Abstract
The authors present a 76-year-old female with high blood pressure and hypercholesterolaemia as cerebrovascular risk factors, who received intravenous thrombolysis for an ischaemic stroke with a progressive neurological improvement. She was asymptomatic at 48 h and she was transferred to the neurology department where antithrombotic treatment was initiated. She began to sit the following day when she suffered a massive pulmonary embolism (PE). Cardiological study showed patent foramen oval persistence and the presence of an atrial septa aneurysm, and paroxysmal atrial fibrillation. The delay of the onset of the antithrombotic treatment could have been determinant for the massive PE. Thromboembolic complications may be seen after intravenous thrombolysis for ischaemic stroke. An accurate treatment is needed in order to avoid potentially threatening complications such as massive PE.
Background
Several cases of concomitant pulmonary embolism (PE) and ischaemic stroke1 2 and an ischaemic stroke following PE3 4 have been described in the literature. We present a patient who suffered a massive PE after intravenous thrombolysis for an ischaemic stroke.
Case presentation
A 76-year-old Caucasian female was admitted on the 2 July 2009 due to a sudden right leg paresis at 9:30 am while out walking. She presented with high blood pressure and hypercholesterolaemia as cerebrovascular risk factors. Neurological status in the emergency department showed a 0–1/5 right leg paresis with hypoesthesia. Brain CT and extra and intracranial angio-CT were normal. With the diagnosis of a left anterior cerebral artery ischaemic stroke, intravenous thrombolysis with 63 mg of recombinant tissular plasminogen was performed at 3 h after the onset of symptoms. The patient was transferred to the Intensive Care Unit. Brain CT after 24 h of intravenous thrombolysis was normal. The patient showed progressive neurological improvement and was asymptomatic at 48 h. She was transferred on the 4th of July to the neurology department where antithrombotic treatment (acetyl-salicylic acid 300 mg daily and low-molecular weight heparin (LMWH) 6000 units per day) was initiated. Our patient began to sit up the following day. At 11 am, after 15 min of sitting up, she lost consciousness and presented hypoxemia (65% oxygen saturation to a 50% Venturi mask), bradycardia and pallor, without signs of venous thrombosis or thrombophlebitis in the legs. Due to haemodynamic instability, the patient was transferred to the intensive care unit. Based on the presumptive diagnosis of a massive PE a bolus of 5000 units of unfractionated heparin was administered at 1 pm followed by a continuous intravenous infusion. One hour later a pulmonary angio-CT confirmed an embolism in the bilateral segmental and subsegmental arteries (figure 1). A transthoracic cardiac echography showed dilated right cardiac cavities with a positive bubble-contrast test. A transesophagic cardiac echography confirmed patent foramen oval (PFO) persistence with slight contrast passage and the presence of an atrial septal aneurysm (ASA). The patient clinically improved and was transferred on the 13th of July to our department, where neurological and cardiological assessment was completed. Cranial MRI confirmed a left anterior cerebral artery stroke (figure 2). A Holter showed a paroxysmal atrial fibrillation (AF), not previously present. The patient was asymptomatic and was discharged with anticoagulant treatment. The delayed thrombophilia study was normal. The patient has remained asymptomatic up to the present date.
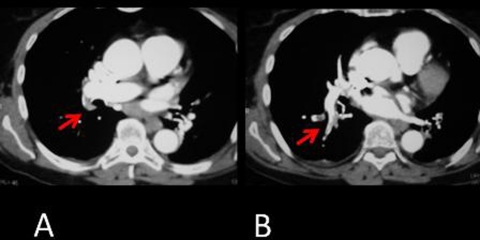
Figure 1.
(A and B) Pulmonary angio-CT confirmed an embolism in the bilateral segmental and subsegmental arteries (red arrows).
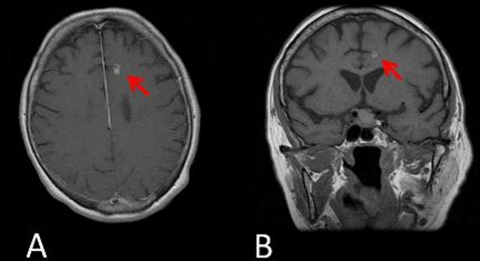
Figure 2.
(A and B) Cranial MRI (T1 sequence) showed a left anterior cerebral artery stroke (red arrows).
Discussion
The role of the PFO in the aetiology of stroke is confusing and contradictory.1 PFO is common in the general adult population and normally is not harmful. However, it allows right to left blood flow when right atrial pressure exceeds left atrial pressure and may result in stroke due to paradoxical embolism.2 3 PFO is associated with a higher incidence of ischaemic stroke and systemic embolism.4 In addition, ASA is often associated with PFO, and the mechanism for stroke in ASA is due to concomitant PFO, and a tendency toward atrial arrhythmias such as AF and thrombus formation.1 In some cases, diagnosis of paradoxical embolism is not an easy task. Caplan5 supports a highly probable the diagnosis of paradoxical embolism in the presence of at least four of the following: (1) situations that promote thrombosis of leg or pelvic veins (2) increased coagulability, (3) sudden onset of stroke during an activity that includes a Valsalva maneuver or promotes right-to-left shunting of blood, (4) PE within a short time before or after the neurologic ischaemic event and (5) the absence of other putative causes of stroke after thorough evaluation. Our patient presented a sudden right leg paresis during walking without either previous situations that promoted thrombosis of the leg (or pelvic veins) or increased coagulability. Moreover, concomitant paroxysmal AF was discovered as another putative cause of stroke. Thus paradoxical embolism in our patient might be a possibility, but the coexistence of AF led us to choose anticoagulant treatment as a secondary prevention ruling out closure of the PFO. Insufficient evidence exists to recommend closure of the PFO for the first stroke,1 above all in older patients without any antithrombotic treatment prior to the ischaemic event, and another presumed cause of stroke. However, it would not be rule out in the case of the recurrence of an embolic stroke.
On the other hand, although deep vein thrombosis is a common complication after stroke due to limb paresis and immobility, and PE is the most feared complication in these patients,6 the precise mechanism of production of PE in our case had not been determined. However, the delay of the commencement of antithrombotic treatment after intravenous thrombolysis in our patient could have been a determinant for the massive PE. This case suggests the need to initiate antithrombotic treatment no more than 24 h after intravenous thrombolysis. A combined antithrombotic treatment (acetyl-salicylic acid and LMWH) may be the best therapeutical approach in all patients after intravenous thrombolysis until the complete study is performed.
Learning points.
-
▶
In conclusion, thromboembolic complications may be seen after intravenous thrombolysis for ischaemic stroke. An accurate treatment is needed in order to avoid potentially threatening complications such as massive PE. This case emphasises the need for antithrombotic treatment after no more than 24 h after intravenous thrombolysis. A combined antithrombotic treatment (acetyl-salicylic acid and LMWH) may be the best therapeutical approach in all patients after intravenous thrombolysis until the complete study is performed.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Irwin B, Ray S. Patent Foramen Ovale-Assessment and Treatment. Cardiovasc Ther. 2011 doi: 10.1111/j.1755-5922.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 2.Lapostolle F, Borron SW, Surget V, et al. Stroke associated with pulmonary embolism after air travel. Neurology 2003;60:1983–5 [DOI] [PubMed] [Google Scholar]
- 3.Pavesi PC, Pedone C, Crisci M, et al. Concomitant submassive pulmonary embolism and paradoxical embolic stroke after a long flight: which is the optimal treatment? J Cardiovasc Med (Hagerstown) 2008;9:1070–3 [DOI] [PubMed] [Google Scholar]
- 4.Bracey TS, Langrish C, Darby M, et al. Cerebral infarction following thrombolysis for massive pulmonary embolism. Resuscitation 2006;68:135–7 [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR. Brain embolism. In: Caplan LR, ed. Caplan’s Stroke. A Clinical Approach. Fourth Edition Philadelphia, USA: Saunders Elsevier; 2009:316–74 [Google Scholar]
- 6.Freeman WD, Dawson SB, Flemming KD. The ABC’s of stroke complications. Semin Neurol 2010;30:501–10 [DOI] [PubMed] [Google Scholar]