Abstract
Majority of children with pandemic influenza A (H1N1)pdm09 experience mild illness with full recovery without treatment. A previously healthy two and a half month-old girl was admitted to our paediatric intensive care unit because of severe respiratory failure with A (H1N1)pdm09 infection. Despite initial clinical improvement all attempts to extubate to non-invasive ventilation were unsuccessful and 2 to 3 weeks after symptom onset she started periods of cardiovascular instability and a progressive neurological deterioration with distal symmetrical progressive motor weakness and areflexia. All investigations were normal except elevated liver enzymes and cerebrospinal fluid examination that revealed elevated protein without pleocytosis. A possible diagnosis of Guillain–Barré syndrome (GBS) was considered and electromyogram was compatible with axonal form of GBS. To our knowledge this is the youngest case of GBS acquired postnatally and the first in children associated with H1N1 virus.
Background
Guillain–Barré syndrome (GBS) has a low incidence of 0.5–2 cases per 100 000 children under 18 years old even though it is the most common cause for acute flaccid paralysis among children.1 2
The diagnosis of GBS is based primarily on the clinical evaluation and the exclusion of important possible alternative diagnoses. GBS is deemed to be an autoimmune disease, and infections as those caused by Campylobacter jejuni, cytomegalovirus (CMV), Epstein–Barr virus (EBV) and Mycoplasma pneumoniae infections are pointed out as important triggers.3 Meanwhile, all new data after 2009 A(H1N1)pdm09 pandemic point out that influenza virus may have an important but previously underestimated role as a triggering factor for GBS during major flu outbreaks.1 2
Case presentation
A previously healthy two-and-a-half-month-old baby girl with normal motor milestones achievements was referred to the emergency department with a 5 day history of feeding difficulties, lethargy and grunting, in November 2009. Pregnancy and delivery were uneventful. Pregnancy routine screening tests were all negative for HIV, syphilis, hepatitis B, toxoplasmosis and rubella. Parents were non-consanguineous and had two previous healthy girls. No family history of autoimmune diseases or other were reported. The baby’s report documented BCG and Hepatitis B vaccination during the first week of life. Neither the baby nor the mother was vaccinated with any flu vaccine.
She was admitted into the ward and intubation, fluid replacement and mechanical ventilation were necessary shortly after. Intravenous antibiotics were started for possible sepsis.
Three days after admission she was transferred to the paediatric intensive care unit (PICU) of our hospital, with the diagnosis of influenza A(H1N1)pdm09 infection and respiratory failure. Treatment with oseltamivir (3 mg/kg twice daily) was introduced.
During her stay in PICU she remained ventilator-dependent. Episodes of bradycardia requiring atropine were frequent after the first week.
After the third week we noticed progressive marked symmetric hypotonia, more severe distally, with absent deep tendon reflexes figuring a floppy infant with frog leg posture. Fasciculations were absent.
Cerebrospinal fluid (CSF) examination and motor nerve conduction studies showed increased protein content, but normal cell count. Motor nerve conduction studies done in the fourth week showed no motor responses of the median, ulnar, peroneal and tibialis posterior nerves, bilaterally.
These findings were suggestive of axonal GBS.
Investigations
Initial investigations before admission in the PICU
The chest x-ray showed bilateral interstitial infiltrates. Cerebral ultrasound and echocardiogram were normal; blood, urine and CSF cultures were sterile. Detection of flu-specific RNA by real-time reverse transcriptase-PCR on nasopharyngeal specimen was positive for A(H1N1)pdm09 virus. Initial laboratory evaluation showed increased liver enzymes (alanine transaminase (ALT) 151 IU/l; aspartate transaminase (AST) 161 IU/l and lactate dehydrogenase (LDH) 2311 U/l), which persisted elevated.
Investigations in the PICU
Serologic studies were negative for all the following: toxoplasmosis, hepatitis B and C, HIV, CMV, herpes simplex virus and EBV.
Fiberoptic bronchoscopy done during the second week excluded airway malacia or obstruction. In the third week, ECG and echocardiography were normal. Brain MRI and ophthalmic examination were also normal. Extensive investigation excluded inborn errors of metabolism and muscle biopsy revealed no alteration.
After onset of neurologic symptoms: CSF examination showed increased protein content (72 mg/dl), but normal cell count (2 leucocytes/mm3). Antiganglioside antibodies were negative in blood and CSF.
Motor nerve conduction studies done in the fourth week showed no motor responses of the median, ulnar, peroneal and tibialis posterior nerves, bilaterally. Antidromic sensory potential of the peroneal nerve and mixed nerve potential of the ulnar nerve were absent, on both sides. Concentric needle sampling (needle electromyogram (EMG)) of both tibialis anterior and first dorsal interosseus disclosed abundant fibrillation and sharp-waves (fibs-sw) with no motor unit recruitment, by stimulating the plantar region or the palm of the hand. Vastus medialis and biceps brachii had no spontaneous activity and limb stimulation showed the recruitment of a few motor units (MU) of normal morphology.
Treatment
After GBS diagnosis intravenous immunoglobulin (1 g/kg/day) was given for 2 days with no clinical improvement.
Outcome and follow-up
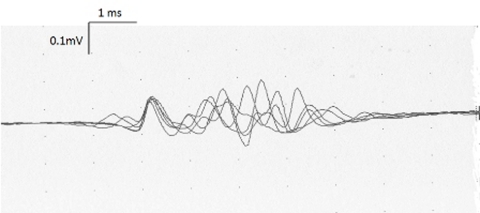
After the treatment with intravenous immunoglobulin a second electromyographic investigation was performed 6 weeks later and did not reveal any distal motor or sensory response. Needle EMG showed fibs-sw in proximal and distal muscles, a few MU typical of recent reinnervation were detected (figure 1).
Figure 1.
Small and polyphasic motor unit recorded in the right tibialis anterior showing a striking instability (500 Hz, high pass filter), typical of recent reinnervation.
Three extubation trials were unsuccessful and bilateral diaphragmatic paralysis was documented by ultrasound examination.
A tracheotomy was performed and the patient transferred to the ward dependent on mechanical ventilation after 2 months in the PICU.
She is at home in a rehabilitation programme but remains fully dependent on mechanical ventilation 2 years after GBS diagnosis.
Discussion
GBS is a peripheral polyneuroradiculopathy with acute onset.1 The clinical presentation of a rapidly progressive and symmetric flaccid tetraparesis, more severe distally, associated with absent deep tendon reflexes, following a viral infection, strongly suggests GBS.
Our patient’s CSF analysis confirmed the classical albumino-cytologic dissociation. Other features supportive for her GBS diagnosis are that there was no disease progression after the fourth week, absence of fever at the onset of neurological symptoms and presence of autonomic dysfunction (bradycardia).1
Antiganglioside antibodies were negative but they are described in only 14–50% of patients with GBS and have no diagnostic value.4
Obviously, the diagnosis of GBS, as the cause of the new onset flaccid paralysis in a 3-month-old baby in the PICU setting requires a high index of suspicion. This is our explanation for the 3 week interval between the first sign/symptom onset and diagnosis.
The main differential diagnosis concerns other forms of acute peripheral neuropathy, severe myopathy, spinal cord lesion or even neuromuscular junction disorders.1 5 The predominant distal weakness and the absence of urinary retention argue against severe myopathy and spinal cord lesion. Regarding other causes of peripheral neuropathy, toxic and metabolic causes were excluded by the history and laboratorial evaluation.
Normal MRI ruled out structural myelopathy or para-sagital cerebral lesion, which can present as acute painful flaccid paraparesis. MRI also excluded demyelinating disorders as acute transverse myelitis and acute disseminated encephalomyelitis. Biochemistry and normal muscle biopsy excluded disorders of muscle.
The lack of motor and sensory responses, and the observation of abundant and symmetric fibrillation and sharp-waves in distal muscles in the electrophysiologic studies supported the diagnosis of axonal GBS – acute motor and sensory axonal neuropathy.1 3 6 A distal conduction block could mimic this condition, but the severe outcome of this infant precludes this hypothesis. For all the above this case meets all three criteria (clinical, electrophysiologic and CSF) of case definition of GBS based on the Brighton Collaboration Criteria.7–12
One could question if GBS onset after a influenza A(H1N1)pdm09 infection is more than a coincidence.
The aetiology of the GBS still remains elusive. Nowadays the leading hypothesis for the pathogenesis of GBS and peripheral nerve damage is for a molecular mimicry, invoking an immune response to antigenic targets that are coincidentally shared by an infectious organism and nerve tissue.13–17 Inflammatory infiltrates, which contain T lymphocytes and macrophages, cause demyelination and subsequent axonal degeneration by direct cytotoxic effects and via release of inflammatory mediators, such as cytokines and nitric oxide.6 Significant infectious antecedent events include C jejuni (4–66%), CMV (5–15%), EBV (2–10%) and M pneumoniae (1–5%) infections.3 Although in 60% cases of GBS related to previous infection the specific organism remains unidentified.3 6 In our case no infection history, negative serologic findings and no previous vaccination ruled out other triggers for GBS except for A(H1N1)pdm09.
Anecdotal reports following influenza virus infection have been recently confirmed by analysis of the UK’s General Practice Research Database showing an association of flu illnesses with GBS.6 7
Grimaldi-Bensouda et al study also found that subjects with a recent history of influenza A/H1N1 infection showed higher odds of GBS, bringing A/H1N1 virus to the list of one of the potential inciting viral mechanism of GBS.7 Surveillance findings showed actually a 16-fold increased risk for GBS within 1 month of an flu-like illness when compared to flu vaccination.18 Whether the A(H1N1)pdm09 2009 pandemic was associated with an increased number of GBS cases is unknown.6 However, sporadic cases of GBS after H1N1 infection have been published previously in a 28-year-old white female who presented GBS – variant acute motor axonal neuropathy.19
In the UK, the GBS surveillance was found between September 2009 and August 2010, five children with GBS after H1N1 influenza (laboratory-confirmed).20 All these new data after 2009 H1N1 pandemic point out that influenza virus may have important but previously underestimated roles as triggering factors for GBS during major flu outbreaks.2
Treatment chosen for our patient was intravenous immunoglobulin taking into consideration her age and weight, however no clinical improvement was observed. Severity of the disease and requirement of artificial ventilation were clear adverse prognostic factors and her clinical course was with permanent neurologic disabilities, which is documented in nearly 20% of patients with GBS.7
Liver function disturbances (LFD) were found in the acute phase and persisted after it but this is also a finding described in GBS.21 The fact that the levels never normalised is not easily explained, almost 2 years after disease onset she still presents raised ALT (174 IU/l), AST (163 IU/l) and LDH (1621 U/l) with normal range alkalic phosphatase, bilirubin and γ glutamyl transferase. Abdominal ultrasound reveals normal liver and all additional investigations excluded Wilson disease, autoimmune and hepatotropic virus disease, α1-antitrypsin deficiency, peroxisomal disorders or lysosomal and glycogen storage disorders. Accordingly to Oomes et al study – Dutch Guillain–Barré study, they found an association between GBS and mild liver function disturbances without obvious cause and also that IgIV treatment originated mild transient liver function disturbances through an unknown mechanism.21 Same results were found in the prospective Massachusetts General Hospital series, where several patients with GBS had abnormal liver function tests without clinical or serologic evidence of hepatotropic virus disease.22 A plausible explanation for the presence of persistent LFD is that it can be from the ongoing muscle deterioration as well as from an immune-mediated dysregulation of the liver in parallel to the one ongoing in the peripheral nervous system.23
To our knowledge this is the youngest case of acquired-GBS as the previously reported baby was 4 months old.24 It is also the first case report associated with the pandemic H1N1 influenza virus in a child.7 25 26 We believe that these two facts are related. We hypothesise that the absence of maternal antibodies to the novel H1N1 influenza virus could explain the occurrence of GBS at such a young age.
Despite only limited experience, it is mandatory to perform lumbar puncture, electrophysiological studies and antiganglioside antibody assay in all critically ill children with the new onset of acute flaccid paralysis, especially if pandemic influenza A(H1N1)pdm09 or some other infection was the reason for PICU admission.
Learning points.
-
▶
GBS is the most common cause for acute flaccid paralysis among children.
-
▶
New data after 2009 A (H1N1)pdm09 pandemic point out that influenza virus may have an important role as a triggering factor for GBS.
-
▶
Lumbar puncture, electrophysiological studies and antiganglioside antibody assay should be performed in all critically ill children with the new onset of acute flaccid paralysis.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Agrawal S, Peake D, Whitehouse WP. Management of children with Guillain-Barré syndrome. Arch Dis Child Educ Pract Ed 2007;92:161–8 [DOI] [PubMed] [Google Scholar]
- 2.Winner SJ, Evans JG. Age-specific incidence of Guillain-Barré syndrome in Oxfordshire. Q J Med 1990;77:1297–304 [DOI] [PubMed] [Google Scholar]
- 3.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet 2005;366:1653–66 [DOI] [PubMed] [Google Scholar]
- 4.Hughes RA, Hadden RD, Gregson NA, et al. Pathogenesis of Guillain-Barré syndrome. J Neuroimmunol 1999;100:74–97 [DOI] [PubMed] [Google Scholar]
- 5.Randall DP. Guillain-Barré syndrome differential diagnosis. Disease-a-month: DM 2010;56:266–78 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20493304 (accessed January 2012). [DOI] [PubMed] [Google Scholar]
- 6.Lehmann HC, Hartung HP, Kieseier BC, et al. Guillain-Barré syndrome after exposure to influenza virus. The Lancet infectious diseases. 2010;10:643–51 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20797646 (accessed January 2012). [DOI] [PubMed] [Google Scholar]
- 7.Grimaldi-Bensouda L, Alpérovitch A, Besson G, et al. ; Lucien Abenhaim for the GBS-PGRx Study Group Guillain-Barre syndrome, influenzalike illnesses, and influenza vaccination during seasons with and without circulating A/H1N1 viruses. Am J Epidemiol 2011;174:326–35 [DOI] [PubMed] [Google Scholar]
- 8.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979;110:105–23 [DOI] [PubMed] [Google Scholar]
- 9.Sejvar JJ, Uyeki TM. Neurologic complications of 2009 influenza A (H1N1): heightened attention on an ongoing question. Neurology 2010;74:1020–1 [DOI] [PubMed] [Google Scholar]
- 10.The Brighton Collaboration. Immunize Safely Basel, Switzerland: Brighton Collaboration Foundation; 2010. (http://www.brightoncollaboration.org). (accessed 25 June 2010). [Google Scholar]
- 11.Asbury AK, Arnason BGW, Karp HR, et al. Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol 1978;3:565–6 [DOI] [PubMed] [Google Scholar]
- 12.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 1990;27:S21–4 [DOI] [PubMed] [Google Scholar]
- 13.Yuki N, Handa S, Taki T, et al. Cross-reactive antigen between nervous tissue and a bacterium elicits Guillain-Barré syndrome: molecular mimicry between gangliocide GM1 and lipopolysaccharide from Penner’s serotype 19 of Campylobacter jejuni. Biomed Res 1992;13:451–53 [Google Scholar]
- 14.Yuki N, Taki T, Takahashi M, et al. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher’s syndrome. Ann Neurol 1994;36:791–3 [DOI] [PubMed] [Google Scholar]
- 15.Aspinall GO, McDonald AG, Raju TS, et al. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem 1993;213:1017–27 [DOI] [PubMed] [Google Scholar]
- 16.Aspinall GO, Fujimoto S, McDonald AG, et al. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect Immun 1994;62:2122–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neisser A, Bernheimer H, Berger T, et al. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect Immun 1997;65:4038–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowe J, Andrews N, Wise L, et al. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol 2009;169:382–8 [DOI] [PubMed] [Google Scholar]
- 19.Kutlesa M, Santini M, Krajinović V, et al. Acute motor axonal neuropathy associated with pandemic H1N1 influenza A infection. Neurocritical Care 2010;13:98–100 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20428968 (accessed January 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verity C, Stellitano L, Winstone AM, et al. Guillain-Barré syndrome and H1N1 influenza vaccine in UK children. Lancet 2011;378:1545–6 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22035553 (accessed January 2012). [DOI] [PubMed] [Google Scholar]
- 21.Oomes PG, van der Meché FG, Kleyweg RP. Liver function disturbances in Guillain-Barré syndrome: a prospective longitudinal study in 100 patients. Dutch Guillain-Barré Study Group. Neurology 1996;46:96–100 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8559429 (accessed January 2012). [DOI] [PubMed] [Google Scholar]
- 22.Ropper AH, Wijdicks EFM, Truax BT. Guillain-Barre syndrome. In: Contemporary Neurology Series. Vol. 34 Philadelphia, PA: FA Davis; 1991 [Google Scholar]
- 23.van Doorn PA, Brand A, Vermeulen M. Clinical significance of antibodies against peripheral nerve tissue in inflammatory polyneuropathy. Neurology 1987;37:1798–802 [DOI] [PubMed] [Google Scholar]
- 24.Smith SA, Ouvrier R. Peripheral neuropathies in children. In: Swaiman KF, Ashwal S, eds. Paediatric Neurology Principles and Practice. Third edition St Louis: Mosby Inc; 1999 [Google Scholar]
- 25.CDC. Neurologic complications associated with novel influenza A (H1N1) virus infection in children – Dallas, Texas, May 2009. MMWR 2009;58:773–8 [PubMed] [Google Scholar]
- 26.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010;38(4 Suppl):e91–7 [DOI] [PubMed] [Google Scholar]