animals were quickly anaesthetized with halothane and then were killed immediately by decapitation. All experimental procedures were approved by local bioethic committee at the Medical University of Silesia.
RT-PCR
The small intestines were completely excised directly after sacrifice and then rinsed with cold phosphate buffer pH 7.3 containing 1.5 mM KCl and 96 mM NaCl. Total mRNA was extracted from manually scrapped 50 mg samples of intestinal mucosa (each sample was taken from one individual) using Trizol TM according to Chomczynski and Sacchi (1987), and then RT-PCR method was used to analyze CYP1A1, CYP2B1/2 and CYP3A1 mRNA expression. Collected RNA samples were transcribed into cDNA during incubation in buffered solution of reverse transcriptase MMLV-RT with RNAsin, oligo-dT and mix of nucleotides at 37°C for 90 min. using DNA Thermal Cycler 480 (Perkin Elmer Inc., Waltham, MA). The PCR was performed in Peltier Thermal Cycler PT-200 (MJ Research Inc., Watertown, MA) for 26 rounds; 1 min. at 94 °C 1 min. at 65°C (CYP1A1), 55.8°C (CYP2B1/2) or 62.3°C (CYP3A1) and finally 90 s. at 72°C. The studied mRNAs and standard internal reference glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA were amplified using the following primers CYP1A1; forward: 5′-GATGCTGAGGACCAGAAGACCGC, reverse: 5′-CAGGAG GCTGGACGAGAATGC; CYP2B1/2; forward: 5′-CCAAGCGCTCCACGAGACTT, reverse: 5′-TTGGGAGCAGGTACCCTC, CYB3A1; forward: 5′-CCGCCTGGATTCTGTGCAGA, reverse: 5′-TGGGAGGTGCCTTATTGGGC, GAPDH; forward: 5′-GTG AACGGATTTGGCCGTATCG, reverse: 5′-ATCACGCCACAGCTTTCCAGAGG. Products of PCR amplification were separated on 2% agarose gel and visualized with ethidium bromide and then were photographed in UV light chamber. Semiquantitative densitometric analysis was performed with the use of OneDScan software (Scanalytics), and the results were expressed on graphs as integrated optical density (IOD).
Enzymatic assay
Microsomal fraction was prepared from the homogenates of intestinal mucosa (0.25 M sucrose in 100 mM phosphate buffer) by centrifugation (100,000×g, 90 min.) of the postmitochondrial supernatant (9,000×g, 20 min.) as previously described (Dallner 1974). Microsomes were resuspended in 20% buffered glycerol and stored at −80°C until analysis. The activities of NADPH-cytochrome P450 and NADH-cytochrome b5 reductases were estimated in microsomal fraction using NADH, NADPH and cytochrome c buffered solutions according to Hodges and Leonard method (1974). All the results were analyzed statistically by Student–Newman–Keuls test (P < 0.05).
Results
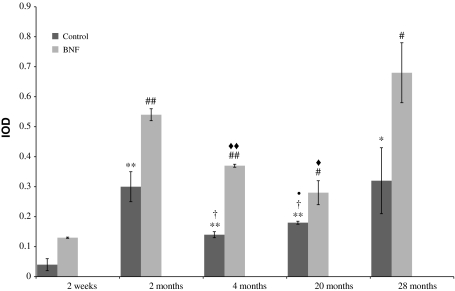
The results show clearly that mRNA expression of the studied intestinal CYP450 isoforms changes its function depending on age (Figs. 1, 2, 3; Tables 1, 2, 3). Constitutive expression of CYP1A1 mRNA was lowest in immature 2-week-old rats. Statistically significant increase in CYP1A1 mRNA expression in comparison with the immature animals was found in 4-, 20- (P < 0.001) and 28-month-old ones (P < 0.01). In sexually mature rats, the level of CYP1A1 mRNA was relatively stable till the advanced age. An increase in CYP1A1 mRNA expression was also observed in individuals treated with β-naphtoflavone. The highest CYP1A1 gene inducibility (increase in the amount of PCR product in induced groups in relation to control groups) was detected in 2-week-old animals (1998% of control) and the lowest in the 28-month-old ones (112% of control). Individuals at age of 2, 4, and 20 months showed similar CYP1A1 gene inducibility: 237, 284 and 266% of control, respectively.
Table 4.
Activity of NADPH-cytochrome P450 reductase with statistical analysis
| Age | 2 Weeks | 2 Months | 4 Months | 20 Months | 28 Months |
|---|---|---|---|---|---|
| C | 0.004 ± 0.002 | 0.015 ± 0.001 | 0.007 ± 0.001 | 0.006 ± 0.001 | 0.02 ± 0.002 |
| PB | 0.004 ± 0.007 | 0.017 ± 0.002 | 0.011 ± 0.002 | 0.011 ± 0.004 | 0.028 ± 0.001 |
| BNF | 0.003 ± 0.001 | 0.012 ± 0.001 | 0.01 ± 0.003 | 0.011 ± 0.001 | 0.024 ± 0.006 |
| DEX | 0.003 ± 0.001 | 0.011 ± 0.003 | 0.008 ± 0.001 | 0.011 ± 0.003 | 0.019 ± 0.004 |
| Control | PB | BNF | DEX | |
|---|---|---|---|---|
| Comparison between age-groups (P-value) | ||||
| 2 Weeks versus 2 months | P < 0.005 | P < 0.01 | P < 0.01 | P < 0.05 |
| 2 Weeks versus 4 months | ns | P < 0.05 | ns | P < 0.01 |
| 2 Weeks versus 20 months | ns | ns | P < 0.01 | P < 0.05 |
| 2 Weeks versus 28 months | P < 0.01 | P < 0.001 | P < 0.05 | P < 0.05 |
| 2 Months versus 4 months | P < 0.005 | P < 0.05 | ns | ns |
| 2 Months versus 20 months | P < 0.01 | ns | ns | ns |
| 2 Months versus 28 months | P < 0.01 | P < 0.05 | ns | ns |
| 4 Months versus 20 months | ns | ns | ns | ns |
| 4 Months versus 28 months | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.05 |
| 20 Months versus 28 months | P < 0.01 | P < 0.05 | ns | ns |
The data shown as micromoles/min/mg of protein (mean ± SD)
ns Statistically not significant
Table 5.
Activity of NADH-cytochrome b5 reductase with statistical analysis
| Age | 2 Weeks | 2 Months | 4 Months | 20 Months | 28 Months |
|---|---|---|---|---|---|
| C | 0.004 ± 0.002 | 0.012 ± 0.001 | 0.006 ± 0.001 | 0.008 ± 0.002 | 0.02 ± 0.002 |
| PB | 0.004 ± 0.001 | 0.013 ± 0.001 | 0.008 ± 0.001 | 0.015 ± 0.005 | 0.033 ± 0.003 |
| BNF | 0.003 ± 0.002 | 0.012 ± 0.001 | 0.006 ± 0.003 | 0.014 ± 0.002 | 0.022 ± 0.004 |
| DEX | 0.006 ± 0.001 | 0.01 ± 0.001 | 0.008 ± 0.001 | 0.015 ± 0.004 | 0.026 ± 0.004 |
| Control | PB | BNF | DEX | |
|---|---|---|---|---|
| Comparison between age-groups (P-value) | ||||
| 2 Weeks versus 2 months | P < 0.05 | P < 0.001 | ns | P < 0.05 |
| 2 Weeks versus 4 months | ns | P < 0.05 | ns | ns |
| 2 Weeks versus 20 months | ns | ns | ns | P < 0.05 |
| 2 Weeks versus 28 months | P < 0.001 | P < 0.05 | ns | P < 0.01 |
| 2 Months versus 4 months | P < 0.01 | P < 0.05 | ns | P < 0.05 |
| 2 Months versus 20 months | P < 0.05 | ns | ns | ns |
| 2 Months versus 28 months | P < 0.05 | ns | P < 0.05 | P < 0.05 |
| 4 Months versus 20 months | ns | ns | P < 0.05 | ns |
| 4 Months versus 28 months | P < 0.01 | P < 0.05 | P < 0.01 | P < 0.05 |
| 20 Months versus 28 months | P < 0.01 | P < 0.05 | P < 0.05 | P < 0.05 |
The data shown as micromoles/min/mg of protein (mean ± SD)
ns Statistically not significant
Fig. 1.
Age-related constitutive and β-naphtoflavone-inducible expression of CYP1A1 mRNA in rat small intestine. Values are expressed as mean integrated optical density (IOD) ± S.D. Significant difference at **P < 0.01 and *P < 0.05 versus control immature 2-week-old rats, at †P < 0.05 versus control 2-month-old rats, at filled circleP < 0.05 versus control 4-month-old rats, at ##P < 0.001 and #P < 0.05 versus induced 2-week-old animals and at filled double diamondP < 0.01 and filled diamondP < 0.05 versus induced 2-month-old individuals
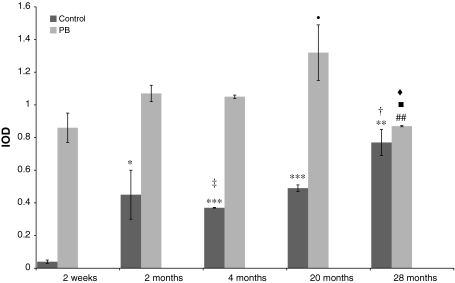
Fig. 2.
Age-related constitutive and phenobarbital-inducible expression of CYP2B1/2 mRNA in rat small intestine. Values are expressed as mean integrated optical density (IOD) ± S.D Significant difference at ***P < 0.001 and **P < 0.01 and *P < 0.05 versus control immature 2-week-old rats, at ‡P < 0.01 and †P < 0.05 versus control 2-month-old rats, at filled circleP < 0.05 versus induced 2-week-old rats, at #P < 0.05 versus induced 2-month-old animals, at filled squareP < 0.01 versus induced 4-month-old rats, at filled diamondP < 0.05 versus induced 20-month-old individuals
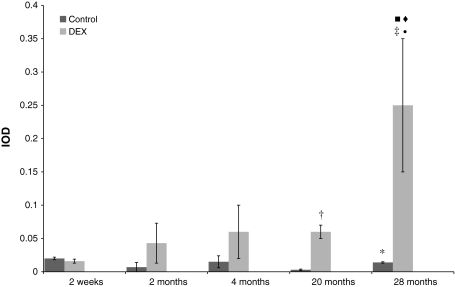
Fig. 3.
Age-related constitutive and dexamethasone-inducible expression of CYP3A1 mRNA in rat small intestine. Values are expressed as mean integrated optical density (IOD) ± S.D. Significant difference at *P < 0.05 versus control immature 2-week-old rats, at ‡P < 0.01 and †P < 0.05 versus induced 2-week-old rats, at filled circleP < 0.05 versus induced 2-month-old rats, at filled squareP < 0.05 versus induced 4-month-old rats, at filled diamondP < 0.05 versus induced 20-month-old individuals
Table 1.
Expression levels of CYP1A1 mRNA in all studied age-groups with statistical analysis
| Age-group | Control | Induced with BNF |
|---|---|---|
| 2 Weeks | 0.04 ± 0.02 | 0.13 ± 0.002 |
| 2 Months | 0.30 ± 0.05 | 0.54 ± 0.02 |
| 4 Months | 0.14 ± 0.01 | 0.37 ± 0.005 |
| 20 Months | 0.18 ± 0.005 | 0.28 ± 0.04 |
| 28 Months | 0.32 ± 0.11 | 0.68 ± 0.43 |
| Comparison between age-groups (P-value) | ||
| 2 Weeks versus 2 months | P < 0.01 | P < 0.001 |
| 2 Weeks versus 4 months | P < 0.01 | P < 0.001 |
| 2 Weeks versus 20 months | P < 0.01 | P < 0.05 |
| 2 Weeks versus 28 months | P < 0.05 | P < 0.05 |
| 2 Months versus 4 months | P < 0.05 | P < 0.01 |
| 2 Months versus 20 months | P < 0.05 | P < 0.05 |
| 2 Months versus 28 months | ns | ns |
| 4 Months versus 20 months | P < 0.05 | ns |
| 4 Months versus 28 months | ns | ns |
| 20 Months versus 28 months | ns | ns |
The data shown as integrated optical density (mean ± SD)
ns Statistically not significant
Table 2.
Expression of CYP2B1/2 mRNA in all studied age-groups with statistical analysis
| Age-group | Control | Induced with phenobarbital |
|---|---|---|
| 2 Weeks | 0.04 ± 0.01 | 0.86 ± 0.09 |
| 2 Months | 0.45 ± 0.15 | 1.07 ± 0.05 |
| 4 Months | 0.37 ± 0.002 | 1.05 ± 0.01 |
| 20 Months | 0.49 ± 0.02 | 1.32 ± 0.17 |
| 28 Months | 0.77 ± 0.08 | 0.87 ± 0.004 |
| Comparison between age-groups (P-value) | ||
| 2 Weeks versus 2 months | P < 0.05 | ns |
| 2 Weeks versus 4 months | P < 0.001 | ns |
| 2 Weeks versus 20 months | P < 0.001 | P < 0.05 |
| 2 Weeks versus 28 months | P < 0.01 | ns |
| 2 Months versus 4 months | P < 0.01 | ns |
| 2 Months versus 20 months | ns | ns |
| 2 Months versus 28 months | P < 0.05 | P < 0.05 |
| 4 Months versus 20 months | ns | ns |
| 4 Months versus 28 months | P < 0.05 | P < 0.01 |
| 20 Months versus 28 months | ns | P < 0.05 |
The data shown as integrated optical density (mean ± SD)
ns Statistically not significant
Table 3.
Expression of CYP3A1 mRNA in all studied age-groups with statistical analysis
| Age-group | Control | Induced with dexamethasone |
|---|---|---|
| 2 Weeks | 0.02 ± 0.02 | 0.016 ± 0.003 |
| 2 Months | 0.007 ± 0.007 | 0.043 ± 0.03 |
| 4 Months | 0.015 ± 0.009 | 0.06 ± 0.04 |
| 20 Months | 0.003 ± 0.001 | 0.06 ± 0.01 |
| 28 Months | 0.014 ± 0.001 | 0.25 ± 0.13 |
| Comparison between age-groups (P-value) | ||
| 2 Weeks versus 2 months | ns | ns |
| 2 Weeks versus 4 months | ns | ns |
| 2 Weeks versus 20 months | ns | P < 0.05 |
| 2 Weeks versus 28 months | ns | P < 0.01 |
| 2 Months versus 4 months | ns | ns |
| 2 Months versus 20 months | ns | ns |
| 2 Months versus 28 months | ns | P < 0.05 |
| 4 Months versus 20 months | ns | ns |
| 4 Months versus 28 months | ns | P < 0.05 |
| 20 Months versus 28 months | P < 0.05 | P < 0.05 |
The data shown as integrated optical density (mean ± SD)
ns Statistically not significant
The lowest constitutive CYP2B1/2 mRNA expression was observed in small intestine of 2-week-old rats, the highest in 2- and 28-month-old animals. Stable and medial level of CYP2B1/2 mRNA was found in 4- and 20-month-old animals. The increase in CYP2B1/2 mRNA level after phenobarbital administration was significant in all studied groups. Although the amount of CYP2B1/2 mRNA in immature rats was low, the inducibility in this age-group was highest (273% of control) and comparable to observed for 4-month animals (266% of control). Significant inducibility was also detected in oldest, 28-month-old rats (208% of control).
The constitutive expression of CYP3A1 mRNA was relatively low in intestinal mucosae from all studied groups of animals. Differences between age-groups were not statistically significant, except 28-month-old rats, where the significant increase in CYP3A1 mRNA expression in comparison with 20-month-old animals was observed (P < 0.005). Dexamethasone did not affect the level of CYP3A1 mRNA in 2-week-old rats; 2- and 4-month-old animals were characterized by higher inducibility of this isoform, 614 and 406% of control, respectively. The highest increase in the amount of CYP3A1 mRNA after dexamethasone induction was found in small intestine of 20- and 28-month-old rats: 2,233 and 1,800% of control, respectively.
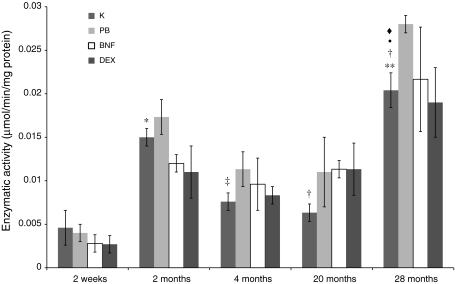
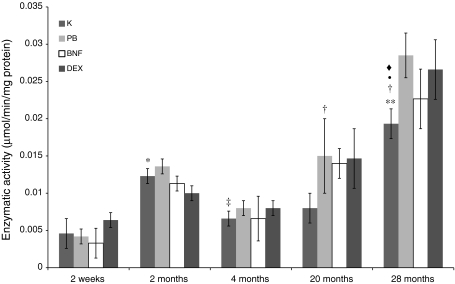
The activities of both studied reductases, NADPH-cytochrome P450 and NADH-cytochrome b5, in rat small intestine apparently showed age-dependent fluctuations, the lowest in 2-week-old and the highest in 28-month-old animals. In intestinal mucosa of 28-month-old rats, the activities of the above enzymes were about 3-fold higher than in 4- and 20-month-old animals. Analogous tendencies were observed in all studied groups after treatment with CYP450 inducers as well as changes in the intestinal activities of studied reductases in induced animals were low. Only 20-month-old rats after β-naphtoflavone administration and 28-month-old animals after phenobarbital treatment showed abundant increase in NADPH-CYP450 reductase activity in comparison with controls (Figs. 4, 5).
Fig. 4.
Age-related differences in NADPH-cytochrome P450 reductase activity in rat small intestine. Values are expressed as micromoles/min/mg of protein ± S.D. Significant difference at **P < 0.001 and *P < 0.005 versus control 2-week-old rats, at ‡P < 0.005 and †P < 0.01 versus control 2-month-old rats, at filled circleP < 0.01 versus control 4-month-old rats, at filled diamondP < 0.01 versus control 20-month-old individuals. The differences in the enzymatic activity between the control and induced animals at the same age were not statistically significant, except for 20- and 28-month-old animals treated with β-naphtoflavone and phenobarbital, respectively. On account of that and also to maintain the lucidity, the graph shows all the values versus control groups only. Complete statistical analysis is shown in Table 4
Fig. 5.
Age-related differences in NADH-cytochrome b5 reductase activity in rat small intestine. Values are expressed as micromoles/min/mg of protein ± S.D. Significant difference at **P < 0.001 and *P < 0.05 versus control 2-week-old rats, at ‡P < 0.01 and †P < 0.05 versus control 2-month-old rats, at filled circleP < 0.01 versus control 4-month-old rats, at filled diamondP < 0.01 versus control 20-month-old rats. The differences in the enzymatic activity between the control and induced animals at the same age were not statistically significant, except for 20- and 28-month-old animals treated with β-naphtoflavone and phenobarbital, respectively. On account of that and also to maintain the lucidity, the graph shows all the values versus control groups only. Complete statistical analysis is shown in Table 5
Discussion
Intestinal biotransformation of drugs and dietary xenobiotics may be modified during maturation and aging. The main purpose of this study was to estimate the level of expression and inducibility of selected intestinal CYP450 mRNAs in various phases of rat life. Biochemical analysis of the cytochrome P450 and cytochrome b5 reductases activity in function with age was the second but also important aim of the presented work. However, it should be explicitly stressed that the results of this experiment do not concern the age-related drug metabolism and disposition in humans. Under conditions of our experiment, constitutive CYP1A1 mRNA expression was lowest in the small intestine of 2-week-old rats and was increased in sexually mature 2-month-old animals. Since the attainment of maturity, the animals showed relatively constant levels of constitutive CYP1A1 mRNA. Such a stable expression persisted till the advanced animal age, when its significant augmentation was observed. These data may indicate that the constitutive intestinal expression of CYP1A1 gene changes during maturation and aging. Inducibility of CYP1A1 gene was evident in 2-, 4- and 20-month-old rats. It is known that the CYP1 family is involved in metabolizing aromatic hydrocarbons to potentially harmful and genotoxic products, the CYP1B1 gene is often overexpressed in colon adenocarcinoma cells (Gibson et al. 2003). The food-derived heterocyclic aromatic amines act as the inducers of intestinal CYP1A1 whereas some components found in Saint John’s wort (Hypericum perforatum), e.g., myricetin, apigenin and quercetin can selectively inhibit this isoform (Hümmerich et al. 2004; Chaudhary and Willett 2006). Relatively high expression and inducibility of intestinal CYP1A1 in aged rats may suggest the existence of analogous pattern in older humans. Perhaps, the flavonoids mentioned above may be considered suitable for administration as chemoprotective and cancer-preventive substances also in aged patients.
Changes in CYP2B1/2 mRNA expression were similar to those observed for CYP1A1, a constitutive mRNA level of this isoform was lowest in 2-week-old rats and highest in 2- and 28-month-old animals. It may reflect the basic activity of CYP2B1/2 in either immature or aged rats; our previous study on the age-related changes in expression of selected intestinal CYP450 proteins showed a parallel profile of the constitutive CYP2B1/2 levels (Pałasz et al. 2003, also unpublished data). The amount of CYP2B1/2 mRNA increased significantly after phenobarbital administration in all studied age-groups, especially in oldest 28-month-old individuals. These findings compare well with our earlier data showing that the oldest rats exposed had a relatively high inducibility of CYP2B1/2 protein (Pałasz et al. 2003). Studies performed by Patel et al. (1998) on rat fetuses, newborns and animals in the early period of postnatal life showed significant increases in CYP2B protein levels in the small intestine during sexual maturation. The results of the present study support this observation and also may suggest that the constitutive intestinal expression of CYP2B in sexually immature rats is only slightly marked in comparison with adult ones. It is possible that the CYP2B level in the digestive tract may be modulated by alterations in steroid hormone concentrations, e.g., testosterone (Fujita et al. 1990), although there are still no available investigations confirming this mechanism.
The CYP3A1 isoform, which has particularly high concentration in the rat liver, was confirmed to be also expressed in the small intestine. The mRNA of this isoform exhibited a completely different profile of intestinal expression and inducibility than previously described CYPs. Dexamethasone had no effect on the level of CYP3A1 mRNA in 2-week-old rats. It supports the conjecture that CYP3A1-dependent I phase xenobiotic biotransformation in the small intestine of sexually immature rats may be considerably limited.
These findings were consistent with a previous study in which Warrington et al. (2004) noted no statistically significant age-dependent changes of CYP3A expression in the rat small intestine. A comparable stability was also observed by Wauthier et al. (2004) in constitutive hepatic CYP3A mRNA content in 3-, 8-, 11- and 18-month-old rats. It seems that such isoforms should be less affected by age-related oxidative injuries, e.g., decrease in reduced glutathione concentration. However, interestingly the oldest 28-month-old animals showed a significant increase in intestinal CYP3A1 mRNA expression after dexamethasone administration.
Results published by Lee and Werlin (1995) showed that in the liver of 5-day-old rats treated with dexamethasone, very high CYP3A1 mRNA expression was observed in comparison with adult animals. These data do not correlate with our results concerning rat small intestine, where the CYP3A1 inducibility was highest in oldest, 28-month-old individuals. The differences between small intestine and liver in age-dependent CYP3A protein expression were also reported by other authors (Warrington et al. 2004) who showed that in the liver of aged rats, a significant decrease in CYP3A occurred, whereas the intestinal expression of this isoform was unchanged. George et al. (1995) observed that the level of hepatic CYP3A protein in human decreases during aging. However, our previous results derived from the first experiment clearly showed that the amount of intestinal CYP3A1 protein as well as its inducibility did not decrease even at advanced stage of rat life (Pałasz et al. 2003). Therefore, it may suggest that the pattern of age-dependent changes in intestinal CYP3A expression is substantially different than observed in the liver. Okabe et al. (2003) studying CYP3A activity in rats after nephrectomy and glycerol or cisplatin intoxication also found that the intestinal expression of this isoform did not change, whereas it significantly decreased in the liver. Assuming that the profile of cytochrome P450-dependent biochemical processes performed by rat intestinal mucosa seems not to be analogous to xenobiotic biotransformation by hepatocytes. Not many food products such as the well-described grapefruit juice act as blockers of this isoform either in rodents or in humans. It seems to be important, that the plasma concentrations of some drugs may be significantly and sometimes dangerously increased by concomitant treatment with this beverage when the medicines are administered orally (Hanley et al. 2011). The well-known members of the CYP3A family are the only isoforms expressed in clinically meaningful amounts in human enteric mucosa. Probably, the parallel profile of the intestinal CYP3A4 isoform action may also take place in older patients.
Reductases, the unique electron donors for catalytic center of CYP450 protein, are important and inseparable components of oxidative phase in xenobiotic biotransformation. The results of our performed study clearly demonstrate that activities of NADPH-cytochrome P450 and NADH-cytochrome b5 reductase in the rat small intestine change in various phases of life. Constitutive activities of both enzymes were lowest in immature 2-week-old rats, and the inducers influenced their activity in this age-group. Nevertheless, this effect was poorly significant. Activity of NADPH-cytochrome P450 reductase in this age-group was slightly decreased after phenobarbital and β-naphtoflavone administration. On the other hand, dexamethasone increased NADH-cytochrome b5 activity in the small intestine of 2-week-old rats. During sexual maturation, the increase in the activity of studied reductases was evident. After attainment of sexual maturity, the activity of both reductases was relatively stable till the 20-month-old age, and then the increase was evident again. The aged, 28-month-old rats were characterized by the highest activity of both NADPH-CYP450 and NADH-cytochrome b5 reductases. Nevertheless, Warrington et al. (2004) suggested an age-dependent decline in NADPH-CYP450 reductase protein expression in the rat small intestine. However, it is proper to notice that this western blotting and immunohistochemical study did not concern the enzymatic activity of both reductases in the gut microsomal fraction. It was performed on young mature (2 months old) rats only and did not include either immature or extremely aged 28-month-old animals. The age-dependent changes of reductase activity in the rat small intestine are similar to those observed by some authors in the liver (Plewka and Kaminski 1992). Studies dealing with effects of age and phenobarbital on hepatic mixed function monooxygenases showed higher NADPH-CYP450 reductase activity in 2-month-old rats in comparison with 2-week-old and 28-month-old animals. We suggest that in the intestinal mucosa of aged rats as well as other mammals, not excluding humans, the augmentation of NADPH/NADH-dependent oxidoreductive processes take place. These reactions are often connected with the generation of free radicals, leading to injury of various cellular structures, e.g., phospholipid bilayers and nuclear chromatin. It may contribute to dysplastic or even neoplastic transformation of gut mucosa frequently observed in aged mammals. A lot of carcinogenic metabolites are the products of CYP450-dependent biotransformation of various agents, for example, polycyclic hydrocarbons (PAHs) and numerous organic food contaminants, heterocyclic aromatic amines, polyphenols, dioxins, urethane, etc. (Hümmerich et al. 2004; Stupans et al. 2001; Guengerich et al. 1994). High inducibility of CYP1A1, CYP2B1/2 and CYP3A1 genes in the small intestine of aged rats could also promote carcinogenesis within intestinal epithelium. Probably, the CYP1A1 isoform plays a particular role in this process. There are suggestions that biotransformation of PAHs and also food-derived xenobiotics into active carcinogens depends on CYP1A1 gene variability (Shimada and Fujii-Kuriyama 2004). It is generally accepted that the high level of oxidative reactions is an important factor in the course of aging processes. Our results suggest that the phenomenon of intestinal aging is reflected by qualitative and quantitative changes in the expression of particular cytochrome P450 isoforms as well as activities of their reductases. Interestingly, the age of animals influences the expression of CYP1A1, CYP2B1/2 and CYP3A1 in a selective manner. The constitutive level and inducibility of studied cytochrome P450 isoforms in the small intestine of immature rats were very low. Sexually mature animals showed high level expression of CYP450, which was not decreased even at advanced phases of life. Therefore, the expression of CYP450 isoforms in the rat small intestine may be one of the new potential markers of sexual maturity. Some studies on cell proliferation and apoptosis in rat intestinal mucosa showed that the equilibrium of these processes is not significantly impaired during aging (Mandir et al. 2005). It is probably one of the reasons for relatively high metabolic activity in this organ even in old individuals. Other results could also indicate that the intestinal activities of both NADPH-cytochrome P450 reductase and NADH-cytochrome b5 reductase in old rats are higher than in young, mature individuals. We hypothesized that the differences in intestinal biotransforming potential of immature and aged rats may affect the character and rate of dietary xenobiotic metabolism in the gastrointestinal tract. The intestinal metabolic rate as well as the efficiency of cytochrome P450-dependent biotransformation is not decreased by the aging processes. It should be taken into consideration that the high level of oxidative reactions within enterocytes may contribute to pathological changes within the intestinal mucosa of aged animals. Perhaps, these biochemical and cytophysiological processes may be supported or blocked by the presence of various potentially harmful food xenobiotics. This brings up the question of what molecular events regulate activation and inhibition of the selected CYP450 genes involved in intestinal carcinogenesis. We finally postulate that the putative contribution of dietary xenobiotics to this phenomenon requires further study.
References
- Baltes MR, Dubois JG, Hanocq M. Application to drug-food interactions of living cells as in vitro model expressing cytochrome P450 activity: enzyme inhibition by lemon juice. Talanta. 2001;54:983–987. doi: 10.1016/S0039-9140(01)00368-X. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Cummins CL, Wu CY. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm. 2004;277:3–9. doi: 10.1016/j.ijpharm.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Bernauer U, Ellrich R, Heinrich-Hirsch B, Teubner W, Vieth B, Gundert-Remy U. Expression of cytochrome P450 enzymes in human colon. IARC Sci Publ. 2002;156:487–489. [PubMed] [Google Scholar]
- Catterall F, McArdle NJ, Mitchell L, Papayanni A, Clifford MN, Ioannides C. Hepatic and intestinal cytochrome P450 and conjugase activities in rats treated with black tea theafulvins and theaflavins. Food Chem Toxicol. 2003;41:1141–1147. doi: 10.1016/S0278-6915(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Willett KL. Inhibition of human cytochrome CYP1 enzymes by flavonoids of St. John’s wort. Toxicology. 2006;217:194–205. doi: 10.1016/j.tox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chomczyński P, Sacchi N. Single step method of RNA isolation by acid guanidium-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Cuciureanu V, Vlase L, Muntean D, Varlan I, Cuciureanu R. Grapefruit juice-drug interactions: importance for pharmacotherapy. Rev Med Chir Soc Med Nat Iasi. 2010;114:885–891. [PubMed] [Google Scholar]
- Dallner G. Isolation of rough and smooth microsomes—general. Methods Enzymol. 1974;32:191–194. doi: 10.1016/0076-6879(74)31021-X. [DOI] [PubMed] [Google Scholar]
- Waziers I, Cugnec PH, Yang C, Leroux JL, Beaune PH. Cytochrome P450 isoenzymes, epoxide hydroxylase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253:387–394. [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue selective chemical toxicity in the respiratory and gastrointestinal tract. Annu Rev Pharmacol Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- Emoto C, Yamazaki H, Yamasaki S, Shimada N, Nakajima M, Yokoi T. Characterization of cytochrome P450 enzymes involved in drug oxidations in mouse intestinal microsomes. Xenobiotica. 2000;30:943–953. doi: 10.1080/00498250050200104. [DOI] [PubMed] [Google Scholar]
- Fujita K. Food-drug interactions via human cytochrome P450 3A (CYP3A) Drug Metabol Drug Interact. 2004;20:195–217. doi: 10.1515/dmdi.2004.20.4.195. [DOI] [PubMed] [Google Scholar]
- Fujita S, Chiba M, Ohta M, Kitani K, Suzuki T. Alteration of plasma sex hormone levels associated with old age and its effect on hepatic drug metabolism in rats. J Pharmacol Exp Ther. 1990;253:369–374. [PubMed] [Google Scholar]
- George J, Byrth K, Farrell GC. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol. 1995;50:727–730. doi: 10.1016/0006-2952(95)00192-3. [DOI] [PubMed] [Google Scholar]
- Gibson P, Gill JH, Khan PA, Seargent JM, Martin SW, Batman PA, Griffith J, Bradley C, Double JA, Bibby MC, Loadman PM. Cytochrome P450 1B1 (CYP1B1) is overexpressed in human colon adenocarcinomas relative to normal colon: implications for drug development. Mol Cancer Ther. 2003;2:527–534. [PubMed] [Google Scholar]
- Guengerich FP, Shimada T, Yun CH, Yamazaki H, Raney KD, Thier R, Coles B, Harris TM (1994) Interactions of ingested food, beverage and tobacco components involving human cytochrome P450 1A2, 2A6, 2E1 and 3a4 enzymes. Environ Health Perspect 102(Suppl 9): 49–53 [DOI] [PMC free article] [PubMed]
- Hakkak R, Ronis MJJ. Effects of enteral nutrition and ethanol on cytochrome P450 distribution in small intestine of male rats. Gastroenterology. 1993;104:1611–1618. doi: 10.1016/0016-5085(93)90636-q. [DOI] [PubMed] [Google Scholar]
- Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7:267–286. doi: 10.1517/17425255.2011.553189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MF. Contributions of hepatic and intestinal P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27:201–214. doi: 10.1016/S0169-409X(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Hidestrand M, Shankar K, Ronis MJ, Badger TM. Effects of light and dark beer on hepatic cytochrome P-450 expression in male rats receiving alcoholic beverages as part of total enteral nutrition. Alcohol Clin Exp Res. 2005;29:888–895. doi: 10.1097/01.ALC.0000164371.91315.2B. [DOI] [PubMed] [Google Scholar]
- Hodges T, Leonard R. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Hümmerich J, Zohm C, Pfau W. Modulation of cytochrome P450 1A1 by food-derived heterocyclic aromatic amines. Toxicology. 2004;199:231–240. doi: 10.1016/j.tox.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim Biophys Acta. 2007;1770:314–329. doi: 10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Iwao T, Inoue K, Hayashi Y, Yuasa H, Watanabe J. Absorption and metabolic extraction of diltiazem from the perfused rat intestine. Drug Metab Pharmacokinet. 2004;19:430–437. doi: 10.2133/dmpk.19.430. [DOI] [PubMed] [Google Scholar]
- Kanazu T, Okamura N, Yamaguchi Y, Baba T, Koike M. Assessment of the hepatic and intestinal first-pass metabolism of midazolam in CYP3A drug–drug interaction model rats. Xenobiotica. 2005;35:305–317. doi: 10.1080/00498250500093786. [DOI] [PubMed] [Google Scholar]
- Kleman MI, Overvik E, Poellinger L, Gustafsson JA. Induction of cytochrome P450 1A isozymes by heterocyclic amines and other food-derived compounds. Princess Takamatsu Symp. 1995;23:163–171. [PubMed] [Google Scholar]
- Kotegawa T, Laurijssens BE, Moltke LL, Cotreau MM, Perloff MD, Venkatakrishnan K, Warrington JS, Granda BW, Harmatz JS, Greenblat DJ. In vitro pharmacokinetic and pharmacodynamic interactions of ketokonazole and midazolam in the rat. J Pharmacol Exp Ther. 2002;302:1228–1337. doi: 10.1124/jpet.102.035972. [DOI] [PubMed] [Google Scholar]
- Lee PC, Werlin SL. The induction of hepatic cytochrome P450 3A in rats; effects of age. Proc Soc Exp Biol Med. 1995;210:134–139. doi: 10.3181/00379727-210-43932. [DOI] [PubMed] [Google Scholar]
- Lindell M, Karlsson MO, Lennernäs H, Påhlman L, Lang MA. Variable expression of CYP and Pgp genes in the human small intestine. Eur J Clin Invest. 2003;33:493–499. doi: 10.1046/j.1365-2362.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- Mandir N, FitzGerald AJ, Goodlad A. Differences in the effects of age on intestinal proliferation, crypts fission and apoptosis in the small intestine and the colon of the rat. Int J Exp Path. 2005;85:125–130. doi: 10.1111/j.0959-9673.2005.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M, Groothius G, Kanter R. Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metab Dispos. 2006;34:1047–1054. doi: 10.1124/dmd.105.009035. [DOI] [PubMed] [Google Scholar]
- Mitschke D, Reichel A, Fricker G, Moenning U. Characterization of cytochrome P450 protein expression along the entire length of the intestine of male and female rats. Drug Metab Dispos. 2008;36:1039–1045. doi: 10.1124/dmd.107.019687. [DOI] [PubMed] [Google Scholar]
- Nekvindova J, Anzenbacher P. Interactions of food and dietary supplements with grug metabolizing cytochrome P450 enzymes. Ceska Slov Farm. 2007;56:165–173. [PubMed] [Google Scholar]
- Okabe H, Hasunuma M, Hashimoto Y. The hepatic and intestinal metabolic activities of P450 in rats with surgery- and drug- induced renal dysfunction. Pharm Res. 2003;20:1591–1594. doi: 10.1023/A:1026131216669. [DOI] [PubMed] [Google Scholar]
- Paine MF, Shen DD, Kunze KLO, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. First-pass metabolism of midazolam by the human intestine. Clin Pharm Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- Paine MF, Widmer WW, Pusek SN, Beavers KL, Criss AB, Snyder J, Watkins PB. Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am J Clin Nutr. 2008;87:863–871. doi: 10.1093/ajcn/87.4.863. [DOI] [PubMed] [Google Scholar]
- Pałasz A, Czekaj P, Wiaderkiewicz A, Wiaderkiewicz R. Expression of cytochrome P450 isoforms in small intestine of aged rats. Pol J Environ Stud. 2003;12(suppl 1):231–234. [Google Scholar]
- Patel HRH, Hewer A, Hayes JD, Philips DH, Campbell FC. Age-dependent changes of metabolic capacity and genotoxic injury in rat intestine. Chem Biol Interact. 1998;113:27–37. doi: 10.1016/S0009-2797(98)00016-7. [DOI] [PubMed] [Google Scholar]
- Perloff MD, Moltke LL, Greenblatt DJ. Ritonavir and dexamethasone induce expression of CYP3A and P-glycoprotein in rats. Xenobiotica. 2004;34:133–150. doi: 10.1080/00498250310001630215. [DOI] [PubMed] [Google Scholar]
- Plewka A, Kaminski M. Wpływ wieku i fenobarbitalu na aktywnosc watrobowego układu oksydaz o funkcji mieszanej u szczurów. Folia Med Cracov. 1992;33:141–154. [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Schauer L, Sahlen GE, Ronquist G, Oliw EH. Expression of CYP4F12 in gastrointestinal and urogenital epithelia. Basic Clin Pharmacol Toxicol. 2004;94:177–183. doi: 10.1111/j.1742-7843.2004.pto940404.x. [DOI] [PubMed] [Google Scholar]
- Stupans I, Murray M, Kirlich A, Tuck KL, Hayball PJ. Inactivation of cytochrome P450 by the food-derived complex phenol oleuropein. Food Chem Toxicol. 2001;39:1119–1124. doi: 10.1016/S0278-6915(01)00060-6. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Yamazaki H, Tanaka Y, Nakajima M, Yokoi T. Catalytic activities of cytochrome P450 enzymes and UDP-glucuronosyltransferases involved in drug metabolism in rat everted sacs and intestinal microsomes. Xenobiotica. 2003;33:43–55. doi: 10.1080/0049825021000022348. [DOI] [PubMed] [Google Scholar]
- Tsunoda SM, Harris RZ, Christians U, Velez RL, Freeman RB, Benet LZ, Warshaw A. Red wine decreases cyclosporine bioavailability. Clin Pharmacol Ther. 2001;70:462–467. doi: 10.1016/S0009-9236(01)70992-7. [DOI] [PubMed] [Google Scholar]
- Uesawa Y, Takeuchi T, Mohri K. Publication bias on clinical studies of pharmaceutical interactions between felodipine and grapefruit juice. Pharmazie. 2010;65:375–378. [PubMed] [Google Scholar]
- Ware WR. Nutrition and the prevention and treatment of cancer: association of cytochrome P450 CYP1B1 with the role of fruit and fruit extracts. Integr Cancer Ther. 2009;8:22–28. doi: 10.1177/1534735408328573. [DOI] [PubMed] [Google Scholar]
- Warrington JS, Greenblatt DJ, Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine and kidney. J Pharmacol Exp Ther. 2004;309:720–729. doi: 10.1124/jpet.103.061077. [DOI] [PubMed] [Google Scholar]
- Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. doi: 10.1016/S0169-409X(97)00041-0. [DOI] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, Buc Calderon P. Age-related changes in protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as their hepatic activities in Wistar rats. What role of oxidative stress? Arch Toxicol. 2004;78:131–138. doi: 10.1007/s00204-003-0526-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Benet LZ. The gut as barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40:159–168. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Raner G, Ding X, Dunbar D, Coon MJ, Kaminsky LS. Characterization of the cytochrome P450 CYP2J4: expression in rat small intestine and role in retinoic acid biotransformation from retinal. Arch Biochem Biophys. 1998;353:257–264. doi: 10.1006/abbi.1998.0654. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Ostrowska A, Zeisloft S, Yang J, Kaminsky LS. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–809. [PubMed] [Google Scholar]