Abstract
The authors describe the case of fatal cumulative toxicities in a 58-year-old AIDS-free, HIV-infected patient, who successively developed under highly active antiretroviral therapy (HAART): severe lipodystrophy, complicated osteoporosis, complicated non-cirrhotic portal hypertension of the liver (with ascites, portal thrombosis, oesophageal varices and protein-losing enteropathy) due to nodular regenerative hyperplasia. These cumulative HAART-related toxicities led to death, despite symptomatic treatment and the switch of antiretrovirals (especially didanosine) putatively involved in the process in these drug-mediated diseases. As morbidity and mortality in HIV infection continue to improve, it appears important to recognise such rare HAART-associated toxicities. This case illustrates the absolute necessity of investigating the long-term side effects of HAART in HIV infection, particularly in patients treated with first generation molecules. The switch strategy (switching old molecules to newer ones) is crucial in case of severe suspected toxicity and has to be discussed in asymptomatic patients largely exposed to first generation molecules, in order to prevent long-term toxicity.
Background
The use of highly active antiretroviral treatment (HAART) in industrialised countries has dramatically decreased mortality in HIV-infected patients since 1996, mainly due to the reduction of occurrence of AIDS-defining illnesses.1 As HIV-infected persons live longer, they are increasingly more likely to develop non-HIV related diseases, such as long-term expected or unexpected HAART-related toxicities. Lipodystrophy has been associated with thymidine analogues and with protease inhibitors2 3; liver toxicity with non-nucleoside reverse transcriptase inhibitors and didanosine4; and osteoporosis with HAART in general and especially with protease inhibitors and tenofovir.5 In most cases of HAART-induced toxicity, the removal of the causative drug improves or stabilises the condition. Some severe forms of toxicity have been described. They mainly occurred in the first week of therapy such as fulminant hepatitis related with nevirapine6 or efavirenz.7 Nodular regenerative hyperplasia (NRH) of the liver is rare, but seems to be an emerging complication in HIV-infected patients. NRH induces non-cirrhotic portal hypertension (NCPH) manifestations and could be fatal. The role of didanosine in NRH and NCPH has been demonstrated in two studies8 9 and a mitochondrial toxicity is suspected to be involved in the process of the disease.4 It is unknown that if switching didanosine to newer drugs is considered to be less toxic, it may improve the prognosis of NCPH due to NHR.
We report the case of an AIDS-free HIV-infected patient, who successively developed several severe HAART-related toxicities, including NCPH due to NHR that lead to death, despite the switch from old drugs to newer ones.
Case presentation
A 50-year-old man, was diagnosed with HIV infection in 2001, following herpes zoster limited to one dermatome. The patient reported no significant personal or family medical history. Physical examination was normal and the weight was 61 kg. CD4 cell count was 204/mm3 (9%) and HIV viral load was 5.64 log10 copies/ml (CDC stage A2). Serological tests showed no evidence of hepatitis C virus (HCV) and hepatitis B virus (HBV) infection. No resistance mutation to antiretrovirals was detected. A combination of zidovudine, lamivudine and nelfinavir was started. Lamivudine was replaced by didanosine 400 mg/day 18 months later, following the identification of M184V mutation on a still detectable plasma HIV-RNA. Didanosine was chosen instead of stavudine or abacavir to avoid the occurrence of: (1) zidovudine-stavudine drug-drug interaction; and (2) severe hypersensitivity reactions associated with abacavir. The patient weighed at that time 64 kg. Twenty three months after initiating HAART, viral load was undetectable, and CD4 cell count was stable at 300/mm3 (15%). One year later, as the patient experienced persistent diarrhoea, nelfinavir was replaced by efavirenz.
In 2005, the patient weighed 57 kg and had lost 11% of his body-weight. HIV-infection was still controlled with stable CD4 cell count (357/mm3, 22%) and HIV viral load (<50 copies/ml). A bilateral gynecomastia appeared, without other clinical signs of lipodystrophy. Complete blood count, hepatic test, lactic acid and lipid profile were normal as well as complete endocrine tests. Efavirenz was replaced by nevirapine, while zidovudine and didanosine were maintained at the same dose (300 mg twice a day, and 400 mg once a day, respectively). A dose reduction of didanosine should have been prescribed, as the patient weighed <60 kg at this stage.
Between 2005 and 2007, the patient’s weight remained unchanged, but general health status was relatively good. Systematic hepatic tests showed an asymptomatic and isolated liver cholestasis around twofold higher than the upper limit of normal (ULN). In particular, there was no thrombocytopaenia. Chest x-ray and abdominal ultrasound did not find any abnormality. Another treatment switch was discussed, especially concerning zidovudine and/or didanosine, but simple clinical and biological monitoring was finally decided on.
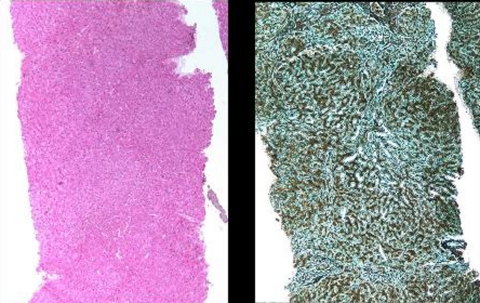
The patient was hospitalised in 2007 for onset of ascites. Physical examination revealed facial lipoatrophy and an increase in gynecomastia. Weight with ascites was 60 kg. Laboratory tests revealed cholestasis up to five times ULN, without cytolysis or signs of hepatic failure. In particular albumin was normal. Grade-2 oesophageal varices were found on gastroscopy. CT scan demonstrated portal vein thrombosis, hepatic perfusion disorder and significant collateral circulation without splenomegaly. Interview revealed neither regular alcohol consumption nor drug abuse. HBs Ag, HBe Ag, HBV DNA, HCV antibodies and HCV RNA were not detected in the serum. Screening was negative for other aetiology of chronic hepatic disorder, including auto-immune hepatitis, hemochromatosis, Wilson’s disease or α-1 antitrypsin deficiency. There was no sign of neoplasm. A liver biopsy exhibited typical findings of NRH without signs of other liver disease, including chronic hepatitis and steatohepatitis (figure 1A,B). At that time, HIV viral load and CD4 cell count were <50 copies/ml and 242/mm3 (21%), respectively. As nevirapine was known to be also potentially associated with delayed hepatotoxicity, it was replaced by boosted lopinavir. Despite lipoatrophy (limited at this stage to the face), zidovudine and didanosine were continued at the same doses. Zidovudine should have been to be switched at this stage (there was no financial, social or other issues that justified the continuation of zidovudine) and again, a dose reduction of didanosine should have been prescribed, as the patient weighed 60 kg at this stage. Anticoagulants, diuretics and β-blockers were initiated, and the clinical and radiographic signs of portal hypertension, including portal vein thrombosis, resolved.
Figure 1.
Liver biopsy showing nodular regenerative hyperplasia: liver parenchyma (A), without any significant inflammation or fibrosis (H&E staining); (B), with discrete nodularity with thick and atrophic cords (reticulin staining).
In 2008, the patient experienced a spontaneous L1 fracture, leading to the discovery of osteoporosis by dual energy x-ray absorptiometry (lumbar spine and hip T-score <2.5). Calcium and vitamin D treatment were initiated.
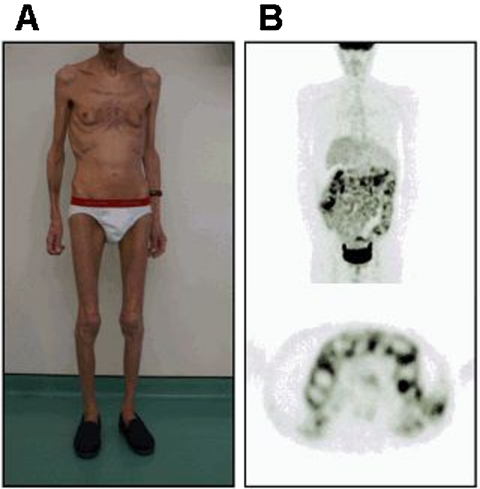
In early 2009, 8 years following initiation of HAART, the patient was hospitalised in a context of deterioration of general health status. The weight was 48 kg, corresponding to a 25% body weight loss. Facial and peripheral lipoatrophy, gynecomastia and clinical ascites were observed (figure 2A). There was no diarrhoea or vomiting and no signs of active infection. An indirect calorimetry demonstrated a profound hypocatabolism (resting energy expenditure 800 kcal/day). Bioelectrical impedance demonstrated a severe fat tissue loss, with a total fat weight <1 kg. HIV infection was still controlled, with an HIV viral load of <50 copies/ml and CD4 cell count measured at 269/mm3 (26%). Hepatic test showed only minor cholestasis, with gGT less than two ULN with normal bilirubin and alkaline phosphatase. There was no sign of biliary obstruction on MRI. The lipid profile and lactic acid level were normal, but fasting hyperglycaemia revealed an insulin resistance. Homeostatic model assessment, used to quantify insulin resistance, was not performed. Blood test demonstrated severe hypoalbuminemia (12 g/l) without nephrotic syndrome or other signs of liver failure. The ascitic protein level was low. The daily stool weight was 278 g/24 h (normal range 150–250 g). Stool examination did not find any bacterial or parasitological pathogen. Faecal pancreatic elastase and chymotrypsine were normal. The intestinal clearance of α-1-antitrypsine demonstrated a protein leakage in stool (117 ml/24 h, normal value <15 ml/24 h). The D-xylose absorption test was not performed. Vitamin B12 and B9 blood levels were normal. The serum iron and ferritin concentration were both low, without other signs of malabsorption. ANCA, antinuclear, antismooth muscle, antimitochondrial, antiendomysial and antitransglutaminase antibodies were negative. Ileo-colonoscopy detected a non-specific macroscopic ulcerative ileitis with a normal colon. Gastroscopy showed grade 1 oesophageal varices, atrophic inflammatory gastritis and no villous atrophy on duodenum. Ileal biopsies revealed mild (10 µm) non-specific hyaline thickening of the basal membrane without intraepithelial lymphocytosis. Duodenal biopsies revealed partial villous atrophy with normal crypt depth and normal basal membrane. Digestive tissue cultures for bacterial and viral pathogens remained negative.
Figure 2.
(A) Photograph of the patient showing lipoatrophy, gynecomastia, cachexia and ascites. (B) 18F FDG PET/CT imaging showing a significant increase in the FDG uptake of the whole bowel (SUVmax 6.3 on the small intestine, 4.2 on the colon).
Whole-body CT scan demonstrated ascites and liver dysmorphia without portal vein thrombosis but was normal otherwise. A 18F fluorodeoxyglucose positron emission tomography (FDG PET)/CT imaging, performed to detect neoplasm, found an increase in the FDG uptake of the whole bowel only (maximal standardised uptake value (SUVmax) of 6.3 on the small intestine, and 4.2 on the colon) (figure 2B).
Clinical management
Symptomatic treatment was performed. Enteral followed by parenteral nutrition was undertaken. Symptomatic treatment of portal hypertension was maintained. As the association between NRH and didanosine exposure was poorly described in 2007, didanosine was maintained at the diagnosis of NRH, after an exposure of 5 years. Nevertheless, the didanosine dose was reduced from 400 mg to 250 mg/day. Finally, 2 years after the diagnosis of NHR, boosted lopinavir, zidovudine and didanosine was replaced by a combination of abacavir, raltegravir and etravirine. No improvement was observed and the patient died in August 2009, 8 years following the introduction of HAART, in extreme cachexia, with refractory ascites.
Discussion
This AIDS-free patient with controlled HIV infection successively presented several iatrogenic complications, which ultimately led to death in 8 years.
First, lipodystrophy was described in 1998, a few years after the large prescription of HAART.2 The prevalence of lipodystrophy is estimated at 50–60% following introduction of stavudine- or zidovudine-based HIV treatment.10 It is composed of peripheral lipoatrophy, which could be associated with central fat accumulation (gynecomastia). Lipoatrophy syndrome has been related to nucleoside reverse transcriptase inhibitors and notably to the thymidine analogues (stavudine and zidovudine).3 Central fat accumulation syndrome is known to be mostly related to protease inhibitors2 and non-nucleoside reverse transcriptase inhibitors.11 The impact of the new classes of antiretrovirals on lipodystrophy is unknown. Prolonged administration of zidovudine and didanosine was thought to explain the severe lipoatrophy observed in this patient, while nelfinavir and efavirenz could both explain the gynecomastia. Currently, the main strategy to improve or reduce the progression of lipoatrophy is to switch from thymidine analogues.12 In our patient, we should have changed zidovudine to abacavir earlier, and its continuation in case of lipoatrophy is to be strongly discouraged.
Osteopenia appears frequent in HIV-infected patients, while pathologic osteoporosis and fracture are less frequently described. The role of HIV infection itself remains discussed. Exposure to HAART,13 protease inhibitors,5 and tenofovir13 have been associated with an increased risk of osteoporosis. In the case presented here, nelfinavir and lopinavir may have played a role for such a manifestation. However, there were no recommendations regarding HAART modifications in patients presenting with osteoporosis, especially in regimens that do not contain tenofovir.
NCPH and NRH are infrequently encountered in HIV infection. They are considered as manifestations of a portal obliterative veinopathy inducing hepatic perfusion disorder.14 Differential diagnosis includes immunosuppressive therapies, auto-immune disorders and lymphoproliferative diseases, none of which were present in the case reported here (notably, the 18F FDG PET/CT scan ruled out the hypothesis of lymphoproliferative disease). Two case control studies, in 2009 and 2011, demonstrated the association of these complications with exposure to didanosine, combined or not with stavudine.8 9 The FDA issued a warning in early 2010 about the association of didanosine administration and NCPH.15 Mechanisms remain unknown, and may include drug-induced mitochondrial toxicity,4 decreased protein-S level due to elevated antiprotein S IgG in HIV-associated NRH,16 or non-remitting vascular injury.17 The hepatic disease, in our case, was probably exacerbated by the prolonged exposure to high doses of didanosine: the patient received 400 mg didanosine daily despite the progressive weight loss that occurred over 5 years. The dose was finally reduced to 250 mg daily in 2007 (body weight 60 kg). Whether as cause or consequence, the Swiss case control study on NCPH found a lower body mass index in the NCPH group, unlike the French study.8 9
NCPH diagnosis is difficult, often made several months or years following the first symptoms of portal hypertension. NRH is characterised on pathology by small infra-lobular nodules originating in the periportal areas, surrounded by atrophic hepatocytes without fibrous septa.18 Some patients are diagnosed years after termination of didanosine.8 Likewise, a recent case report of didanosine-associated NRH showed persistence of marked signs of portal hypertension on MRI more than 7 years after termination of didanosine.19 This suggests that NRH can evolve spontaneously, and the reversibility of the phenomenon is uncertain even after termination of the culprit drug. Systematic anticoagulant therapy may be indicated for NRH.20 In case of severe portal hypertension, liver transplantation is the only therapeutic option, being is feasible in HIV-infected patients.21
The severe weight loss and hypoprotidemia presented by our patient at the beginning of 2009 was a very unusual manifestation. There was no sign of pancreatic exocrine failure, celiac disease, gastrointestinal infection or inflammatory bowel disease. A complete investigation was consistent with the diagnosis of protein-losing enteropathy without evidence of malabsorption. The diagnosis of microscopic colitis appeared unlikely despite the description of a mild thickening of the basal membrane on ileal biopsies in the absence of intraepithelial lymphocytosis. FDG uptake in the bowels was a striking finding in this patient, confirming the severity of its gastrointestinal involvement, without providing information about aetiology. A recent Italian study analysed FDG uptake in several HIV subjects according to their immunologic or virologic status and did not show a mesenteric uptake, even in the immunological non-responder subgroup.22 Similar findings were reported by another group comparing HIV-negative patients, HIV-infected patients with undetectable viral load and HIV-infected patients without HAART.23 A toxic enteropathy could be suspected in our case, but this diagnosis was ruled out in the absence of long-term treatment by at least 1 drug known as a recognised enterotoxic. The association between protein-losing enteropathy and portal hypertension was described more than 50 years ago but still remains debatable.24 However, case reports of reversal of protein-losing enteropathy following hepatic transplantation suggest that such an association shares a common if not aetiologic factor.25 In the absence of other identified aetiology, we hypothesise that this patient died from protein-losing enteropathy secondary to NCPH caused by didanosine induced-NRH.
Long-term administration, uncorrected dosage of didanosine despite body weight loss and pursuit of didanosine with zidovudine following the diagnosis of NRH are factors that could possibly explain the fatal evolution in this patient. However, one cannot exclude a particular susceptibility factor in this patient who successively presented manifestations of HAART toxicity in different organs.
Some data underline the beneficial effect of drug replacement in case of long-term toxicity. Replacement of thymidine analogues has demonstrated a benefit in patients with HAART-associated lipoatrophy.12 The FDA issued a warning in early 2010 about the association of didanosine administration and NCPH. Following this warning, the didanosine prescription label was updated, recommending recommending the discontinuation of the drug in patients with evidence of NCPH.19 In 2011, continuation of zidovudine in patients with lipoatrophy, and didanosine in patients suspected to have NHR, was strongly discouraged.12 19 To date, there are no recommendations regarding HAART modifications in patients with osteoporosis. Since the severe symptomatology presented by our patient in early 2009 was presumed to be iatrogenic, replacement of every drug in his HAART appeared mandatory, despite the absence in 2009 of specific recommendations for such a case, except for switching from zidovudine. However, HAART modifications, combined with symptomatic treatment of NCPH and with supportive care and nutrition failed to reverse the pathogenic process.
The present case is remarkable for the association of several serious iatrogenic conditions, and the severe evolution of a rare condition. It highlights the need to detect HAART’s long-term side-effects as soon as possible to adapt dosage or to switch to other drugs of lower known toxicity. However, despite the fact that recent antiretrovirals appeared better tolerated in the short term than the older ones, a postmarketing survey appears necessary to identify new and rare toxicities. Since the role of didanosine in NRH took nearly 20 years to be recognised, cautious follow-up is required regarding the long-term effect of more recent drugs.
Learning points.
-
▶
Long-term treatment toxicity can occur in HIV infected patients and can lead to serious injury and to death, even when the infection appears virologically controlled.
-
▶
Didanosine-associated NRH is a rare but potentially fatal drug-induced liver injury in HIV-positive patients, which can occur years after treatment interruption and may evolve despite drug interruption.
-
▶
The implication of a particular drug in combination therapy, when long term toxicity is suspected, is often difficult to prove, especially in the case of rare disease such as NRH and protein-losing enteropathy.
-
▶
In case of HAART-associated long-term toxicity due to long exposure of old drugs (such as didanosine), switching old drugs to newer ones is mandatory.
Acknowledgments
Thomas Perpoint, André Boibieux, Florence Ader and Didier Barnoud were also involved in the patient’s care. Michele Chevallier and Brigitte Bancel performed the analysis of the liver and bowel biopsies, respectively.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Bhaskaran K, Hamouda O, Sannes M, et al. ; CASCADE Collaboration Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 2008;300:51–9 [DOI] [PubMed] [Google Scholar]
- 2.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998;12:F51–8 [DOI] [PubMed] [Google Scholar]
- 3.Mallal SA, John M, Moore CB, et al. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS 2000;14:1309–16 [DOI] [PubMed] [Google Scholar]
- 4.Soriano V, Puoti M, Garcia-Gascó P, et al. Antiretroviral drugs and liver injury. AIDS 2008;22:1–13 [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–74 [DOI] [PubMed] [Google Scholar]
- 6.CDC Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after hiv exposures-worldwide, 1997–2000. Morb Mortal Wkly Rep 2001;49:1153–6 [PubMed] [Google Scholar]
- 7.Abrescia N, D’Abbraccio M, Figoni M, et al. Fulminant hepatic failure after the start of an efavirenz-based HAART regimen in a treatment-naive female AIDS patient without hepatitis virus co-infection. J Antimicrob Chemother 2002;50:763–5 [DOI] [PubMed] [Google Scholar]
- 8.Cotte L, Bénet T, Billioud C, et al. The role of nucleoside and nucleotide analogues in nodular regenerative hyperplasia in HIV-infected patients: a case control study. J Hepatol 2011;54:489–96 [DOI] [PubMed] [Google Scholar]
- 9.Kovari H, Ledergerber B, Peter U, et al. ; Swiss HIV Cohort Study Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis 2009;49:626–35 [DOI] [PubMed] [Google Scholar]
- 10.Hammond E, McKinnon E, Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: prevalence and metabolic consequences. Clin Infect Dis 2010;51:591–9 [DOI] [PubMed] [Google Scholar]
- 11.Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother 2009;64:910–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyle GJ, Sabin CA, Cartledge J, et al. ; RAVE (Randomized Abacavir versus Viread Evaluation) Group UK A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS 2006;20:2043–50 [DOI] [PubMed] [Google Scholar]
- 13.Grund B, Peng G, Gibert CL, et al. ; INSIGHT SMART Body Composition Substudy Group Continuous antiretroviral therapy decreases bone mineral density. AIDS 2009;23:1519–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapport yeni 2010, prise en charge des patients infectes par le vih, editions flammarion, 2010
- 15.Podcast for healthcare professionals: serious liver disorder associated with the use of videx/videx ec. FDA, 2010
- 16.Mallet VO, Varthaman A, Lasne D, et al. Acquired protein S deficiency leads to obliterative portal venopathy and to compensatory nodular regenerative hyperplasia in HIV-infected patients. AIDS 2009;23:1511–8 [DOI] [PubMed] [Google Scholar]
- 17.Schiano TD, Uriel A, Dieterich DT, et al. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch 2011;458:231–5 [DOI] [PubMed] [Google Scholar]
- 18.Steiner PE. Nodular regenerative hyperplasia of the liver. Am J Pathol 1959;35:943–53 [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmaenner D, Kovari H, Weber A, et al. Nodular regenerative hyperplasia of the liver associated with didanosine persists for years even after its interruption. BMJ Case Reports 2011; doi:10.1136/bcr.03.2011.3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bihl F, Janssens F, Boehlen F, et al. Anticoagulant therapy for nodular regenerative hyperplasia in a HIV-infected patient. BMC Gastroenterol 2010;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateo M, Sebagh M, Bralet MP, et al. A new indication for liver transplantation: nodular regenerative hyperplasia in human immunodeficiency virus-infected patients. Liver Transpl 2008;14:1194–8 [DOI] [PubMed] [Google Scholar]
- 22.Lucignani G, Orunesu E, Cesari M, et al. FDG-PET imaging in HIV-infected subjects: relation with therapy and immunovirological variables. Eur J Nucl Med Mol Imaging 2009;36:640–7 [DOI] [PubMed] [Google Scholar]
- 23.Brust D, Polis M, Davey R, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS 2006;20:985–93 [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos P, Mowat C, McMillan DC, et al. Is portal hypertension associated with protein-losing enteropathy? J Gastroenterol Hepatol 2005;20:103–7 [DOI] [PubMed] [Google Scholar]
- 25.Wong WM, Hui CK, Yuen MF, et al. Reversal of protein-losing enteropathy by liver transplantation. J Clin Gastroenterol 2003;36:86–7 [DOI] [PubMed] [Google Scholar]