Abstract
The monosomy 1p36 syndrome is a cause of syndromic obesity. It is characterised by psychomotor delay, hypotonia and typical craniofacial dysmorphism. Other features commonly associated are behavioural anomalies including hyperphagia and self-injuring, seizures, congenital heart disease and hypothyroidism. The authors report the case of a 9-year and 5-month-boy referred to the paediatric endocrinology clinics for morbid obesity. Clinical findings were generalised obesity with a body mass index >95th centile, acanthosis nigricans of the neck, arms with self inflicted lesions, deep-set eyes, straight eyebrows, broad nasal bridge and pointed chin. He was unable to walk and had no expressive language. Cytogenetic analysis identified 1p36.33-pter deletion (~139 Mb terminal deletion in chromosome 1 short arm) and Y chromosome duplication. The blood analysis showed insulin resistance and dyslipidaemia. The authors emphasise the need to consider monosomy 1p36 as a cause of severe psychomotor delay and obesity.
Background
Monosomy 1p36 is the most common subtelomeric microdeletion syndrome.1–3 It is associated with congenital hypotonia and feeding difficulties, psychomotor delay, usually severe, with poor expressive language and typical craniofacial phenotype with midface hypoplasia, deep-set eyes and straight eyebrows, broad nasal root and bridge and pointed chin. Other features commonly described are behavioural disorders such as hyperphagia and self-injuring, seizures, structural brain anomalies, congenital heart disease and cardiomyopathy, orofacial clefting, deafness, visual anomalies (strabismus, refractive errors), genital anomalies and hypothyroidism. Generalised obesity associated with hyperphagia is frequently described as Prader–Willi like phenotype.2 4 Management of patients requires multi-disciplinary approach with neurodevelopment assessment including hearing and vision, cardiac evaluation, endocrine follow-up of thyroid function and obesity and support by physical and occupational therapy.1–3
Case presentation
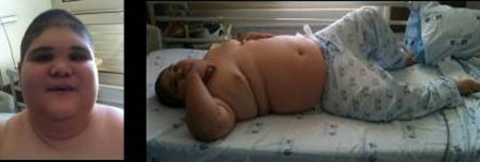
A 9-year and 5-month boy was referred to the paediatric endocrinology clinics for evaluation of morbid obesity (figure 1). He was the first child of consanguineous parents, the father had psoriatic arthritis and the mother was obese. Several family members were affected with γ-sarcoglicanopathy; genetic testing for previous identified familial mutation in SGCG gene revealed that father was carrier and the mother was not. The pregnancy was irregularly monitored. He had a preterm vaginal delivery at 36 weeks of gestation, the Apgar score was 9/10 and the newborn somatometry was adequate for gestacional age (weight 2480 g, 25–50th centile; length 42 cm, 5th centile; head circumference 33 cm, 50th centile).
Figure 1.
(a) Notice round face with narrow bifrontal diameter, straight eyebrows, deep-set eyes, convergent strabismus, downturned and narrow palpebral fissures, epicantus, broad nasal root, flat nasal bridge, short nose, small mouse and pointed chin; (b) General obesity. Hands and feet are short.
Psychomotor developmental delay and facial dysmorphism were noticed in the first month of life. Hypertension was diagnosed at 5 years and treated with captopril. He was submitted to an adenotonsillectomy because of obstructive sleep apnoea. He had a history of recurrent respiratory infections and at 5 years he had an admission at paediatric intensive care unit for ventilatory support, maintaining since then non-invasive ventilation at night (bilevel positive airway pressure).
Observation at 5 years and 11 months revealed severe psychomotor delay, with no walking or speech, aggressive behaviour, generalised obesity with body mass index (BMI) of 339 kg/m2 (>>95th centile, weight > 97th centile and height in 97th centile), dysmorphic facial features with straight eyebrows, deep-set eyes, broad nasal root, flat nasal bridge and pointed chin and prepubertal genitalia.
Investigations
He had a normal metabolic evaluation in the first month of life. At 3 years of age brain CT scan and cardiac evaluation were normal. Blood tests revealed, at first appointment, insulin resistance, with insulin 43.9 mUI/l and HOMA-IR 6,8; normal fasting glucose and HbA1c, insulin-like growth factor 1, cortisol and thyroid function. He had total cholesterol of 209 mg/dl, high density lipoprotein 49 mg/dl, low density lipoprotein 111 mg/dl and triglycerides 278 mg/dl.
Lymphocyte karyotype was 47XYY, which did not justify his phenotypic changes.5 Fluorescence insitu hybridisation (FISH) analysis for both Prader–Willi syndrome and Smith–Magenis syndrome were normal. Like all the previous research was normal, we proceeded the study with subtelomeric FISH which identified a translocation chromosome 2 long arm to chromosome 1 short arm whose terminal portion is deleted at 1p36 result: ish der(1)t(1;2)(pter-;qter+), del(2)(qter-)dn, Yp11.32 (DXY129x2). The patient’s parents had normal lymphocyte karyotype and subtelomeric FISH analysis. Further characterisation of the patient genome by oligoarray CGH confirmed a terminal deletion with 139 Mb corresponding to the region 1p36.33 with break points starting at 825 513 Mb and ending at 2 224 320 Mb. Thus, monosomy 1p36 syndrome was diagnosed.
Treatment
He started therapy with metformin and topiramate (because of aggressive behaviour). He was medicated since 5 years old with captopril. Orlistat was proposed but not initiated because of economic difficulties. Currently, he is medicated with metformin, topiramate, captopril and budesonide.
Outcome and follow-up
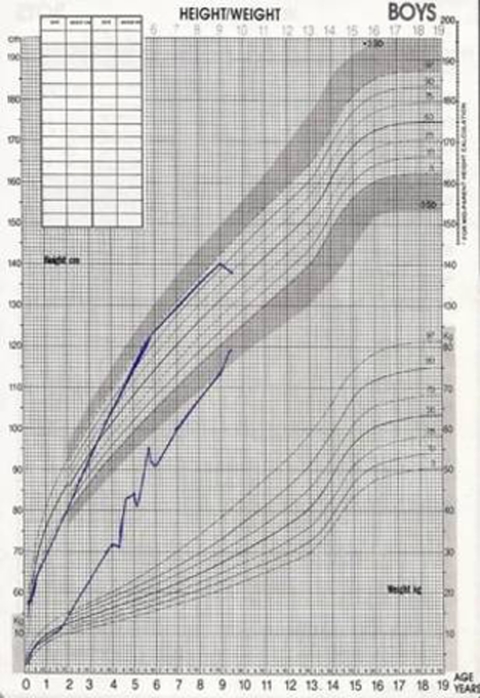
He maintained regular follow-up in paediatric endocrinology with great difficulty in weight control, maintaining BMI above the 95th centile (figure 2). He maintain insulin resistance with insulin 226 ng/ml (HOMA-IR 44,1) and triglycerides of 622 mg/dl. He has paediatric pulmonology, paediatric cardiology and ophthalmology follow-up. He is also followed in genetics, where genetic counselling to the family was carried out.
Figure 2.
Growth chart showing progressive weight gain. Weight >>97th centile since 2 years old.
Discussion
The incidence of 1p36 monosomy has been estimated to be 1 in 5000 live born children.6 More females than males have been reported. Despite 100 reported cases, little is known about its natural history.1–3
Development delay is almost invariable, and is usually severe, with more severe impairment for speech compared with motor development.2 3 Language skills are commonly delayed and expressive language is normally absent. Behavioural difficulties and self injury have been described.
Pre and postnatal growth retardation, with or without microcephaly has been noted in a significant number of cases. Paradoxically, children with obesity and hyperphagia resembling Prader–Willi syndrome have also been reported with terminal 1p deletions.3 4
The most common described dysmorphic features included a large anterior fontanel, prominent forehead (also described as bossed), deep set eyes, and short, narrow or slanting palpebral fissures. A flat nasal bridge and midface hypoplasia were also frequent. Orofacial clefting has been described in 10–40% of patients with 1p36 monosomy.1–4
Hypotonia has been consistently documented and epilepsy has been found in the majority of children.1 2 6 In some children, seizures started in infancy and ceased in the first few years of life whereas others have had persistent convulsions requiring long-term medication.2 6
Structural cardiac malformations were present in 43% of patients and patent ductus arteriosus, tetralogy of Fallot, septal defects and Ebstein anomaly have been described. Infantile dilated cardiomyopathy was reported in 23% of subjects and has responded to treatment in some cases.
Visual defects consist of strabismus, sixth nerve palsy, amblyopia, refractive errors, anomalous optic discs, lachrymal defects, bilateral cataracts, iris colobomas and optic atrophy. Sensorineural or conductive hearing loss have been reported to be quite frequent.
Hypothyroidism may be present and thyroid function should be routinely tested.
When obesity is present, it should be managed by paediatric endocrinologists and include screening of co morbidities, such as hypertension, dyslipidaemia, impaired glucose tolerance/insulin resistance (which predicts the development of diabetes), hepatic steatosis/steatohepatitis, hypoventilation syndrome and sleep apnoea. Nutritional intervention is essential and pharmacological treatment may be needed, such as metformin in cases with impaired glucose tolerance. Management of paediatric dyslipidaemia is difficult because statins and fibrates are not routinely used in very young children.7 However, statins can be initiated in patients as young as 8 year of age with severe dyslipidaemia who have failed non-pharmacologic therapy. Although data are limited on the use of fibric acid derivatives in children, it is recommended by the American Heart Association as the preferred class of drugs in treating children with severe triglycerides elevation.7 8
The variable nature of features that occur in patients with 1p36 monosomy requires a multi-disciplinary approach with neurodevelopmental assessment including hearing and vision, cardiac evaluation, endocrinology follow-up of thyroid function and obesity.
Learning points.
-
▶
Obesity is not always essential or idiopathic.
-
▶
Studying rearrangements of 1p36 deletions may elucidate some genetic markers of obesity.
-
▶
1p 36 deletion may be considered in patients with obesity and mental retardation.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Battaglia A, Shaffer LG. 1p36 Deletion Syndrome. GeneReviews 2008 [Google Scholar]
- 2.Slavotinek A. Chromosome 1p36 deletions. Orphanet encyclopedia 2003:1–4 [Google Scholar]
- 3.Battaglia A, Hoyme HE, Dallapiccola B, et al. Further delineation of deletion 1p36 syndrome in 60 patients: a recognizable phenotype and common cause of developmental delay and mental retardation. Pediatrics 2008;121:404–10 [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo CS, Kohl I, Varela MC, et al. Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia. Am J Med Genet A 2010;152A:102–10 [DOI] [PubMed] [Google Scholar]
- 5.Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis 2010;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld JA, Crolla JA, Tomkins S, et al. Refinement of causative genes in monosomy 1p36 through clinical and molecular cytogenetic characterization of small interstitial deletions. Am J Med Genet A 2010;152A:1951–9 [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Cardiovascular risk reduction in high-risk pediatric populations. Pediatrics 2007;119:618. [DOI] [PubMed] [Google Scholar]
- 8.McCrindle BW, Urbina EM, Dennison BA, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee; American Heart Association Council of Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular Nursing Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation 2007;115:1948–67 [DOI] [PubMed] [Google Scholar]