Abstract
A 29 year old female HIV-positive patient presented in emergency with acute right lower quadrant abdominal pain, fever, tenderness and positive Blumberg sign. Laboratorial tests revealed eosinophilia, anaemia and leukocytosis. She underwent exploratory laparotomy followed by appendectomy. The pathological analysis of the appendix revealed acute appendicitis, accentuated eosinophilia and infestation by Strongyloides stercoralis and Enterobius vermicularis. She did well after surgery and adequate treatment. To the authors’ knowledge, this is the first case of eosinophilic acute appendicitis caused by these two parasitic worms reported in the medical literature.
Background
Appendicitis is defined as an inflammation of the inner lining of the vermiform appendix which spreads to its other parts. Acute appendicitis is the most frequent cause of emergency abdominal surgery worldwide,1 with its peak during the second and third decades of life.2 Nearly 7.0% of individuals will undergo appendectomy during their lives.3 4
Obstruction of the appendix has less commonly been associated with bacteria, foreign material, tumours and parasites. Many different types of parasites can be found in the appendicular lumen. Of these, Enterobius vermicularis (pinworms/threadworms) is the most commonly found and Strongyloides stercoralis2 3 5 is a very unusual finding.
Case presentation
A 29-year-old Caucasian HIV-positive female presented in emergency with a history of intermittent fever, flank rebound pain and abdominal pain in the right iliac fossa. The pain was dull and spasmodic in character; it was not associated with nausea, vomiting, dysuria or diarrhoea. In addition, elected progressive weight loss (15 kg) and anorexia were found in recent months. On examination, the patient was febrile (39.5°C) and haemodynamically stable. On palpation of the abdomen, there was flatness, with involuntary muscle contraction, tenderness and positive Blumberg’s sign.
Investigations
Admitting blood tests found C reactive protein 420 mg/l, white cell count 12.3×103/mm3, haemoglobin 7.5 g/dl and eosinophilia (19%). HIV type 1 antibody was positive, HIV viral load was 5210 copies/ml and CD4+ count was 380 cells/mm3. The urine dip stick was negative for blood, leucocytes and nitrites. β-human chorionic gonadotropin test was negative. The chest x-ray was normal.
Ultrasound of the abdomen demonstrated a non-perforated and inflamed appendix, associated with cecum and ascending colon wall thickening. CT of the abdomen confirmed the results of the ultrasound, and revealed a small amount of periappendiceal fluid.
Differential diagnosis
-
▶
Acute appendicitis
-
▶
Tubo-ovarian abscess
-
▶
HIV related opportunistic infections.
Treatment
In view of the continuing pain and the persistence of the patients’ symptoms it was decided to proceed to diagnostic laparoscopy. At operation, the uterus, the ovaries and the small bowel were normal. There was minimal fluid in the pelvis but the tip of appendix was turgid with a purulent serosa. An uncomplicated appendicectomy was performed and the specimen was send to pathological examination.
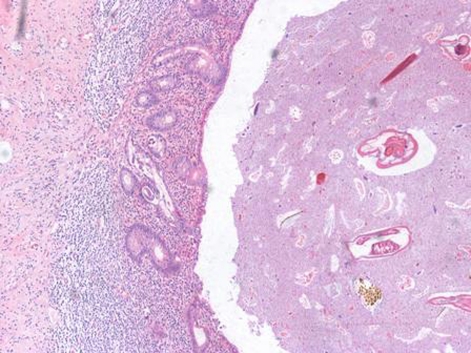
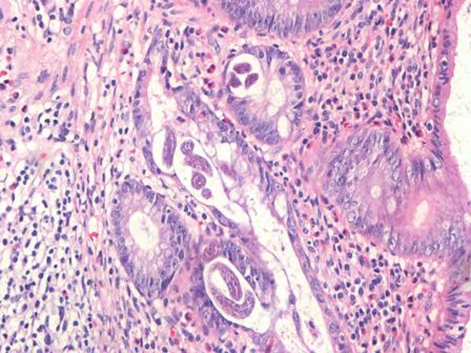
Externally the appendix showed a fibrinopurulent exudate on serosa and prominent vessels, with no areas of perforation. Microscopically, the appendicular lumen contained several parasites, which present lateral alae, causing obstruction. These features were compatible with infection by E vermicularis (figure 1). At higher power view, within the appendicular glands, were found larvae compatible with co-infection by S stercoralis (figure 2), with a mixed dense inflammatory infiltrate in the lamina propria (neutrophils and eosinophils), dense neutrophils in muscularis propria with focal necrosis and congestion.
Figure 1.
Strongyloides stercoralis are seen within the appendiceal glands, while Enterobius vermicularis are within the overlying luminal debris (H&E stain, 100x magnifications).
Figure 2.
Higher power view of larvae (Strongyloides stercoralis) within the appendiceal glands, with a eosinophilic inflammatory infiltrate in the lamina propria (H&E stain, 400x magnifications).
Outcome and follow-up
Postoperatively, the patient and family were treated with mebendazole and on review in the outpatient clinic in 4 weeks’ there was no recurrence of symptoms, CD4+ count was 400 cells/mm³, viral load was 5300 copies/ml, stool examination for parasites and their eggs (ova) was negative and chest x-ray was normal.
Discussion
Chronic infections by parasites are very common in South-American countries. It is associated with poor sanitarian conditions, ingestion of contaminated food and exposure to contaminated water.
Among the infectious causes of acute appendicitis, S stercoralis is extremely unusual.2 This is the eleventh case reported in the existing literatutre,3 5 and only one of these was HIV-positive.6 E vermicularis is a relatively common finding within appendix.2 The ability of this worm to cause damage to the mucosa, however, has been discussed,7 8 and only considered as an aetiological agents of appendicitis when it causes obstruction of the lumen of the appendix9–11 or when there is frank invasion of the mucosa.
There is also some discussion regarding the minimum criteria for the diagnosis of acute appendicitis: a group of researchers believes that is necessary to have a neutrophil inflammatory infiltrate through the muscle itself, while others believe to be the neutrophil infiltrate in the mucosa associated with superficial ulceration of the same criterion earlier. The most accepted hypothesis and the one which seems to correlate better with the symptoms of the patient appears to be the first,12 13 even in patients who have some form of immunosuppression.
The incidence of acute appendicitis among HIV-positive patients is the same as among uninfected individuals. Most cases of appendicitis in HIV patients are caused by obstruction, small stool or lymphoid hyperplasia. On the other hand, infections in that same group caused by microbiological entities are responsible only for 30% of the cases reported. The clinical presentation and postoperative morbidity, however, are similar in both groups, making no difference in the way of treatment.
In summary, we report the first case of acute appendicitis caused by S stercoralis in patients with HIV, in which there is the rare finding of co-infection with E vermicularis.
Learning points.
-
▶
Parasitic infection is an important cause of acute appendicitis.
-
▶
Intestinal parasites infection may cause acute inflammation by luminal obstruction of the appendix.
-
▶
The incidence of acute appendicitis among HIV-positive patients is the same as among uninfected individuals.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Aydin Ö. Incidental parasitic infestation in surgically removed appendices: a retrospective analysis. Diagn Pathol 2007;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamps LW. Appendicitis and infections of the appendix. Semin Diagn Pathol 2004;21:86–97 [DOI] [PubMed] [Google Scholar]
- 3.Komenaka IK, Wu GCH, Lazar EL, et al. Strongyloides appendicitis: unusual etiology in two sibilings with chronic abdominal pain. J Ped Surgery 2003;38:1–3 [DOI] [PubMed] [Google Scholar]
- 4.Flum DR, Koepsell T. The clinical and economic correlates of misdiagnosed appendicitis: nationwide analysis. Arch Surg 2002;137:799–804; discussion 804. [DOI] [PubMed] [Google Scholar]
- 5.Noodleman JS. Eosinophilic appendicitis. Demonstration of Strongyloides stercoralis as a causative agent. Arch Pathol Lab Med 1981;105:148–9 [PubMed] [Google Scholar]
- 6.Felekouras E, Kontos M, Kyriakou V, et al. Strongyloides stercoralis infection as a cause of acute granulomatous appendicitis in an HIV-positive patient in Athens, Greece. Scand J Infect Dis 2002;34:856–7 [DOI] [PubMed] [Google Scholar]
- 7.Yildirim S, Nursal TZ, Tarim A, et al. A rare cause of acute appendicitis: parasitic infection. Scand J Infect Dis 2005;37:757–9 [DOI] [PubMed] [Google Scholar]
- 8.Wiebe BM. Appendicitis and Enterobius vermicularis. Scand J Gastroenterol 1991;26:336–8 [DOI] [PubMed] [Google Scholar]
- 9.Herd ME, Cross PA, Dutt S. Histological audit of acute appendicitis. J Clin Pathol 1992;45:456–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinniah B, Leopairut J, Neafie RC, et al. Enterobiasis: a histopathological study of 259 patients. Ann Trop Med Parasitol 1991;85:625–35 [DOI] [PubMed] [Google Scholar]
- 11.Mogensen K, Pahle E, Kowalski K. Enterobius vermicularis and acute appendicitis. Acta Chir Scand 1985;151:705–7 [PubMed] [Google Scholar]
- 12.Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol 2000;4:46–58 [DOI] [PubMed] [Google Scholar]
- 13.Williams RA, Myers P. Pathology of the Appendix and its Surgical Treatment. London: Chapman and Hall Medical Press; 1994 [Google Scholar]