Abstract
Spinocerebellar ataxia type 2 (SCA2) is a progressive neurodegenerative disorder, characterised by ataxic gait, slow saccades and peripheral neuropathy. Levodopa-responsive parkinsonism could be a clinical phenotype of SCA2, especially those of Chinese origin. In addition to these motor symptoms, SCA2 has been associated with depression and cognitive dysfunction, with only rare reports of psychosis. The authors report the presence of severe psychosis, major depression and multiple system atrophy in affected subjects of a Taiwanese family with intermediate CAG repeats within the ATXN2 gene. The identification of this rare and distinctive SCA2 phenotype expands the current knowledge of the phenotypic variability of SCA2 and suggests that modifier genes could influence the clinical phenotype of SCA2.
Background
Abnormal CAG trinucleotide repeat expansion within the spinocerebellar ataxia type 2 (SCA2) gene was recognised as a cause of parkinsonism in some families, especially those of Chinese origin.1–3 The SCA2 related parkinsonism phenotypes include levodopa-responsive Parkinson’s disease (PD) without obvious cerebellar signs, although mild ataxic features or slow eye movements could become manifest in the later stage.1 3 As compared with the cerebellar phenotype predominant SCA2 patients, patients with parkinsonism variant often have a shorter CAG repeat number and have CAA triplet interruptions within an expanded CAG repeat, suggesting this mixed CAG/CAA repeat may stabilise the mutant ataxin-2 protein in the neurons.3 The mechanism why CAG/CAA repeat related ataxin-2 protein resulting in less neuronal death remains unclear.
In addition to motor impairments, patients with SCA and extracerebellar pathology were sometime characterised by poor frontal executive function, especially SCA 1, 2, 3, 6 and 17.4 Burk et al documented dementia in up to 25% in patients with SCA2 and impairments in verbal memory and phonemic word fluency in a non-demented SCA2 series.5 Affective symptoms, especially depression, were also frequently associated with this devastating disease process. One recent study demonstrated up to 7.6% of patients with SCA2 fitted the criteria of major depression, which ataxia severity and female sex independently predicted depressive status in patients with SCA.6 Although cognitive dysfunction and affective symptoms have been frequently described in patients with SCA2, psychosis feature was rarely reported.
We herein report a Taiwanese SCA2 family whose family members presented with multiple system atrophy (MSA), cognitive impairment, major depression and severe psychosis features. We also reviewed previous literature reporting patients with SCA2 and parkinsonism-predominant phenotypes and compared with our reported patients.
Case presentation
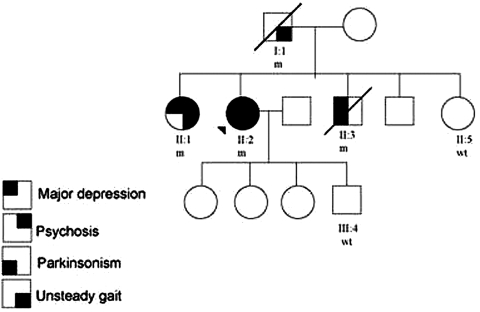
The index patient, case II:2 (figure 1), is a 70-year-old female who was well before, except idiopathic bilateral vocal cord palsy found accidentally at the age of 65. She had progressive slow movement and several episodes of falling down since age 63 years. Parkinsonism was diagnosed and she received low dose of levodopa (150 mg per day) without amantadine or anticholinergics. Depression and anxiety were also diagnosed at the same time by a psychiatrist. She was diagnosed as major depression according to DSM-IV criteria and the geriatric depression scale was 12, indicating severe depression. She received sertraline (50 mg per day) for her depressive symptoms. Two years later, she developed visual and auditory hallucinations that she saw persons not existed beside her and heard voices outside the head told her to attack her children. She also had persecutory delusion that she believed that her son would harm and sexually harassed her. She was then admitted to neurology ward. On mental status examination, she had a depressed affect with ideas of worthlessness, referential and persecutory delusions and second-person auditory hallucinations of derogatory content. No evidence of dementia on minimal mental status examination. Neurological examination revealed apparently normal findings in cranial nerves except marked mask face and slow saccadic eye pursuit. Her muscle power was full and symmetric. Deep tendon reflex and sensory systems were normal. Symmetric rigidity and bradykinesia were noted over bilateral limbs. Her righting reflex was poor. The patient also presented bilateral dysmetria in finger-nose-finger test and poor tandem gait performance. In addition, orthostatic hypotension was also noted during hospitalisation. Her diastolic blood pressure dropped more than 15 mm Hg and systolic blood pressure dropped more than 10 mm Hg while changing position from lying to sitting but she did not feel dizziness or any subjective discomfort. In the lab findings, brain MRI showed marked atrophy in bilateral cerebellum and brainstem without ‘hot cross bun’ sign. Brain perfusion single-photon emission CT (SPECT) showed decreased tracer perfusion in bilateral frontal and parietal regions, suggesting frontal-parietal cortex degeneration. Electrophysiological study showed no evidence of polyneuropathy. In the autonomic functional assay, the sympathetic skin response was present in palms and soles, but the RR interval variability was below normal limits both at rest and during deep breathing, indicating mild autonomic dysfunction. She was therefore clinically diagnosed as possible cerebellar type of MSA-C according to the consensus diagnostic criteria of MSA,7 which fulfilling the criteria of prominent cerebellar dysfunction plus parkinsonism feature and autonomic dysfunction.
Figure 1.
Pedigree of index family with ATXN2 mutation. Black symbols denote family members affected with parkinsonism, unsteady gait, major depression and psychosis. Patients studied are numbered and the proband was marked with an arrow head. ‘m’ denoted as subjects with ATXN2 mutations and ‘wt’ denoted as normal CAG repeat number within ATXN2 gene.
Review her family history, father of thse proband, Case I:1 (figure 1) had gait disturbance and dementia since 60 years old and passed away due to respiratory distress at age 75 years. No any psychotic or parkinsonsim features were noted by the family. Case II:3, the younger brother of the proband (figure 1), is a patient of relatively young-onset PD with initial presentation of akinetic rigidity at age 50 years. His eye movements showed slow saccade and persuit. His gait was shuffling with mild ataxic feature. His akinetic rigidity had good response to levodopa treatment under the dose of 600 mg per day. The Unified Parkinson’s Disease Rating Scale motor score improved from 23 to 5 after levodopa treatment. Meanwhile, he also had major depression according to the DSM-IV criteria at the age of PD diagnosed. He was seriously depressed even under antidepressant treatment and committed suicide at age 68 years. Another sibling of the proband, case II:1, was a 73-year-old lady. She had prominent psychosis with vivid auditory, visual hallucinations and persecutory delusion since early twenties and was clinically diagnosed as schizophrenia. Dementia developed at age 50 years and there were no obvious parkinsonism symptoms. She is now dependent in her daily activities and is a resident in a home for people with mental health problems.
Investigations
Given that the autosomal dominant trait of hereditary ataxia with parkinsonism symptoms, the proband received genetic testing for SCA 1, 2, 3 and 17 and the results showed expanded CAG repeats in the ATXN2 gene. The CAG repeats in the ATXN2 locus of our index patient revealed an intermediate expanded allele with 32 repeats and a normal allele with 19 repeats (range in normal population, 15-26).8 The size of repeats was lower than the CAG repeats in the cerebellum ataxic variant SCA2 patients (range 38–51) in our population. DNA analyses also demonstrated abnormal CAG repeats of ATXN2 gene in the other family members (figure 1, CAG repeats 32/20 for case I:1, 35/21 for II:1 and 34/20 for II:3) but normal in the asymptomatic sibling and offspring (case II:5 and III:4).
Differential diagnosis
A SCA2 family clinically presented with MSA and prominent psychotic features confirmed by genetic analysis.
Outcome and follow-up
The index patient (case II:2) received low dose of risperidone (1 mg per day) and the psychotic features resolved gradually. The patient was now treated with 450 mg/day of levodopa with partially resolution of both rigidity and bradykinesia 2 years after MSA was diagnosed. The current Hoehn–Yahr stage of parkinsonism was stage III.
Discussion
Our study highlights the intrafamily phenotypic variability in a genetic confirmed SCA2 family, including MSA with autonomic dysfunction, cognitive impairment and prominent psycho-affective symptoms. SCA2 is a cerebellar degeneration disease with abnormal CAG repeats expansion within ATXN2 gene. CAG repeats which expanded to more than 37 repeats phenotypically presented with progressive cerebellar ataxia, slow saccadic eye movement and generalised hyporeflexia in the fourth decade. However, alleles with intermediate repeats to 32 and 33 CAG copies are often associated with parkinsonism presentation with onset age in the fifth decade (see literature review in table 1).9 As summarised in the table 1, most of the reported SCA2 related parkinsonism patients have an intermediate to low repeat CAG number which is mostly below 40 repeats. Clinically, most of the SCA2 related parkinsonism patients have an onset age less than 60 years and some may also have cognitive impairment. In accordance with previous reports, the CAG repeat number in our index patient with parkinsonism-predominant phenotype was 32, which is an intermediate repeat number. Additionally, the combined presence of parkinsonism and cerebellar ataxia can clinically be mistaken for MSA, particularly when autonomic dysfunction and vocal cord palsy were also present at the same time. Similar conditions have been reported in SCA3, 8 and 17.10–12 Recent reports have shown that the parkinsonism features in SCA2 are related to degeneration outside the cerebellum, including substantia nigra and striatum.13 14 These pathology and neuroimaging evidence of degeneration of either presynpatic or postsynaptic dopaminergic system provide bedrocks to explain beneficial treatment effects of levodopa in SCA2 parkinsonism. In addition, since the SCA2 related parkinsonism features were initially reported in Chinese patients, it seemed that patients of Asian origin, especially Chinese, were more vulnerable to parkinsonism phenotypes than Caucasians. However, many cases of Caucasian origin were reported in recent years, that 7 out of 17 related studies are from western countries (table 1). Further large-scale studies are warranted to clarify the ethnic difference in the parkinsonism or parkinsonism-plus syndrome phenotypes of SCA2.
Table 1.
Clinical phenotypes and CAG repeat numbers of patients with parkinsonism-predominant SCA2 in previous literature
| Reference | Ethnicity | Number of patients (N) | CAG repeat numbers | Age at onset (years) | Clinical phenotypes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Parkinsonism (n/N) | Ataxia (n/N) | Dementia (n/N) | Affective symptoms (n/N) | Psychosis (n/N) | |||||
| Gwinn-Hardy et al1 | Chinese | 8 | 33, 35, 36, 43 | 19, 20, 36, 38, 43, 44, 54, 61 | 8/8 | 4/8 | NT | NT | NT |
| Shan et al2 | Taiwanese | 2 | 36,37 | 44, 41 | 2/2 | 0/2 | NT | NT | NT |
| Furtado et al18 | Alberta, Canada | 6 | 39 | 31, 46, 47, 62, 84, 86 | 6/6 | 0/6 | NT | NT | NT |
| Lu et al19 | Taiwanese | 1 | 36 | 40 | 1/1 | 0/1 | NT | NT | NT |
| Pirker et al20 | Caucasian, America | 1 | 37 | 46 | 1/1 | 1/1 | NT | 1/1 (Depression) | NT |
| Payami et al21 | Caucasian, America | 2 | 33, 35 | 36, 60 | 2/2 | 0/2 | NT | NT | NT |
| Lee et al22 | Korean | 17 | 44.9±5.3 | 32.8±12.7 | 2/17 | 17/17 | 5/17 | NT | NT |
| Lu et al3 | Taiwanese | 7 | 35, 36, 38 | 79, 34, 40, 40, 43, 50, 38 | 7/7 | 3/7 | NT | NT | NT |
| Wilkins et al23 | Caucasian, UK | 1 | 42 | 31 | 1/1 | 0/1 | NT | NT | NT |
| Furtado et al24 | Caucasian, Canada | 4 | 33, 34, 38, 39 | 70, 82, 45, 43 | 4/4 | 2/4 | 0/4 | NT | |
| Shan et al25 | Chinese | 1 | 37 | 56 | 1/1 | 0/1 | NT | NT | NT |
| Ragothaman et al26 | Indian | 11 | 37, 36, 36, 37, 39, 45, 44, 48, 31, (35/37), (36/39) | 75, 60, 72, 30, 20, 19, 15, 37, 27, 22, 15 | 5/11 | 7/11 | NT | NT | NT |
| Lim et al27 | Singapore | 1 | 36 | 50 | 1/1 | 0/1 | NT | 0/1 | 0/1 |
| Modoni et al28 | Italian | 1 | 38 | 48 | 1/1 | 0/1 | 0/1 | NT | NT |
| Rottnek et al16 | Afro-Caribbean | 1 | 39 | 27 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| Socal et al29 | Brazilian | 4 | (34/33), (34/33), (34/33), (44/33) | 41, 46, 35, 40 | 4/4 | 3/4 | 1/4 | NT | NT |
| Ragothaman et al30 | Indian | 2 | (35/37), (36/39) | 22, 15 | 2/2 | 2/2 | 2/2 | 1/2 | 1 |
In our reported family, the CAG repeat number of the index patient (patient II:2) is 32/19. The CAG repeat number of other family members are 32/20 for case I:1 (father of the index patient), 35/21 for case II:1 (elder sister of the index patient) and 34/20 for II:3 (younger brother of the index patient). The normal allele with 20 CAG repeats of case II:3 is apparently inherited from the normal allele of the affected father and the normal alleles with 19 and 21 CAG repeats of other family members are obviously from the unaffected mother, who did not receive the genetic examination. Notably, we found the pathogenic CAG repeat number of case I:1 and the index patient (case II:2) was the same (32 repeats) but the onset age was 12 years earlier in the index patient. This observation seems not fit the inverse correlation between the size of the CAG repeat and the age at disease onset in CAG repeat disease, such as SCA2. Compatible with our reported family, recent studies have observed a wide range of age at disease onset under similar CAG repeat number, suggesting CAG repeat number alone cannot fully explain this clinical variability. A recently identified novel epigenetic regulatory mechanism controlled by DNA methylation level of ataxin2 gene could influence the ATXN2 protein expression level in the cells that may aggravate disease severity and influencing the onset age even under the same CAG repeat number.15
Psychiatric presentation, especially positive symptoms of psychosis, which has traditionally been associated with dementia or late stage of parkinsonsim was rarely reported in patients with cerebellar disease, especially in patients with SCA2.14 Similar to our reported case (figure 1 case II:1), Rottnek et al had presented a case of 22-year-old man who had been diagnosed as having schizophrenia and became cerebellar ataxia when he was 32 years old (table 1).16 The reported patient had prominent somatic and paranoid delusions, auditory hallucination and idea of reference, which were only partially responsive to antipsyhotic treatments. These psychotic symptoms, especially auditory hallucination and somatic delusion, were rarely reported in typical PD or MSA. In addition to case II:1, our index patient also had prominent psychotic features 2 years after parkinsonism was diagnosed under low dose of levodopa (150mg per day). In contrast to the reported case by Rottnek et al and case II:1 in our family, the psychotic symptoms of our proband were easily controlled by low dose of risperidone. These observations confirmed the clinical heterogenisity in the SCA2 related phenotypes. Although visual hallucinations and jealous delusions are a common feature of Lewy body parkinsonism, occurring in up to 40% of patients with PD, it is rare to observe auditory hallucinations and persecutory delusions with complex content in patients with parkinsonism. Given that there was no high dose of levodopa or other possible trigger medication for psychosis, we believe the psychotic features reported in our index patient are part of her clinical phenotypes of ATXN2 mutation. The previously reported psychosis features in cerebellar diseases are mainly paranoid and visual hallucinatory symptoms.17 This concept is supported by the increasingly recognised role of cerebellum in the pathophysiology of schizophrenia, suggesting ‘cognitive dysmetria’ with a general dyscoordination of sensorimotor and mental processes.17 These observations reinforced the role of extracerebellar structures in the clinical presentations of SCA2. There are growing evidence showed that degeneration of extracerebellar structures play a role in the pathogenesis of SCA2. A TRODAT-1 SPECT image study showed decreased dopamine transporter uptake in parkinsonism variant SCA2 patients and brain MRI also revealed generalised cortex atrophy except infratentorial structures. Besides, brain perfusion SPECT also showed decreased tracer signals in bilateral fronto-parietal regions, as demonstrated in our index patient, reinforcing the degeneration of supratentorial structures in SCA2. We hypothesised that the hypofunciton of dopaminergic system and decreased striatum γ-aminobutyric acid innervation from the cerebellar Purkinje fibres would dysregulate the functional circuits of basal ganglia, implicating its role in motor control as well the executive function. In addition, the atrophy of midbrain, which involves central cholinergic pathway, such as pedunculopontine nucleus, is another possible mechanism to support the visual hallucination in patients with spinocerebellar atrophy. These studies provided the evidence of cerebellum and brainstem in the development of psychosis. Further studies are warranted to clarify the pathogenetic mechanism. However, the possibility of co-morbid schizophrenia in our SCA2 family should also be considered. Given that the obvious family history of ataxic gait in our index family and presence of psychotic-affective symptoms in family members in the fifth decade (case II:2 and II:3), which onset age is uncommon for schizophrenia, we considered the psycho-affective symptoms are the uncommon clinical phenotypes of our SCA2 family.
The finding of our SCA2 pedigree showing parkinsonism, affective and psychotic features highlight the cerebellar-affective-psychotic syndrome of SCA2. Further functional study and more clinical information are needed to clarify the role of cerebellum in the pathogenesis of these psychotic symptoms in SCA2 patients. SCA2 should be considered in the differential diagnosis of cases resembling MSA, especially those with atypical features and positive family history.
Learning points.
-
▶
SCA2 may present symptomatically as prominent psychosis features and can clinically be misdiagnosed as MSA and schizophrenia.
-
▶
SCA2 should be considered in the differential diagnosis of cases resembling parkinsonism, especially those with atypical features and positive family history.
-
▶
Patient with familial MSA or atypical parkinsonism feature should receive genetic analysis of ATAX2 gene mutations.
Acknowledgments
The authors thank the patients who have participated this study and the staff of the Second Core Lab, Department of Medical Research of National Taiwan University Hospital for technical support during the study.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Gwinn-Hardy K, Chen JY, Liu HC, et al. Spinocerebellar ataxia type 2 with parkinsonism in ethnic Chinese. Neurology 2000;55:800–5 [DOI] [PubMed] [Google Scholar]
- 2.Shan DE, Soong BW, Sun CM, et al. Spinocerebellar ataxia type 2 presenting as familial levodopa-responsive parkinsonism. Ann Neurol 2001;50:812–15 [DOI] [PubMed] [Google Scholar]
- 3.Lu CS, Wu Chou YH, Kuo PC, et al. The parkinsonian phenotype of spinocerebellar ataxia type 2. Arch Neurol 2004;61:35–8 [DOI] [PubMed] [Google Scholar]
- 4.Klinke I, Minnerop M, Schmitz-Hübsch T, et al. Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum 2010;9:433–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bürk K, Globas C, Bösch S, et al. Cognitive deficits in spinocerebellar ataxia 2. Brain 1999;122 (Pt 4):769–77 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Hübsch T, Coudert M, Tezenas du Montcel S, et al. Depression comorbidity in spinocerebellar ataxia. Mov Disord 2011;26:870–6 [DOI] [PubMed] [Google Scholar]
- 7.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–8 [DOI] [PubMed] [Google Scholar]
- 8.Lin CH, Hwu WL, Chiang SC, et al. Lack of mutations in spinocerebellar ataxia type 2 and 3 genes in a Taiwanese (ethnic Chinese) cohort of familial and early-onset parkinsonism. Am J Med Genet B Neuropsychiatr Genet 2007;144B:434–8 [DOI] [PubMed] [Google Scholar]
- 9.van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Mov Disord 2011;26:792–800 [DOI] [PubMed] [Google Scholar]
- 10.Munhoz RP, Teive HA, Raskin S, et al. CTA/CTG expansions at the SCA 8 locus in multiple system atrophy. Clin Neurol Neurosurg 2009;111:208–10 [DOI] [PubMed] [Google Scholar]
- 11.Lin IS, Wu RM, Lee-Chen GJ, et al. The SCA17 phenotype can include features of MSA-C, PSP and cognitive impairment. Parkinsonism Relat Disord 2007;13:246–9 [DOI] [PubMed] [Google Scholar]
- 12.Nirenberg MJ, Libien J, Vonsattel JP, et al. Multiple system atrophy in a patient with the spinocerebellar ataxia 3 gene mutation. Mov Disord 2007;22:251–4 [DOI] [PubMed] [Google Scholar]
- 13.Robitaille Y, Lopes-Cendes I, Becher M, et al. The neuropathology of CAG repeat diseases: review and update of genetic and molecular features. Brain Pathol 1997;7:901–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duggal HS. Cognitive affective psychosis syndrome in a patient with sporadic olivopontocerebellar atrophy. J Neuropsychiatry Clin Neurosci 2005;17:260–2 [DOI] [PubMed] [Google Scholar]
- 15.Laffita-Mesa JM, Bauer PO, Kourí V, et al. Epigenetics DNA methylation in the core ataxin-2 gene promoter: novel physiological and pathological implications. Hum Genet 2011. (In Press). [DOI] [PubMed] [Google Scholar]
- 16.Rottnek M, Riggio S, Byne W, et al. Schizophrenia in a patient with spinocerebellar ataxia 2: coincidence of two disorders or a neurodegenerative disease presenting with psychosis? Am J Psychiatry 2008;165:964–7 [DOI] [PubMed] [Google Scholar]
- 17.Picard H, Amado I, Mouchet-Mages S, et al. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 2008;34:155–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furtado S, Farrer M, Tsuboi Y, et al. SCA-2 presenting as parkinsonism in an Alberta family: clinical, genetic, and PET findings. Neurology 2002;59:1625–7 [DOI] [PubMed] [Google Scholar]
- 19.Lu CS, Wu Chou YH, Yen TC, et al. Dopa-responsive parkinsonism phenotype of spinocerebellar ataxia type 2. Mov Disord 2002;17:1046–51 [DOI] [PubMed] [Google Scholar]
- 20.Pirker W, Back C, Gerschlager W, et al. Chronic thalamic stimulation in a patient with spinocerebellar ataxia type 2. MovDisord 2003;18:222–5 [DOI] [PubMed] [Google Scholar]
- 21.Payami H, Nutt J, Gancher S, et al. SCA2 may present as levodopa-responsive parkinsonism. Mov Disord 2003;18:425–9 [DOI] [PubMed] [Google Scholar]
- 22.Lee WY, Jin DK, Oh MR, et al. Frequency analysis and clinical characterization of spinocerebellar ataxia types 1, 2, 3, 6, and 7 in Korean patients. Arch Neurol 2003;60:858–63 [DOI] [PubMed] [Google Scholar]
- 23.Wilkins A, Brown JM, Barker RA. SCA2 presenting as levodopa-responsive parkinsonism in a young patient from the United Kingdom: a case report. Mov Disord 2004;19:593–5 [DOI] [PubMed] [Google Scholar]
- 24.Furtado S, Payami H, Lockhart PJ, et al. Profile of families with parkinsonism-predominant spinocerebellar ataxia type 2 (SCA2). Mov Disord 2004;19:622–9 [DOI] [PubMed] [Google Scholar]
- 25.Shan DE, Liu RS, Sun CM, et al. Presence of spinocerebellar ataxia type 2 gene mutation in a patient with apparently sporadic Parkinson’s disease: clinical implications. Mov Disord 2004;19:1357–60 [DOI] [PubMed] [Google Scholar]
- 26.Ragothaman M, Sarangmath N, Chaudhary S, et al. Complex phenotypes in an Indian family with homozygous SCA2 mutations. Ann Neurol 2004;55:130–3 [DOI] [PubMed] [Google Scholar]
- 27.Lim SW, Zhao Y, Chua E, et al. Genetic analysis of SCA2, 3 and 17 in idiopathic Parkinson’s disease. Neurosci Lett 2006;403:11–4 [DOI] [PubMed] [Google Scholar]
- 28.Modoni A, Contarino MF, Bentivoglio AR, et al. Prevalence of spinocerebellar ataxia type 2 mutation among Italian Parkinsonian patients. Mov Disord 2007;22:324–7 [DOI] [PubMed] [Google Scholar]
- 29.Socal MP, Emmel VE, Rieder CR, et al. Intrafamilial variability of Parkinson phenotype in SCAs: novel cases due to SCA2 and SCA3 expansions. Parkinsonism Relat Disord 2009;15:374–8 [DOI] [PubMed] [Google Scholar]
- 30.Ragothaman M, Muthane U. Homozygous SCA 2 mutations changes phenotype and hastens progression. Mov Disord 2008;23:770–1 [DOI] [PubMed] [Google Scholar]