Abstract
Cannibalism is widespread in natural populations of fishes, where the stomachs of adults frequently contain conspecific juveniles. Furthermore, field observations suggest that guardian males routinely eat offspring from their own nests. However, recent genetic paternity analyses have shown that fish nests often contain embryos not sired by the nest-tending male (because of cuckoldry events, egg thievery, or nest piracy). Such findings, coupled with the fact that several fish species have known capabilities for distinguishing kin from nonkin, raise the possibility that cannibalism by guardian males is directed primarily or exclusively toward unrelated embryos in their nests. Here, we test this hypothesis by collecting freshly cannibalized embryos from the stomachs of several nest-tending darter and sunfish males in nature and determining their genetic parentage by using polymorphic microsatellite markers. Our molecular results clearly indicate that guardian males do indeed consume their own genetic offspring, even when unrelated (foster) embryos are present within the nest. These data provide genetic documentation of filial cannibalism in nature. Furthermore, they suggest that the phenomenon may result, at least in part, from an inability of guardians to differentiate between kin and nonkin within their own nests.
Cannibalism is widespread in nature (1) and is particularly evident in fishes, where conspecific juveniles are often found among the partially digested stomach contents of adults (2–5). The evolutionary ramifications of this behavior would seem to depend critically on the genetic relatedness between the cannibal and the embryos or fry consumed, and two extreme categories in this regard should be distinguished: “heterocannibalism,” the consumption of unrelated conspecific individuals, and “filial cannibalism,” the consumption of one's own offspring. Heterocannibalism has been documented in a variety of fish species (6–8), and filial cannibalism is often suspected based on behavioral field observations (9).
Ostensibly, there should be strong selection against cannibalizing one's own progeny. However, filial cannibalism can be favored when the fitness benefits outweigh the fitness costs of this otherwise counterintuitive parental behavior (10). For instance, Rohwer (9) proposed that male fishes might increase their net lifelong reproduction by eating some of their own genetic offspring and using the energy thereby derived to enhance current and/or future spawning success. One such scenario posits that a nest-attendant male who temporarily leaves his clutch (e.g., to forage) may suffer devastating losses to nest predators, whereas a guardian male who eats some of his progeny while remaining on the nest might, by so doing, enhance his cumulative lifetime fitness (3, 5, 9). Females, in general, are presumably somewhat more reluctant to consume their own young, because they have a greater gametic investment (4). Under this view, by the nature of anisogamy, cannibalistic males can also be viewed as parasitizing the efforts of females.
Through a series of ingenious laboratory experiments on brook sticklebacks, Salfert and Moodie (11) documented filial cannibalism conclusively. Gravid females were injected with a radioactive solution of NaOH, and guardian males known to have fertilized the radiolabeled eggs often then acquired the label themselves. These experiments proved that filial cannibalism occurred in the lab, but they did not address the phenomenon in free-swimming populations.
Despite controlled experiments and a large body of theory on the phenomenon, there has been no direct genetic confirmation that filial cannibalism truly occurs in nature or how often. Furthermore, there are reasons to be suspicious. Recent molecular paternity analyses indicate that the nests of many fish species commonly contain embryos that are unrelated to the guardian male, because of instances of egg thievery, nest takeovers, and stolen fertilizations (cuckoldry) via sneaker or other males (12, 13). In addition, there is considerable evidence that at least some fish species (and many other organisms) are capable of finely discriminating kin from nonkin under certain circumstances (14–17). Thus, it is possible that parental males who cannibalize embryos from their own nests might merely be eating embryos that they themselves had not sired!
Here, we describe a critical test of this hypothesis, and our results provide genetic documentation of filial cannibalism in nature. The tessellated darter (Etheostoma olmstedi) was the primary test species (but we also note the phenomenon in two sunfish species: Lepomis auritus and Lepomis punctatus). In these species, males build nests, externally fertilize the eggs that one or more females deposit in the nest, and tend the resulting embryos (which typically number in the hundreds per nest). Prior genetic analyses in our laboratory have documented that guardian males in darters and sunfish occasionally are the foster (rather than biological) parents of some of the embryos they tend (13).
Materials and Methods
We used standard electroshocking techniques to capture 16 nest-guarding darter males and, subsequently, 610 embryos or juveniles from their respective nests in Fourmile Creek, a tributary of the Savannah River near Barnwell, South Carolina. In a previous report, we used allelic data from six microsatellite loci to deduce which individual embryos in each nest were sired by the guardian male and which were not. Individuals not sired by the guardian male typically resulted either from cuckoldry by other males in the population or from nest takeovers wherein a reproductively mature male has “adopted” a nest that already contained fertilized embryos (18, 19).
In the current study, we dissected stomachs from each of 15 nest-guarding males, who, as we concluded from the genetic evidence, were almost certainly the true sires of at least some of the embryos from their respective nests. (The embryos from the one remaining nest were probably not sired by the guardian; see ref. 19.) Stomach contents of each male were detailed, and stomach fullness was rated as 0, 25, 50, 75, or 100%. A total of 38 conspecific cannibalized embryos were discovered in a sufficient state of preservation to enable successful genotyping at most or all of the six polymorphic microsatellite loci. In tandem, these loci yielded a combined average exclusion probability greater than 0.90 (19).
Genotypes were compared among the cannibalized embryos, each guardian male, and his custodial embryos. The custodian was deemed the true sire if and only if the multilocus genotypes of the cannibalized embryos proved to be consistent with genetic paternity by that male. To evaluate maternity of the cannibalized embryos, the dam's gametotypes (deduced in each case by subtracting the paternally derived alleles from an embryo's genotype) were compared with those of the embryos taken from the associated nest. If the two embryos' cohorts (those from the nest proper and those from the cannibal's stomach) collectively shared maternal gametotypes, then we assumed a common maternity for them both.
Results
Cannibalized darter embryos were found in the stomachs of 11 guardian males, and six of these stomachs were at least 50% full. Indeed, cannibalized young seemed to be a major food source for these males, because putative conspecific offspring were in each case the dominant food item in the stomach. Prey items other than fish embryos (namely, insects) were found in only two guardian males. The effect of offspring numbers on stomach fullness is also documented by a near-linear relationship between these two variables (r2 = 0.83; P < 0.001; n = 15). The young fish eaten by the cannibalistic guardians ranged in developmental stage from recently fertilized embryos to viable, near-hatching fry (Fig. 1).
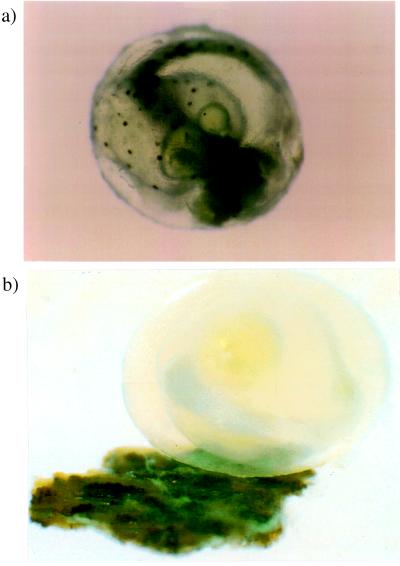
Figure 1.
Tessellated darter embryos recovered from the stomachs of cannibalistic nesting males. Such remains suggest that the embryos were generally in good health before being eaten and probably had been free of fungal disease (see ref. 18). (a) This embryo (displaying spotted pigment areas) was retrieved from the stomach of its biological father; the yolk sac can be seen above the prominent eyes and below the long curved tail. (b) This embryo and egg case, also retrieved from the stomach of its sire, was cemented to a small piece of black wood from the nest, suggesting that it may have been attached to the nest at the time of consumption (see text).
We successfully collected genetic data on 38 cannibalized embryos from the stomachs of 5 of the 11 attendant darter males. In every case, the multilocus genotype of a cannibalized embryo was consistent with paternity by its cannibalistic guardian. Moreover, in most cases the maternal alleles present in cannibalized embryos were a subset of those detected in noncannibalized embryos from the same nest, further indicating that the cannibalized embryos came from the guardian male's nest (rather than that of a neighbor). Of the 11 cannibalistic males, 10 sired all of the offspring collected from their respective nests, suggesting that filial cannibalism is a common behavior among these nest-guarding fishes.
We also recovered conspecific embryos from the stomachs of guardian spotted sunfish (L. punctatus) and redbreast sunfish (L. auritus). However, cannibalized embryos were consistently more degraded, perhaps because these species spawned in much warmer waters than did the darters (19, 20). (In small poikilothermic animals such as fish, water temperature probably influences digestion rates.) Consequently, the genetic data retrieved from these sunfishes were sporadic, usually limited to single-locus genotypes. Nevertheless, those data also were consistent with paternity by cannibalistic guardian males.
Discussion
During most of the year, the diet of E. olmstedi at our study site consists primarily of aquatic invertebrates (unpublished data). However, as gauged by our physical and genetic analyses of stomach contents of nest-tending adults, these nesting guardian males apparently gain much of their nourishment by preying heavily on their own offspring.
Were the Consumed Progeny Viable?
Filial cannibalism would make considerable sense if the embryos eaten were already sick or dying. For these fish, at least two biological possibilities immediately come to mind. First, nest-tending darter males are known to eat fungus-infected embryos (18); thus, filial cannibalism may have evolved in response to fitness advantages of combating the infectious spread of fungal disease within the nest. Nevertheless, as gauged by the physical condition and appearance of cannibalized embryos in the parent's gut, many of these embryos in our study seemed to be “healthy” at the time of death and not fungal infected (Fig. 1a). Furthermore, other studies of filial cannibalism indicate that this behavior “occurs on a scale that exceeds the simple consumption of diseased or dead eggs” (11). Second, perhaps the darter embryos eaten by their guardian males were already physically dislodged from the nest and would otherwise have washed downstream and died. We cannot eliminate this possibility, but it is worth noting that some of the embryos retrieved from the males' stomachs were still secured to small pieces of wood from the nest (Fig. 1b), suggesting that they had not dislodged naturally.
Can Cannibals Distinguish Kin?
Nearly 20% of the darter nests were composed of at least a few embryos not sired by the guardian male. In such nests, it would clearly be to the guardian male's advantage to cannibalize the unrelated embryos preferentially. Our collection of cannibals happened to be comprised mostly of individuals who sired all of the progeny in their respective nests. Nevertheless, data from one of the cannibalistic guardians indicated that he had not preferentially consumed nonrelatives. That male's stomach contained six embryos, all of which he sired, despite the fact that 60% of the embryos in his nest had been fathered by an unrelated second male (19). Perhaps the embryos consumed by the nest attendant were physically grouped within the nest, such that the feeding events were not independent with respect to paternity. In any event, this outcome at face value is in significant opposition to the expectation that males preferentially consume nonkin when afforded the option. It also raises the question as to whether tessellated darter guardians are capable of distinguishing their own embryos from others in the nest.
Considerable evidence suggests that some fishes can distinguish close kin from nonkin (reviewed in ref. 15). For example, both Atlantic and coho salmon can discriminate between siblings and nonsiblings (21, 22). Perhaps the acute olfactory system of these species (as evidenced by their precise natal homing) also allows kin discrimination on the basis of subtle chemosensory cues. Similarly, Midas cichlids can distinguish their own young from those of unrelated conspecifics (23). In this species, the kin-discrimination ability is lost when an adult's nares are plugged with cotton and quickly regained when the nares are unplugged, again suggesting that the kin recognition cues are chemical (olfactory). Although the genetic data presented here fail to support the hypothesis that male darters preferentially avoid eating their own offspring in a nest, additional studies will be required to assess more adequately the rates and patterns of filial cannibalism in this nest-tending species as well as the degree to which fish parents in general can distinguish their own progeny from foster juveniles. In future extensions of the current research approach, genetic analyses of filial cannibalism may prove especially instrumental in laboratory-based studies of kin recognition.
Is Filial Cannibalism Adaptive?
The fact that guardian males routinely cannibalize their own progeny raises the possibility that the lifelong fitness benefits of filial cannibalism to the nest owner may often outweigh the immediate costs of this behavior (and, conversely, that the fitness costs associated with cannibalizing nonkin in another male's nest may often outweigh the benefits of heterocannibalism). Most guardian males (>80%) sampled from this darter population sired all of their custodial embryos (19). Thus, each of those males would have to leave his nest to forage on unrelated juveniles, thereby exposing his own offspring to predation by conspecific or heterospecific intruders. If such predation risks (or other dangers associated with straying from the nest) are normally great, it may behoove a guardian male to spend most of his time at home, even if that means meeting energetic needs by consuming some of his own progeny. Thus, under such ecological conditions, perhaps behavioral tendencies toward filial cannibalism are under positive selection as a fitness-enhancing strategy for guardian males otherwise faced with nutritional weakness or starvation. Whether darter males are indeed often energy-limited during nesting might be addressed, in part, by future research examining their lipid profiles in appropriate experimental designs.
Alternatively, perhaps no particular selective rationale for filial cannibalism need be sought. Thus, filial cannibalism per se might be maladaptive, its presence merely a behavioral byproduct of more general tendencies for voraciousness, which themselves are probably selectively favored in many ecological settings. Another possibility is that filial cannibalism is essentially a neutral behavior with respect to a guardian male's fitness, because it may have negligible consequences for the total successful reproductive output of a typical nest (especially when offspring numbers and mortality from other sources are both high, as they are in darters and most fish species). Overall, whether filial cannibalism itself exerts strong selection pressures on nest guardians to distinguish biological from foster progeny depends on whether this phenomenon has positive, negative, or nearly-neutral fitness consequences to the cannibals. Of course, many other behavioral considerations during a fish's life may also influence the nature and intensity of natural selection for kin recognition capabilities.
Synopsis.
In recent years, PCR-based genetic assays have found applications in forensic analyses of a wide variety of natural animal and plant products (24) ranging from single feathers (25), hairs (26), and egg-shell membranes (27) to tiny invertebrate larvae (28), preserved foods in retail markets (29), material retrieved from digestive tracts (30), and bits of modern and ancient feces (31–33). Here, we have used PCR-based methods in another unorthodox context: paternity assignment of juveniles recovered from the stomachs of conspecific nest-tending fishes. By so doing, we have provided genetic documentation of the phenomenon of filial cannibalism in the wild. Furthermore, our results prove the previously untested notion that filial cannibalism in nature does occur even when a nest-tending male has ample opportunity to dine on unrelated juveniles within his nest. Such methods also hold great promise for future studies of kin recognition in fish as well as other organisms.
Acknowledgments
We thank M. Asmussen, L. Blumer, A. Fiumera, A. Keyser, M. Mackiewicz, B. McCoy, D. Pearse, B. Porter, O. Rhodes, D. Walker, and two anonymous reviewers for valuable input. This work was supported by the Pew Foundation, funds from the University of Georgia, and by Financial Assistance Award No. DE-FC09-96SR18546 between the U.S. Department of Energy and the University of Georgia.
References
- 1.Elgar M A, Crespi B J, editors. Cannibalism: Ecology and Evolution Among Diverse Taxa. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 2.Nemtzov S C, Clark E. Bull Mar Sci. 1994;55:133–141. [Google Scholar]
- 3.Hoelzer G A. Bull Mar Sci. 1995;57:663–671. [Google Scholar]
- 4.Okuda N. Anim Behav. 1999;58:273–279. doi: 10.1006/anbe.1999.1148. [DOI] [PubMed] [Google Scholar]
- 5.Lindström K. Evolution (Lawrence, Kans) 2000;54:617–627. doi: 10.1111/j.0014-3820.2000.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 6.Hynes H B N. J Anim Ecol. 1950;19:36–58. [Google Scholar]
- 7.Jewell E D. Wash State Dep Fish Res Pap. 1968;3:27–36. [Google Scholar]
- 8.Semler D E. J Zool. 1971;165:291–302. [Google Scholar]
- 9.Rohwer S. Am Nat. 1978;112:429–440. [Google Scholar]
- 10.Hamilton W D. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 11.Salfert I G, Moodie E E. Behavior. 1985;93:82–100. [Google Scholar]
- 12.Avise J C, editor. DNA-Based Profiling of Mating Systems and Reproductive Behaviors in Poikilothermic Vertebrates. Oxford: Oxford Univ. Press; 2001. , in press. [PubMed] [Google Scholar]
- 13.DeWoody, J. A. & Avise, J. C. (2001) J. Hered., in press. [DOI] [PubMed]
- 14.Lazzaretto I, Salvato B. Mar Biol. 1992;113:579–582. [Google Scholar]
- 15.Brown G E, Brown J A. Rev Fish Biol Fish. 1996;6:201–219. [Google Scholar]
- 16.Evans T A. Proc R Soc London Ser B. 1999;266:287–292. [Google Scholar]
- 17.Pfennig D W. Proc R Soc London Ser B. 1999;266:57–61. [Google Scholar]
- 18.Constantz G D. Environ Biol Fishes. 1985;14:175–183. [Google Scholar]
- 19.DeWoody J A, Fletcher D E, Wilkins S D, Avise J C. Copeia. 2000;2000:740–747. [Google Scholar]
- 20.DeWoody J A, Fletcher D E, Wilkins S D, Nelson W E, Avise J C. Proc R Soc London Ser B. 2000;267:2431–2437. doi: 10.1098/rspb.2000.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn T P, Busack C A. Anim Behav. 1985;33:51–56. [Google Scholar]
- 22.Moore A, Ives M J, Kell L T. Proc R Soc London Ser B. 1994;255:173–180. [Google Scholar]
- 23.McKaye K R, Barlow G W. Copeia. 1976;1976:276–282. [Google Scholar]
- 24.Avise J C. Molecular Markers: Natural History and Evolution. New York: Chapman & Hall; 1994. [Google Scholar]
- 25.Taberlet P, Bouvet J. Auk. 1991;108:959–960. [Google Scholar]
- 26.Vigilant L, Pennington R, Harpending H, Kocher T D, Wilson A C. Proc Natl Acad Sci USA. 1989;86:9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce J M, Fields R L, Scribner K T. J Field Ornithol. 1997;68:471–481. [Google Scholar]
- 28.Silberman J D, Walsh P J. Mol Mar Biol Biotech. 1992;1:195–205. [PubMed] [Google Scholar]
- 29.Baker C S, Palumbi S R. Science. 1994;265:1538–1539. doi: 10.1126/science.265.5178.1538. [DOI] [PubMed] [Google Scholar]
- 30.Nelson W S, Dean T, Avise J C. Mol Ecol. 2000;9:809–813. doi: 10.1046/j.1365-294x.2000.00929.x. [DOI] [PubMed] [Google Scholar]
- 31.Höss M, Kohn M, Pääbo S, Knauer F, Schroder W. Nature (London) 1992;359:199. doi: 10.1038/359199a0. [DOI] [PubMed] [Google Scholar]
- 32.Poinar H N, Hofreiter M, Spaulding W G, Martin P S, Stankiewicz A, Bland H, Evershed R P, Possnert G, Pååbo S. Science. 1998;281:402–406. doi: 10.1126/science.281.5375.402. [DOI] [PubMed] [Google Scholar]
- 33.Farrell L E, Roman J, Sunquist M E. Mol Ecol. 2000;9:1583–1590. doi: 10.1046/j.1365-294x.2000.01037.x. [DOI] [PubMed] [Google Scholar]