Abstract
Selenium (Se) is essential for human health. Despite evidence that Se intake affects inflammatory responses, the mechanisms by which Se and the selenoproteins modulate inflammatory signalling, especially in the gut, are not yet defined. The aim of this work was to assess effects of altered Se supply and knock-down of individual selenoproteins on NF-κB activation in gut epithelial cells. Caco-2 cells were stably transfected with gene constructs expressing luciferase linked either to three upstream NF-κB response elements and a TATA box or only a TATA box. TNFα and flagellin activated NF-κB-dependent luciferase activity and increased IL-8 expression. Se depletion decreased expression of glutathione peroxidase1 (GPX1) and selenoproteins H and W and increased TNFα-stimulated luciferase activity, endogenous IL-8 expression and reactive oxygen species (ROS) production. These effects were not mimicked by independent knock-down of either GPX1, selenoprotein H or W; indeed, GPX1 knock-down lowered TNFα-induced NF-κB activation and did not affect ROS levels. GPX4 knock-down decreased NF-κB activation by flagellin but not by TNFα. We hypothesise that Se depletion alters the pattern of expression of multiple selenoproteins that in turn increases ROS and modulates NF-κB activation in epithelial cells, but that the effect of GPX1 knock-down is ROS-independent.
Keywords: Selenoprotein, Inflammatory signalling, NF-κB, GPx1, GPx4, Selenium
Introduction
The micronutrient selenium (Se) is essential for human health (Bellinger et al. 2009; Fairweather-Tait et al. 2011). Low Se status has been reported to be associated with increased risk of colorectal cancer (CRC) whereas in contrast higher Se intake and status are associated with lower risk of colonic adenoma recurrence (Russo et al. 1997; Jacobs et al. 2004; Rayman 2005; Peters and Takata 2008). Supplementation with 200 μg Se per day resulted in reduced CRC mortality, especially in those individuals with low Se status prior to supplementation (Clark et al. 1996). However, the biochemical and cellular mechanisms that link Se intake gut epithelial cell function and carcinogenesis are poorly understood.
In humans, Se is incorporated as the amino acid selenocysteine into ~25 selenoproteins (Bellinger et al. 2009) that play important roles in cell protection mechanisms. The selenoproteins include a family of glutathione peroxidases (GPx1, GPx2 and GPx4) that protect cells from reactive oxygen species (ROS), the thioredoxin reductases (TR) that function in redox control (Brigelius-Flohé 2006; Arnér 2009) and members of a novel series of thioredoxin-like proteins that have been proposed to have antioxidant functions (Bellinger et al. 2009). There is evidence that Se has anti-inflammatory effects in macrophages and immune cells (Hoffmann and Berry 2008; Vunta et al. 2008) and Se supplementation has been found to modulate activation of the transcription factor NF-κB, which plays a pivotal role in regulation of inflammatory pathways (Jeong et al. 2002; Zamamiri-Davis et al. 2002; Prabhu et al. 2002; Gasparian et al. 2002, Christensen et al. 2007; Vunta et al. 2007).
However, critically, the in vitro effects of Se supplementation on NF-κB signalling are distinct in different cell lines. Thus, for example, supplementation of the cell culture medium lowered activation of NF-κB in human bronchial and prostate cells (Gasparian et al. 2002, Christensen et al. 2007) but had no effect on NF-κB translocation in human endothelial cells and led to increased NF-κB translocation and increased NF-κB response in macrophages (Prabhu et al. 2002; Vunta et al. 2007). These observations correspond closely to the suggestion that in vivo NF-κB pathways are regulated in a cell-type dependent mechanism (Smale 2011). Gut epithelial cells such as the Caco-2 cell line respond to bacterial and host inflammatory challenges by activation of NF-κB pathways (Kelly et al. 2004), but the NF-κB signalling response of such cells to altered Se supply or changes in selenoprotein expression are not known. Indeed, the effects of Se on inflammatory signalling in the gut and the roles of selenoproteins, especially in the epithelial cells, have not yet been defined.
However, transcriptomic analysis of samples from a recent mouse experiment examined the response of mouse colon gene expression to sub-optimal Se intake and identified the expression of genes associated with the NF-κB signalling pathway as being sensitive to dietary Se (Kipp et al. 2009). In view of the links between gut inflammation and susceptibility to colorectal cancer (Klampfer 2011), and the possible benefits of increased Se intake (Russo et al. 1997; Jacobs et al. 2004; Rayman 2005; Bellinger et al. 2009; Fairweather-Tait et al. 2011), it is important to elucidate whether Se supply affects NF-κB signalling in gut epithelial cells. The aim of the present work was to use an in vitro model of the gut epithelium to test the hypothesis that alteration of Se supply modulates NF-κB activation in response to host and microbial factors, and the to test whether the effects are due to changes in selenoprotein expression.
Materials and methods
Cell culture
Human colon adenocarcinoma Caco-2 cells were grown at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (with 4.5 g/l glucose and Glutamax) supplemented with 10% (v/v) foetal calf serum (Sigma, Poole, UK) and 1% (v/v) (100 μg/ml) penicillin–streptomycin. For siRNA knockdown experiments, cells were grown in the same medium. In Se depletion/supplementation experiments, cells were transferred to serum-free medium containing 0.1% (v/v) penicillin–streptomycin, 1% (v/v) (100 units/ml) non-essential amino acids (Gibco), insulin (5 μg/ml) and transferrin (5 μg/ml) without (selenium-deficient medium) or supplemented with 7 ng/ml sodium selenite (equivalent to 40 nM; selenium-repleted medium), as described previously (Pagmantidis et al. 2005; Crosley et al. 2007). Cells were cultured in Se-deficient or Se-supplemented medium for 3 days and then tested for responses to treatment with either 20 ng/ml TNFα (Sigma) for or 100 ng/ml Salmonella typhimurium flagellin for 0–8 h.
Luciferase constructs and transfection
Luciferase constructs were obtained by modification of a previously established construct (Carlsen et al. 2002), which contains three NFκB binding sites and a TATA box linked to a luciferase coding sequence (CDS). The entire 3× NF-κB-TATA box-luciferase CDS sequence was isolated by Hind III/ApaI restriction digestion and ligated into pBLUE-TOPO plasmid vector (Invitrogen). The control construct was obtained by isolation of TATA box-luciferase CDS sequence by BamHI/ApaI restriction digestion and fusion with pBLUE-TOPO vector. The correct insertion of the 3× NF-κB-luciferase and TATA-luciferase sequences were verified by sequencing (data not shown). Stable transfections of Caco-2 cells (1 × 106 cells/transfection) with luciferase constructs (test or control) used 2–6 μg of plasmid DNA combined with 5 μl Lipofectamine™ 2000 (Invitrogen). Stably transfected cells were selected in medium containing 750 μg/ml G418 (Sigma) for 6 weeks (medium changed every 2 days) and harvested as a mixed population.
siRNA transfection
Knock-down of expression of selenoprotein genes GPX1, GPX4, selenoprotein W (SELW) and selenoprotein H (SELH) was achieved by transient transfection of Caco-2 cells with siRNA duplexes (Ambion), specific to the mRNAs of these targets The relevant siRNA sequences were: SELH sense GCGACCGUUGUUAUCGAGCAUUGCA and antisense UGCAAUGCUCGAUAACAACGGUCGC; GPX1 sense GGUACUACUUAUCGAGA AUUU and antisense AUUCUCGAUAAGUAGUACCUU; SELW sense CCACCGGGUUCUUUGAAGUGAUGGU and antisense ACCAUCA CUUCAAAGAACCCGGUGG GPX4 sense UUCGAUAUGUUCAGCAAGAUU and antisense UCUUGCUGAACAUAUCGAAUU negative control sense GUUCAAUAUUAUCAAGCGGUU and antisense CCGCUUGAUAAUAUUGAACUU. According to Ambion’s specifications, 5 × 105 cells were grown in 2 ml of serum-free medium supplemented with 5% (v/v) foetal calf serum in the absence of antibiotics. A siRNA/transfection reagent complex was formed at 37°C by combining siRNA oligomer (30–45 nM) with 5 μl (2 μg/ml) Lipofectamine™ 2000 transfection reagent (Invitrogen) in 0.5 ml Optimem medium (Gibco), and this was applied to cells for 3 days until they were harvested. Control cells were transfected with a non-specific ‘scrambled’ siRNA duplex (Ambion).
Luciferase assay
NFκB activation in Caco-2 cells was determined by measuring luciferase activity in the reporter cells stably transfected with the 3× NF-κB-luciferase or TATA-luciferase constructs. Cells were washed twice in 1× PBS, mixed with 1× Reporter lysis buffer (Promega), frozen and thawed once three times, and lysed by vortexing vigorously for 15 s. Cell lysate was harvested as the supernatant fluid after centrifugation at 12,000g for 2 min at 4°C. Twenty microliters of cell lysate was mixed with 80 μl of 5× Luciferase assay reagent (Promega) and luciferase activity was measured on a TD-20e luminometer (Turner Designs). Protein concentration was quantified using the bicinchoninic acid protein assay (Sigma). Specific luciferase activity was calculated as luciferase activity (relative light units; RLU) per mg protein lysate.
RTPCR
The mRNA expression of the selenoproteins GPX1, GPX2, GPX4, SELW and SELH, the house-keeping gene GAPDH and the NFκB target gene IL8 were determined by semi-quantitative RT-PCR using primer oligomers as listed in Table 1. Reverse transcription was carried out amplifying 1 μg total RNA using Transcriptor Reverse Transcriptase kit (Roche) and p(dT)15 primer for cDNA Synthesis (Roche) according to manufacturer’s instructions. PCR was then carried out using 0.05 U/μl BIOTAQ polymerase (Bioline), 2 mM MgCl2, 1 mM total dNTP, 1–2 pmol/μl primer, and cDNA template. PCR was carried out using the appropriate amplification cycles so that amplification was in the linear range (Table 1). Following separation of RTPCR products by gel electrophoresis and staining with ethidium bromide, gels were visualised on a GelDoc 1000 and the intensity of bands measured using UV Band software. mRNA levels were normalised against GAPDH and expressed relative to the control conditions.
Table 1.
Primers used for RTPCR analysis of GPX1, SELW, SELH, GPX4, GPX2, GAPDH and IL8 transcripts
| Primers | Sequences | Tm |
|---|---|---|
| GPX4 for | 5′-CGA TAC GCT GAG TGT GGT TTG C-3′ | 66°C |
| GPX4 rev | 5′-CAT TTC CCA GGA TGC CCT TG-3′ | 29cyc |
| GAPDH for | 5′-TGA AGG TCG GAG TCA ACG GAT TTG-3′ | 55°C |
| GAPDH rev | 5′-CAT GTA AAC CAT GTA GTT GAG GTC-3′ | 29cyc |
| GPX1 for | 5′-CAG TCG GTG TAT GCC TTC TCG-3′ | 56°C |
| GPX1 rev | 5′-TGT CAG GCT CGA TGT CAA TG-3′ | 27cyc |
| GPX2 for | 5′-GGC TTT CAT TGC CAA GTC CTT C-3′ | 60°C |
| GPX2 rev | 5′-CTA TAT GGC AAC TTT AAG GAG GCG C-3′ | 27cyc |
| SELW for | 5′-GTT TAT TGT GGC GCT TGA GGC-3′ | 60°C |
| SELW rev | 5′-GAA CAT CAG GGA AAG ACC ACC-3′ | 27cyc |
| SELH for | 5′-GCT TCC AGT AAA GGT GAA CCC G-3′ | 62°C |
| SELH rev | 5′-ACC CAA ATC TCC CTA CGA CAG G-3′ | 27cyc |
| IL8 for | 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ | 60°C |
| IL8 rev | 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′ | 31cyc |
ROS measurement
Total cellular ROS levels were determined using the Image-iT™ LIVE Green Reactive Oxygen Species (ROS) Detection Kit (Invitrogen) according to manufacturer’s specifications. A total of 6 × 104 or 3 × 104 cells were seeded in each well of a 96-well plate and grown in Se−/Se+ medium, or treated with GPX1 siRNA/control siRNA, respectively. Cells were washed with 1× HBSS and incubated with 50 μl staining solution containing 25 μM 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) and 1 μM Hoechst 33342 (control staining of nucleic acid) for 30 min at 37°C. Cells were washed with 1× HBSS and ROS levels measured at 495/529 nm (for carboxy-H2DCFDA) and 350/461 nm (for Hoechst 33342) wavelengths on a BMG LABtech Fluostar Omega luminometer.
Western blotting
Total protein was extracted from Caco-2 cells by washing twice with ice-cold PBS, resuspension in PBS containing protease inhibitor, centrifugation at 150 rpm, 4°C for 5 min and then lysis in 25 mM HEPES buffer (pH 7.6) containing 3 mM MgCl2, 40 mM KCL, 2 mM DTT, 5% glycerol and 0.5% NP40. Protein concentration was determined using the Bradford protein assay procedure. Twenty micrograms of protein was subjected to SDS-PAGE, transferred to a PVDF membrane (ROCHE), and incubated overnight in PBS containing 5% dried milk, 0.05% Tween20 and primary antibody (Anti-GPx1 from AbCam diluted 1/500, Anti-GPx4 from Labfrontier, Korea diluted 1/500, monoclonal Anti-βactin from Sigma diluted 1/5,000). After four washes in PBS containing 0.05% Tween20, the membrane was incubated with secondary antibody (polyclonal Anti-rabbit for Anti-GPx1 and Anti-GPx4, polyclonal Anti-mouse for Anti-βactin) linked to horseradish peroxidase (from Sigma, UK diluted 1/5000) for 1 h, washed 4 times and bound antibody detected by chemiluminescence.
Statistical analysis
Groups were compared by Mann–Whitney U-test using SPSS 17.0 software.
Results
TNFα and flagellin activate NF-κB signalling in Caco-2 cells
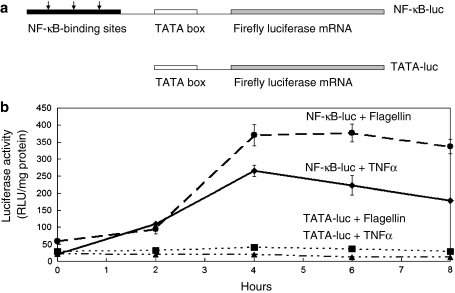
Previous work has shown that it is possible to monitor activation of NF-κB signalling in vivo in transgenic mice expressing a gene in which a luciferase reporter is linked to an upstream region containing three copies of a NF-κB response element (Carlsen et al. 2002). In the present work, we adopted a similar reporter gene approach to monitor NF-κB activation in the Caco-2 gut epithelial cell line. Two gene constructs were produced (see Fig. 1a), as described in "Materials and methods". In the first, the luciferase coding region was linked to three upstream NF-κB response elements and a TATA box (NF-kB-luciferase construct). In addition, a control construct was made in which the three NF-κB response elements were absent and the region upstream of the luciferase coding sequences contained only a TATA box (TATA-luciferase construct). Caco-2 cells were transfected with either the NF-κB-luciferase construct or the control TATA-luciferase construct and stable transfectant cell populations selected. When cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal calf serum, a low level of luciferase activity was detected in both transfectants (Fig. 1b, time point 0). However, as shown in Fig. 1b, addition of either TNFα or bacterial flagellin resulted in a striking ~ten fold increase in luciferase activity, with the appearance of a peak activity ~4 h after addition of TNFα and 4–6 h after addition of flagellin (see Fig. 1b). These results indicate that both these compounds activate NF-κB signalling in Caco-2 cells.
Fig. 1.
Activation of NF-κB-luciferase constructs in Caco-2 cells. a Schematic illustration of the NF-κB-luciferase and control TATA-luciferase constructs. The NF-κB-luciferase construct contained the firefly luciferase coding region, a TATA box and three NF-κB binding sites whereas the NF-κB binding sites were deleted in the control. b Caco-2 cells were transfected with either the NF-κB-luc or TATA-luciferase construct. Stable transfectants grown in Se-supplemented medium were incubated with flagellin (100 ng/ml) or TNFα (20 ng/ml) for up to 8 h and cell extract prepared for measurement of luciferase activity. Both flagellin and TNFα elicited a stimulation of luciferase activity in the NF-κB-luciferase cells but not in the TATA-luciferase controls (TNFα dashline with triangle and flagellin dashline with square)
Se depletion activates NF-κB signalling in Caco-2 cells
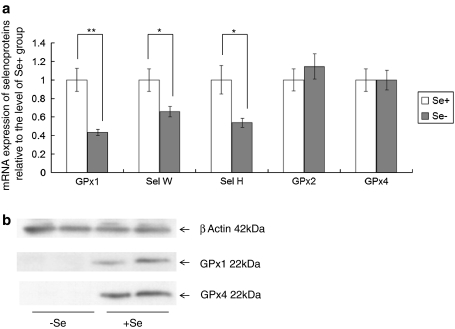
To assess the effects of Se supply on NF-κB signalling in Caco-2 cells, cells were grown in either Se-depleted medium or medium supplemented with 40nM sodium selenite as described previously (Pagmantidis et al. 2005; Crosley et al. 2007). When grown in either medium, cells were maintained, despite the absence of serum, by the addition of insulin and transferrin and effects of Se supplementation could be specifically detected since the two media differed only in their Se content. Expression of GPX1, SELW and SELH, known to be very sensitive to Se supply (Pagmantidis et al. 2005; Kipp et al. 2009), was determined by semi-quantitative RTPCR. As shown in Fig. 2, expression of GPX1, SELW and SELH was significantly decreased in cells grown under Se-deficient conditions compared with those grown in Se-supplemented medium, indicating that the Caco-2 cells grown in Se-deficient or Se-supplemented media differ in Se status. In contrast, there was no significant change in expression of GPX2 [known to be maintained in low Se conditions (Pagmantidis et al. 2005; Kipp et al. 2009)] in response to alterations in Se supply (Fig. 2). In addition, GPx1 and GPx4 protein expression was undetectable by Western blotting in cells grown in Se-deficient medium (Fig. 2b).
Fig. 2.
Selenoprotein mRNA expression in Caco-2 cells during Se depletion. a Caco-2 cells were grown for 3 days in either Se-depleted or Se-supplemented (40 nM) medium, RNA extracted and mRNA levels assessed by semi-quantitative RTPCR. Values were normalised to GAPDH and calculated relative to the expression in the Se-supplemented group. Expression of GPX1, SELH and SELW was significantly lower in the Se-depleted cells, but expression of GPX2 and GPX4 was unaffected. *P < 0.05, **P < 0.01. b Western blotting of total cell protein from cells grown for 3 days in either Se-depleted or Se-supplemented medium. Note that the levels of GPx1 and GPx4 are both undetectable in Se-depleted cells
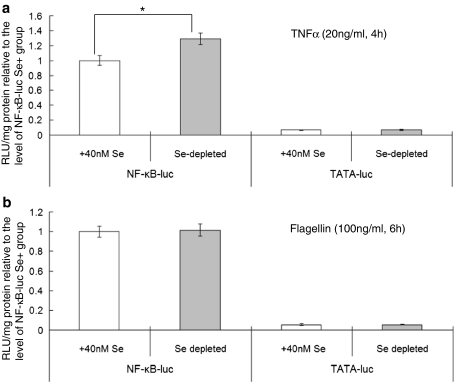
To determine whether changes in Se supply affect NF-kB signalling, luciferase activity was measured in cell extracts from cells grown under Se-supplemented or deficient conditions. As shown in Fig. 3, in cells transfected with the NF-κB-luciferase and grown in Se-deficient conditions, the increase in luciferase activity following TNFα treatment was 30% greater compared with cells grown in Se-supplemented medium (P < 0.05; see Fig. 3). However, there was no change in luciferase activity in response to addition of flagellin. In addition, control TATA-luciferase cells showed no alteration in the response in luciferase activity after addition of either activator whether cells were grown in Se-deficient or Se-supplemented medium.
Fig. 3.
Effect of Se depletion on TNFα and flagellin stimulated NF-κB-driven reporter activity. Stably transfected Caco-2 cells were grown for 3 days in either Se-depleted or Se-supplemented (40 nM) medium and then stimulated with a 20 ng/ml TNFα for 4 h or b 100 ng/ml flagellin for 6 h. Luciferase activity was measured in cell extracts, calculated per mg cell protein and expressed relative to the activity in the Se-supplemented NF-κB-luciferase cells. *P < 0.05
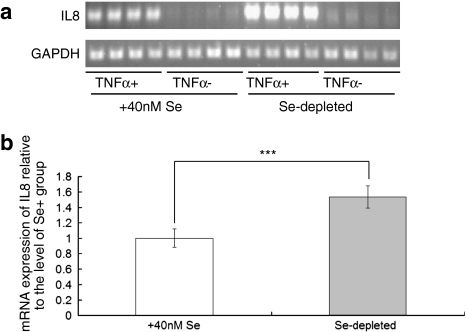
Expression of the cytokine interleukin-8 (IL-8) has been shown to be controlled by NF-κB (Kunsch and Rosen 1993; Kelly et al. 2004). To determine whether changes in Se supply affect NF-κB-induced expression, we measured IL-8 mRNA expression in Caco-2 cells in response to TNFα under Se-deficient and Se-supplemented conditions. As shown in Fig. 4, addition of 20 ng/ml TNFα to the culture medium of NF-κB-luciferase cells led to an increase in IL-8 expression as assessed by semi-quantitative RT–PCR, and this increase was significantly larger (P < 0.001) under Se-deficient conditions compared with Se-supplemented conditions. This increase in IL-8 response under Se depletion conditions is compatible with the data from the luciferase reporter experiments that indicate increased activation of NF-κB (Fig. 3a). On the contrary, in the absence of TNFα, changes in Se supply had no effect on IL8 expression.
Fig. 4.
Effects of Se depletion on interleukin 8 expression. a Semi-quantitative RTPCR amplification of NF-κB target gene IL8 and housekeeping gene GAPDH. Cells were grown in Se-depleted or 40 nM Se-supplemented culture medium (3 days) and either stimulated with TNFα for 1 h or treated with carrier. b Quantification of IL8 mRNA levels in cells grown in Se+ and Se− media, normalised against GAPDH and related to expression in the Se-supplemented cells. ***P < 0.001
GPX1 knock-down compromises TNFα-mediated activation of NF-κB signalling
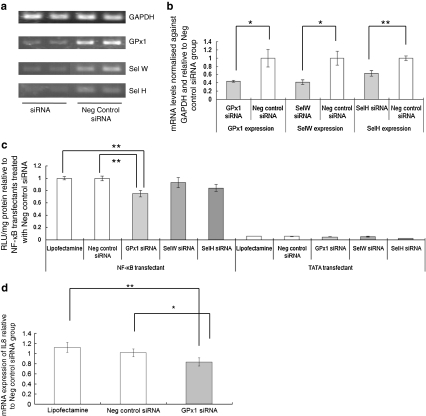
To investigate whether these effects of Se depletion on NF-κB signalling could be accounted for by lower selenoprotein expression, GPX1, SELW and SELH were knocked down by addition of 80 pmol siRNA. Knock-down of SELW or SELH expression by 40–60%, respectively, (Fig. 5a, b) had no significant effect on the luciferase response of NF-κB-luciferase cells to 20 ng/ml TNFα and no effect on the luciferase activity in TATA-luciferase cells (Fig. 5c). Thus, the effects of Se depletion on the NF-κB response to TNFα do not appear to be due to the reduction of SELW and SELH expression known to occur in colonic cells and tissue in Se depletion. Similarly, transfection with an siRNA against GPX1 did not increase the luciferase response to 20 ng/ml TNFα. Thus, the effects of Se depletion on the NF-κB-driven luciferase response to TNFα cannot be explained as being due to effects of Se depletion on expression of GPX1, SELW or SELH.
Fig. 5.
Effect of selenoprotein knock-down on TNFα stimulated NF-κB-driven reporter activity and interleukin 8 expression. a Semi-quantitative RTPCR amplification of mRNA levels for GPX1, SELW and SELH after knockdown of the respective gene with 80 pmol siRNA and in control cells. Total RNA was extracted, RTPCR carried out and the products separated by gel electrophoresis. b The intensity of bands corresponding to the amplified products was measured using UV Band software, normalised to the housekeeping gene GAPDH and expression then calculated relative to the cells treated with the negative control siRNA negative control. c Luciferase activity was measured in NF-κB-luc transfectant or TATA-luc transfectant Caco-2 cells grown in Se-supplemented medium but after GPX1, SELW or SELH siRNA knockdown and stimulation with 20 ng/ml TNFα for 4 h. Luciferase activity was measured in cell extracts, calculated per mg cell protein and expressed relative to the activity in the NF-κB-luciferase cells treated with negative control siRNA. *P < 0.05, **P < 0.01. d Cells were either stimulated with TNFα for 1 h or treated with carrier (phosphate-buffered saline). RNA was extracted and subjected to semi-quantitative RTPCR amplification of NF-κB target gene IL8 and housekeeping gene GAPDH. After separation of the amplified products by gel electrophoresis, intensity of bands was measured using UV Band software. IL8 mRNA levels were quantified, normalised against GAPDH and expression related to that in the cells treated with the negative control siRNA. *P < 0.05, **P < 0.01
On the contrary, transfection with 80 pmol siRNA against GPX1 resulted in approximately 60% decrease in GPx1 expression and a 20% decrease in the response of luciferase activity to TNFα stimulation in NF-κB-luciferase cells compared with the addition of a scrambled control siRNA (see Fig. 5c). Quantification of IL-8 expression after TNFα stimulation of Caco-2 cells showed a reduced cytokine response after GPX1-knock-down compared with the response in cells treated with the control scrambled siRNA (Fig. 5d). It thus appears that GPx1 is required for the full NF-κB response to TNFα.
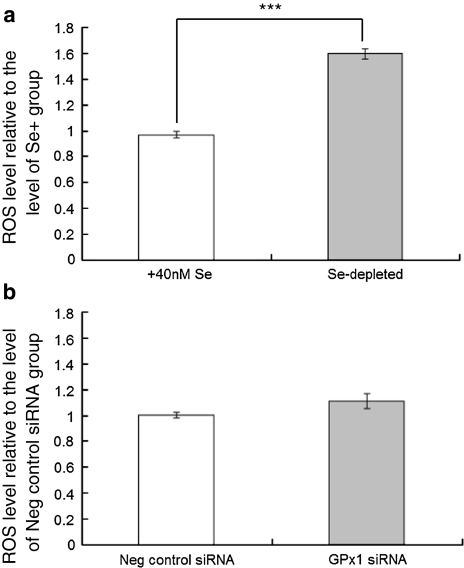
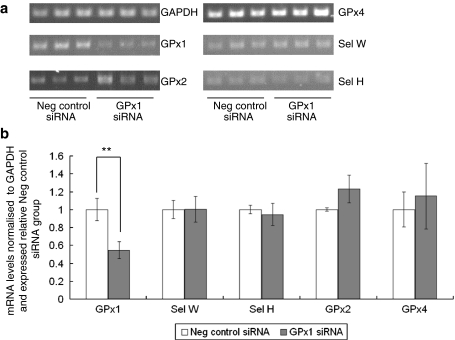
ROS, particularly superoxide radicals and hydrogen peroxide, are known to regulate NF-κB signalling pathways (Schreck et al. 1991; Li et al. 2001; Kabe et al. 2005). One possible explanation for the different effects of Se depletion and GPX1 knockdown on NF-κB activation is that the two treatments have dissimilar effects on superoxide levels and the ability to maintain redox homoeostasis because of the continued activities of other selenoproteins in the knock-down experiments but not in Se-depleted cells. Therefore, the effects of both Se depletion and GPX1 knock-down on total cellular ROS levels were investigated. As shown in Fig. 6, Se depletion led to an increase in baseline ROS levels in Caco-2 cells but GPX1 knock-down had no detectable effect. Additionally, GPX1 knock-down was found to have no effect on expression of GPX2 and GPX4 mRNAs (Fig. 7); the lack of effect of GPX1 knockdown on ROS is likely due to the ability of these selenoproteins to compensate for the lower GPX1 expression.
Fig. 6.
Effects of Se depletion and GPX1 knock-down on levels of reactive oxygen species in Caco-2 cells. ROS levels were determined in extracts of Caco-2 cells using carboxy-H2DCFDA fluorescent indicator. a Comparison between cells grown in Se-depleted and 40 nM Se-supplemented medium. b Comparison between cells grown in Se-supplemented medium but treated with 80 pmol negative control siRNA or GPX1 siRNA.***P < 0.001
Fig. 7.
Selenoprotein mRNA expression in Caco-2 cells after GPX1 siRNA treatment. a Caco-2 cells grown in Se-supplemented medium were treated with 80 pmol GPX1 siRNA or negative control siRNA for 48 h, RNA extracted and selenoprotein mRNA levels assessed by semi-quantitative RTPCR. b After separation of the RTPCR products by gel electrophoresis, the intensity of bands corresponding to the amplified products was measured using UV Band software. Selenoprotein mRNA expression was normalised to GAPDH and calculated relative to the expression in the negative control siRNA group. Expression of GPX1 was significantly lower in GPX1 siRNA treated cells but expression of SELH, SELW, GPX4 and GPX2 was unaffected. **P < 0.01
GPX4 knock-down compromises flagellin-mediated activation of NF-κB signalling
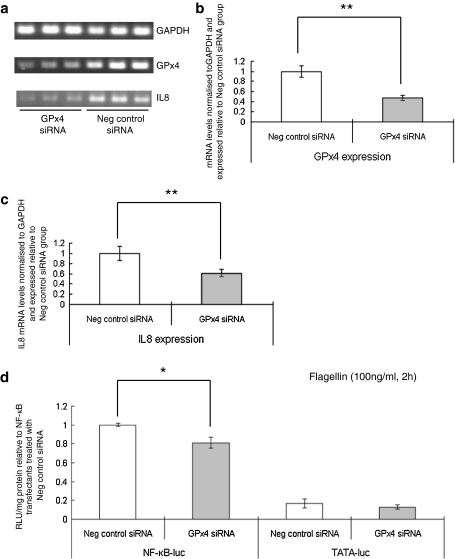
Since GPx4 has been implicated in lipid hydroperoxide and leukotriene metabolism (Brigelius-Flohé 2006; Bellinger et al. 2009), we tested whether knock-down of GPX4 affected NF-κB signalling. Gene knock-down with a GPX4-specific siRNA led to a 50% decrease in GPX4 expression as assessed by RTPCR (Fig. 8a), but knock-down of this selenoprotein had no effect on the response of luciferase activity to TNFα stimulation in NF-κB-luciferase cells compared with addition of a scrambled control siRNA (results not shown). In contrast, GPX4 knock-down caused a reduction in flagellin-activated luciferase activity and IL-8 expression (Fig. 8b, c).
Fig. 8.
Effect of GPX4 knock-down on flagellin stimulated NF-κB- reporter activity and endogenous interleukin 8 expression. a Semi-quantitative RTPCR amplification of GPX4 mRNA in cells treated with either a specific GPX4 siRNA (120 pmol) or the negative control. Total RNA was extracted, RTPCR carried out and the products separated by gel electrophoresis. b The intensity of bands corresponding to the amplified GPX4 product was measured using UV Band software, normalised to the housekeeping gene GAPDH and expression then calculated relative to the cells treated with the negative control siRNA. c Quantification of IL8 expression by semi-quantitative RTPCR. Bands corresponding to the amplified IL8 products were normalised to the housekeeping gene GAPDH and expression then calculated relative to the cells treated with the negative control siRNA. d Luciferase activity measured in NF-κB-luc transfectant or TATA-luc transfectant Caco-2 cells after GPX4 siRNA knockdown and stimulation with 100 ng/ml flagellin for 2 h. Luciferase activity was measured in cell extracts, calculated per mg cell protein and expressed relative to the activity in the NF-κB-luciferase cells treated with negative control siRNA. *P < 0.05, **P < 0.01
Discussion
Results from both luciferase reporter assays and measurement of endogenous IL-8 expression showed that both TNFα and flagellin elicited an activation of NF-κB signalling in the gut epithelial Caco-2 cell line. This confirms earlier data showing that these enterocytic cells activate inflammatory pathways in response to such molecules (Kelly et al. 2004). In addition, the present data show that the response of NF-κB activation to TNFα was modulated by Se supply such that Se depletion led to increased luciferase activity in cells expressing the reporter driven by NF-κB response elements and to increased expression of endogenous IL-8, a chemokine that is released from epithelial cells and is key in determining neutrophil chemotaxis (Eckmann et al. 1993). These observations are consistent with recent transcriptomic data that suggest that a marginal dietary Se deficiency in the mouse causes altered expression of genes in inflammatory NF-κB signalling pathways in the colon (Kipp et al. 2009) but in addition provide the first direct evidence that NF-κB activation in a gut epithelial cell line is sensitive to Se depletion/supplementation. Moreover, Se depletion affected the response of NF-κB activation to TNFα, but not the response to bacterial flagellin, indicating modulation of responses to endogenous inflammatory mediators rather than modulation of the responses to exogenous bacterial activators via Toll-like receptors.
Earlier studies have shown that alterations in Se supply lead to changes in activation of the transcription factor NF-κB but that the effects differ between cell lines, with increased NF-κB response to Se supplementation in macrophages (Zamamiri-Davis et al. 2002; Prabhu et al. 2002; Youn et al. 2008) but lower activation of NF-κB in human bronchial and prostate cells (Jeong et al. 2002; Gasparian et al. 2002; Christensen et al. 2007). The present results show that in gut epithelial cell lines, the effect of Se on the NF-κB response to TNFα is comparable to that in bronchial and prostate epithelial cell lines and distinct from that found in macrophages, a finding consistent with the view that regulation of NF-κB differs between epithelial and immune cell types (Pasparakis 2009; Smale 2011).
Expression of GPX1, SELH and SELW in the mouse colon and Caco-2 cells has been shown to be highly sensitive to Se depletion (Pagmantidis et al. 2005; Kipp et al. 2009). However, knock-down of expression of these selenoprotein genes either failed to affect the response to of NF-κB activation to 20 ng/ml TNFα (SELH, SELW) or in the case of GPX1 knock-down, it caused a reduction in the NF-κB-dependent response. Indeed, the data in Figs. 3 and 5 show opposite effects of Se depletion and GPX1 knock-down on the NF-κB response despite both treatments lowering GPX1 expression (see Fig. 2; Pagmantidis et al. 2005). Thus, the effect of Se deficiency on NF-κB activation cannot be accounted for by a reduction of expression of SELH, SELW or GPX1 individually.
Measurement of total Caco-2 cell ROS levels indicated that Se depletion resulted in an increase in ROS levels, but there was no increase after GPX1 knock-down. A key difference between GPX1 knock-down and Se depletion is that Se depletion alters the expression of multiple selenoproteins (Fig. 2), but that knock-down of GPX1 does not (Fig. 7). Thus, after GPX1 knock-down when Se supply is adequate, expression of these other selenoproteins is expected to be maintained and we speculate that their activity can compensate for the lower GPX1 expression; as a result, any elevation of a particular species of oxidative signal, such as superoxide radicals, will be transient and rapidly corrected, whereas in Se-depleted cells, the rise in ROS cannot be corrected. Superoxide radicals are known to modulate inflammatory signalling pathways, and activation of NF-κB is redox sensitive (Schreck et al. 1991; Takada et al. 2003; Brigelius-Flohé et al. 2004; Kabe et al. 2005) with modulation of hydrogen peroxide clearance affecting IKK activity (Li et al. 2001). Therefore, our hypothesis is that in gut epithelial cells, the TNFα-induced, IKK-mediated activation of NF-κB following Se depletion is modulated by increased superoxide levels as a result of Se supply altering expression of multiple selenoproteins.
In contrast, when Se supply was adequate, knock-down of GPX1 expression led to lower NF-κB activation in response to TNFα but had no effect on baseline ROS levels. This suggests that GPx1 can have a ROS-independent effect on TNFα-induced activation of NF-κB. ROS-independent activation of NF-κB has been reported in other cell types (Legrand-Poels et al. 1997), and knock-out of GPX1 has opposite effects on ROS-dependent and reactive nitrogen species-dependent signalling pathways (Fu et al. 2001). In addition, GPX4 knock-down affected activation of NF-κB by bacterial flagellin, suggesting that changes in GPx4 expression modulate interactions between bacterial microbiota and the host. Thus, the results indicate that in Caco-2 cells, both GPx1 and GPx4 are both required for full response of NF-κB, but it appears that GPx1 modulates pathways operating through the TNFα receptor and GPx4 pathways through Toll-like receptor 5 in response to flagellin. These differences in response of NF-κB activation after GPX1 and GPX4 knock-down may reflect their different products derived from lipid peroxides (Seiler et al. 2008).
In conclusion, the present results indicate that Se depletion affects the response of NF-κB activation to TNFα, but not the response to bacterial flagellin, and that GPX1 knock-down and GPX4 knock-down have distinct effects on activation of NF-κB. We hypothesise that Se depletion affects the pattern of expression of multiple selenoproteins and that the combined changes in expression lead to increased superoxide levels that in turn modulate NF-κB signalling. In contrast, the effect of GPX1 knock-down alone is ROS-independent. The complex roles of selenoproteins in modulating these inflammatory pathways is further illustrated by the finding that knock-down of GPX4 lowers flagellin-induced activation of NF-κB but has no effect on TNFα-induced activation. Effects of Se on inflammatory signalling in macrophages have been linked both to effects of 15-deoxy-delta12,14 prostaglandin J2 and to Toll-like receptor pathways and IκB-kinaseß (Vunta et al. 2007; Youn et al. 2008), and so future work on gut epithelial cell lines should explore the possible links between Se, selenoproteins, pathways of activation of NF-κB and eicosanoid metabolism but bearing in mind that regulation of NF-κB differs between epithelial and immune cell types (Pasparakis 2009). Since gut inflammation has been found to be associated with subsequent tumour development (Klampfer 2011), these effects of Se supply and selenoproteins on inflammatory pathways in gut epithelial cells provide a potential mechanistic link between the effects of Se intake on susceptibility to colorectal cancer, inflammation and carcinogenesis.
Acknowledgments
We thank the Wellcome Trust for financial support (ref: 081380) and Professor Rune Blomhoff (Oslo University, Norway) for the kind gift of the vector used as a source of NFκB-luciferase sequences for the DNA cloning. We thank Chris Blackwell for carrying out the Western blotting.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Arnér ES. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422:11–22. doi: 10.1042/BJ20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Banning A, Kny M, Böl GF. Redox events in interleukin-1 signaling. Arch Biochem Biophys. 2004;423:66–73. doi: 10.1016/j.abb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Carlsen H, Moskaug JØ, Fromm SH, Blomhoff R. In vivo imaging of NF-kappa B activity. J Immunol. 2002;168:1441–1446. doi: 10.4049/jimmunol.168.3.1441. [DOI] [PubMed] [Google Scholar]
- Christensen MJ, Nartey ET, Hada AL, Legg RL, Barzee BR. High selenium reduces NF-kappaB-regulated gene expression in uninduced human prostate cancer. Nutr Cancer. 2007;58:197–204. doi: 10.1080/01635580701328701. [DOI] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. J Am Med Assoc. 1996;276:1957–1963. doi: 10.1001/jama.1996.03540240035027. [DOI] [PubMed] [Google Scholar]
- Crosley LK, Méplan C, Nicol F, Rundlöf AK, Arnér ES, Hesketh JE, Arthur JR. Differential regulation of expression of cytosolic and mitochondrial thioredoxin reductase in rat liver and kidney. Arch Biochem Biophys. 2007;459:178–188. doi: 10.1016/j.abb.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxid Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Fu Y, Sies H, Lei XG. Opposite roles of selenium-dependent glutathione peroxidase-1 in superoxide generator diquat- and peroxynitrite-induced apoptosis and signaling. J Biol Chem. 2001;276:43004–43009. doi: 10.1074/jbc.M106946200. [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Yao YJ, Lü J, Yemelyanov AY, Lyakh LA, Slaga TJ, Budunova IV. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-kappa B) in prostate cancer cells. Mol Cancer Ther. 2002;1:1079–1087. [PubMed] [Google Scholar]
- Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ET, Jiang R, Alberts DS, Greenberg ER, Gunter EW, Karagas MR, Lanza E, Ratnasinghe L, Reid ME, Schatzkin A, Smith-Warner SA, Wallace K, Martínez ME. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- Jeong DW, Yoo MH, Kim TS, Kim JH, Kim IY. Protection of mice from allergen-induced asthma by selenite: prevention of eosinophil infiltration by inhibition of NF-kappa B activation. J Biol Chem. 2002;277:17871–17876. doi: 10.1074/jbc.M200808200. [DOI] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- Kipp A, Banning A, van Schothorst EM, Méplan C, Schomburg L, Evelo C, Coort S, Gaj S, Keijer J, Hesketh JE, Brigelius-Flohé R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res. 2009;53:1561–1572. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- Klampfer L (2011) Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets, 2011 Jan 19 [epub ahead of print] [DOI] [PMC free article] [PubMed]
- Kunsch C, Rosen CA. NF-kB subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand-Poels S, Zecchinon L, Piret B, Schoonbroodt S, Piette J. Involvement of different transduction pathways in NF-kB activation by several inducers. Free Radical Res. 1997;27:301–309. doi: 10.3109/10715769709065768. [DOI] [PubMed] [Google Scholar]
- Li Q, Sanlioglu S, Li S, Ritchie T, Oberley L, Engelhardt JF. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal. 2001;3:415–432. doi: 10.1089/15230860152409068. [DOI] [PubMed] [Google Scholar]
- Pagmantidis V, Bermano G, Villette S, Broom I, Arthur J, Hesketh J. Effects of Se-depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett. 2005;579:792–796. doi: 10.1016/j.febslet.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- Peters U, Takata Y. Selenium and the prevention of prostate and colorectal cancer. Mol Nutr Food Res. 2008;52:1261–1272. doi: 10.1002/mnfr.200800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu KS, Zamamiri-Davis F, Stewart JB, Thompson JT, Sordillo LM, Reddy CC. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem J. 2002;366:203–209. doi: 10.1042/BJ20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–542. doi: 10.1079/PNS2005467. [DOI] [PubMed] [Google Scholar]
- Russo MW, Murray SC, Wurzelmann JI, Woosley JT, Sandler RS. Plasma selenium levels and the risk of colorectal adenomas. Nutr Cancer. 1997;28:125–129. doi: 10.1080/01635589709514563. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, Peterson DG, Reddy CC, Prabhu KS. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12, 14-prostaglandin J2 in macrophages. J Biol Chem. 2007;282:17964–17973. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res. 2008;52:1316–1323. doi: 10.1002/mnfr.200700346. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lim HJ, Choi YJ, Lee JY, Lee MY, Ryu JH. Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol. 2008;8:495–501. doi: 10.1016/j.intimp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Zamamiri-Davis F, Lu Y, Thompson JT, Prabhu KS, Reddy PV, Sordillo LM, Reddy CC. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med. 2002;32:890–897. doi: 10.1016/S0891-5849(02)00775-X. [DOI] [PubMed] [Google Scholar]