Abstract
We investigated the role of the chondrocyte primary cilium in mechanotransduction events related to cartilage extracellular matrix synthesis. We generated conditionally immortalized wild-type (WT) and IFT88orpk (ORPK) mutant chondrocytes that lack primary cilia and assessed intracellular Ca2+ signaling, extracellular matrix synthesis, and ATP release in response to physiologically relevant compressive strains in a 3-dimensional chondrocyte culture system. All conditions were compared to unloaded controls. We found that cilia were required for compression-induced Ca2+ signaling mediated by ATP release, and an associated up-regulation of aggrecan mRNA and sulfated glycosaminosglycan secretion. However, chondrocyte cilia were not the initial mechanoreceptors, since both WT and ORPK cells showed mechanically induced ATP release. Rather, we found that primary cilia were required for downstream ATP reception, since ORPK cells did not elicit a Ca2+ response to exogenous ATP even though WT and ORPK cells express similar levels of purine receptors. We suggest that purinergic Ca2+ signaling may be regulated by polycystin-1, since ORPK cells only expressed the C-terminal tail. This is the first study to demonstrate that primary cilia are essential organelles for cartilage mechanotransduction, as well as identifying a novel role for primary cilia not previously reported in any other cell type, namely cilia-mediated control of ATP reception.—Wann, A. K. T., Zuo, N., Haycraft, C. J., Jensen, C. G., Poole, C. A., McGlashan, S. R., Knight, M. M. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes.
Keywords: cartilage, extracellular matrix, polycystin-1, polycystin-2, purine receptors
Physiological joint loading and the associated compression of articular cartilage produces a number of interrelated physicochemical events that influence both chondrocyte metabolism and extracellular matrix (ECM) synthesis (1, 2). However, the process of chondrocyte mechanotransduction is only partially understood (3–7). A prime candidate for cellular mechanosensation is the primary cilium, an organelle present on nearly all eukaryotic cell types, including chondrocytes (8, 9). It consists of a membrane-coated axoneme that projects from the cell surface into the extracellular microenvironment and an intracellular basal body (9–11). Assembly and maintenance of the cilium depend on the bidirectional intraflagellar transport (IFT) system (12). Disruption of genes involved in IFT, such as IFT88 and Kif3a, causes defective ciliogenesis, which results in a wide range of developmental and postnatal abnormalities (13–15). In cartilage, primary cilia are essential for regulation of joint development such that loss of cilia results in skeletal patterning abnormalities, disruption of cell polarity and organization, and ECM defects (16, 17).
Primary cilia are known to function as mechanosensory organelles in several cell types, in particular those subjected to fluid flow, such as cholangiocytes, vascular endothelium, and renal epithelia (18–23). We hypothesize that the primary cilium is also involved in chondrocyte mechanosensation in response to compressive loading. Chondrocyte cilia are ideally placed to sense associated deformations since they are structurally associated with collagen fibers, express collagen anchoring receptors including integrins, and are mechanically deflected by interactions with the ECM (24, 25). Although several studies have examined the role of primary cilia as flow sensors, no studies have examined the role of the cilium in response to other types of physiologically relevant mechanical loads. Here we present data on postnatal chondrocyte primary cilia and show that they are essential for the transduction of mechanically regulated signals in response to compression. Furthermore, we show that this occurs via a novel cilia mechanotransduction pathway involving modulation of ATP-induced Ca2+ signaling via purine receptors.
MATERIALS AND METHODS
Antibodies and fluorescent probes
The study used the following antibodies: P2X2, P2X4, P2X7, P2Y1, and P2Y2 (Alomone Laboratories, Jerusalem, Israel); acetylated α-tubulin (clone 6-11B-1, Sigma, Auckland, New Zealand; and C3B9, gift from T. Sherwin, University of Auckland); large T-antigen SV40 (BD Biosciences, Auckland, New Zealand); polycystin-1 (PC1; H-260) and polycystin-2 (PC2; H-280; Santa Cruz Biotechnologies, Santa Cruz, CA, USA); goat anti-rabbit Dylight 488 and goat anti-mouse Dylight 594 (Jackson ImmunoResearch, West Grove, PA, USA); and sheep anti-rabbit horseradish peroxidase (Chemicon, Auckland, New Zealand). Probes: Hoescht 1:500 (Sigma); Fluo-4 AM, calcein AM, and ethidium homodimer-2 (Invitrogen, Auckland, New Zealand).
Generation of ciliated and nonciliated chondrocyte cell lines
Heterozygous Tg737ORPK mutant mouse lines were generated as described previously (26, 27). Mice were maintained on a mixed genetic background according to approved protocols at the Medical University of South Carolina. Heterozygous Oak Ridge polycystic kidney (ORPK) mice were bred with heterozygous Immortomouse mice (H-2Kb-tsA58), which harbor a temperature-sensitive SV40 large T-antigen transgene under the control of an interferon-γ-inducible H-2Kb promoter (H-2Kb-tsA58) to produce orpk/Immortomouse compound heterozygous mice (28). Heterozygous ORPK females were bred with heterozygous/Immortomouse orpk males. Chondrocytes were isolated from the sterna of 4-d-old mice by digestion with collagenase type II (2 mg/ml) at 37°C for 4 h. All mice were genotyped by PCR from tail biopsy DNA. Western blot analysis was conducted to confirm the expression of SV40 large T antigen in chondrocytes in the presence of IFN-γ at 33°C.
Chondrocytes were cultured in DMEM supplemented with 10% FCS, 88 U/ml penicillin, 90 μg/ml streptomycin, 10 ng/ml INF-γ, and 2.5 mM l-glutamine (Invitrogen). Immortalized cells were grown to 90% confluence in 5% CO2 at 33°C with 10 nM IFN-γ, then cultured in nonpermissive conditions at 37°C (without IFN-γ) for 5 d before seeding in 5- × 5-mm cylindrical agarose gels (4). Cell-agarose constructs were cultured for up to 24 h before loading experiments. Cell viability was assessed by calcein AM/ethidium homodimer-2 labeling and fluorescence microscopy.
Compressive loading
To analyze the effect of mechanical loading on ECM synthesis and ATP release, we used a loading rig mounted within a cell culture incubator at 37°C in 5% CO2, driven by a Bose mechanical compressive strain system (Bose Corp., Eden Prairie, MN, USA). This system has proven to be highly accurate and repeatable, as indicated by load values measured from cells beneath the 24-well plates. Each construct was hydrated in culture medium and loaded within individual wells of a 24-well plate. Control constructs were also mounted in the loading rig but without application of cyclic compression. Samples were allowed to rest for 1 h under pins before loading commenced. Loading was conducted in a sinusoidal fashion with a peak compressive strain of 15% at 1 Hz (29–31). To examine mechanically induced Ca2+ signaling, a separate microscope-mounted loading system was used, as described previously (32, 33).
Total sGAG and DNA quantification
sGAG concentration was assessed within both constructs and culture medium following a 24-h period of cyclic compression. Constructs were digested, and the total sGAG content was quantified using the 1,9-dimethylmethylene blue (DMB; Sigma, Poole, UK) assay (34). To assess cell proliferation, DNA was quantified using Hoechst 33258 fluorescence as described previously (4).
Gene expression
Expression of two key cartilage-specific ECM molecules, aggrecan and collagen II mRNA, was examined in unloaded cell-agarose constructs and after 1 h cyclic loading. Constructs were placed in QG buffer (Qiagen, Crawley, UK) and frozen in liquid N2. RNA extraction from agarose constructs was adapted from the Qiagen RNeasy minikit. Genomic DNA cleanup with a DNase kit (Sigma, Poole, UK) was conducted, and reverse transcription reactions were carried out using products from Sigma. Primer sequences were synthesized by Sigma: collagen IIa, forward 5′-GGCAACAGCAGGTTCACATA and reverse 5′-ATGGGTGCGATGTCAATAAT; aggrecan, forward 5′-CACGCTACACCCTGGACTTTG and reverse 5′-CCATCTCCTCAGCGAAGCAGT; GAPDH, forward 5′-GACAAAATGGTGAAGGTCGG and reverse 5′-TCCACGACATACTCAGCACC. For qPCR, a fast 2-step SYBR kit (KAPA Biosystems, Boston, MA, USA) was used with a reference dye, ROX. Fluorescence data, normalized to ROX, were collected during annealing and analyzed using MxPro QPCR 3.0 software (Stratagene, La Jolla, CA, USA). Cycle threshold (Ct) values for each triplicate reaction were averaged. Relative quantification of aggrecan and collagen type II was normalized to GAPDH and to the mean of calibrator (control) samples from the same preparation by a comparative Ct approach. Expression ratios were expressed as log2.
Extracellular ATP measurements
Following a 1-h period of cyclic mechanical loading of chondrocyte-agarose constructs, the medium was removed and kept on ice for immediate ATP measurement, using a luciferase ATP assay kit (Sigma, Poole, UK) and a luminescence plate reader (35).
Intracellular calcium signaling
Chondrocyte-agarose constructs were labeled with 5 μM Fluo-4 AM plus 0.1% Pluronic for 1 h at room temperature, followed by a 30-min deesterification period. Constructs were mounted in the compression rig and visualized using confocal fluorescence microscopy. In a separate set of experiments, chondrocyte-agarose constructs were exposed to either 1 or 100 μM ATP (Sigma, Auckland, New Zealand). Images were acquired every 6 s over a 5-min period of either compressive loading (20% strain) or ATP exposure. A subset of constructs was also incubated with 10 μM suramin (Sigma) for 30 min prior to imaging. Control constructs remained unstrained or untreated. The mean fluorescent intensity within individual cells was recorded and plotted against time. Characteristic Ca2+ transients were manually identified as in previous studies (32, 33, 36).
Western blotting
Expression of SV40, P2 receptors, PC1, and PC2 was determined by Western blotting. Monolayer chondrocytes were extracted using RIPA buffer containing protease inhibitors (Roche, Auckland New Zealand) for 1 h at 4°C. Total protein was measured using the Bio-Rad DC Protein Assay (Bio-Rad, Gladesville, NSW, Australia). Supernatants were heated to 70°C for 10 min, and protein was separated on NuPAGE 4–12% gels (Invitrogen) or 3–8% Tris-bis gels and transferred onto PVDF membranes (Hybond-P; Amersham Biosciences, Little Chalfont, UK). Membranes were blocked with 5% nonfat milk powder for 1 h at room temperature, then primary antibody overnight at 4°C, followed by a horseradish peroxidase secondary for 2 h at room temperature. Peroxidase activity was visualized using enhanced chemiluminescence (ECL Plus; Amersham Biosciences). Values were normalized to GAPDH.
Immunofluorescence and confocal microscopy
Chondrocyte-agarose constructs were fixed in 3.7% (w/v) paraformaldehyde and processed in paraffin wax, as described previously (37). Sections (12 μm thick) underwent microwave antigen retrieval, followed by dual immunofluorescent labeling with antibodies against either γ-tubulin or PC1 or PC2 and acetylated α-tubulin, as described previously (37). Sections were imaged using a Leica TCS SP2 confocal microscope using a ×63, 1.3-NA lens (Leica Microsystems, Wetzlar, Germany; ref. 37). Monolayer cells grown on glass coverslips were fixed and stained in a similar manner.
Statistics
Statistical analyses involved Student's t tests, Mann-Whitney U tests, or analysis of variance (ANOVA) to examine the differences between two or more groups, respectively. For Ca2+ studies, χ2 analysis was used to compare proportions of responding cells in each treatment group. In all cases, 2-tailed tests were employed, with statistically significant differences indicated at P < 0.05, P < 0.01, and P < 0.001. In the figures, asterisks (*) are employed to note significance for tests indicating the influence of loading, and plus symbols (+) are employed for comparisons between wild-type (WT) and ORPK groups.
RESULTS
Characterization of WT and ORPK transgenic chondrocytes
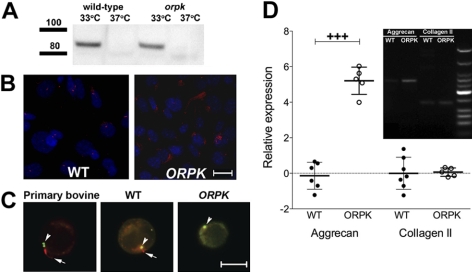
Western blots from conditionally immortalized chondrocyte lysates confirmed that under permissive conditions (IFN-γ, 33°C), WT and ORPK cells expressed the SV40 large T antigen, which was no longer detectable when cells were switched to nonpermissive conditions (37°C) for 3 d (Fig. 1A). In monolayer culture, 85 ± 5% of WT cells expressed primary cilia, which was reduced to 20 ± 8% when cells were cultured in agarose constructs for 24 h. In both monolayer and agarose cultures, the hypomorphic mutation of IFT88 in ORPK chondrocytes caused disruption of ciliogenesis, resulting in disorganized cytoplasmic α-acetylated tubulin and stunted or absent cilia (Fig. 1B, C). Both WT and ORPK cells showed a rounded morphology, similar to primary bovine chondrocytes, in 3-dimensional (3D) culture (Fig. 1C), and viability was >80% during all experiments. No statistically significant difference was found in chondrocyte proliferation between WT and ORPK cells cultured for 24 h in agarose, as determined by DNA quantification.
Figure 1.
Deletion of IFT88 results in loss of primary cilia expression in ORPK chondrocytes but does not affect expression of cartilage-specific matrix molecules aggrecan and collagen type II. A) Western blot shows loss of SV40 at 37°C in the absence of INF-γ after 5 d in nonpermissive conditions. B) Acetylated α-tubulin labeling of primary cilia (red) in WT monolayer chondrocytes. Stunted or no cilia were detected in ORPK cells. Nuclei (blue) are labeled with DAPI. C) Acetylated α-tubulin labeling of primary cilia of primary bovine and WT mouse chondrocytes in 3D agarose constructs (red; arrows) and γ-tubulin labeling of centrioles (green; arrowheads). Cilia were either stunted or absent in ORPK cells (red; arrow). D) RT-PCR (gel inset) and real-time qPCR (graph) show aggrecan and collagen type II gene expression in both WT and ORPK cells. ORPK aggrecan mRNA was significantly up-regulated compared to WT cells. Relative expression ratios were logged to the base 2 and plotted as individual points. Error bars = sd. Scale bars = 10 μm. +++P < 0.001.
Chondrocyte phenotype, for both WT and ORPK cells, was confirmed by expression of aggrecan and collagen type II mRNA, measured by RT-PCR (Fig. 1D). No significant difference was found in collagen type II expression between WT and ORPK cells, whereas aggrecan expression was 5.2-fold greater in ORPK cells relative to WT (P<0.001; Fig. 1D). In contrast, sGAG synthesis was reduced by 33% in ORPK cells (see Fig. 2B) compared with WT cells (P<0.001).
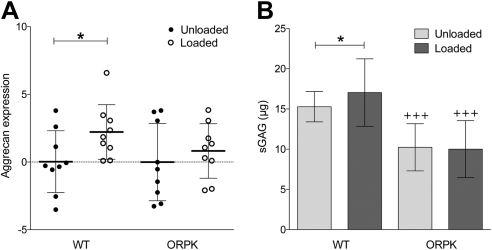
Figure 2.
Primary cilia are required for mechanosensitive up-regulation of proteoglycan synthesis. Cyclic compression (1Hz, 0–15%) significantly up-regulates aggrecan gene expression (A) and sGAG synthesis (B) in WT but not ORPK chondrocytes. mRNA was quantified after 1 h loading by real-time qPCR and normalized to unloaded controls. sGAG synthesis was measured after 24 h of loading. Bars represent mean ± sd values (n=36 constructs). Experiments were repeated 3 times. *P < 0.05. +++P < 0.001 vs. corresponding WT.
Primary cilia are required for mechanically induced up-regulation of matrix synthesis
Following 1 h of cyclic compression, qPCR analysis showed that aggrecan mRNA expression in WT cells significantly increased by 2.2 fold (P<0.05), whereas no increase was found in ORPK cells (Fig. 2A). No significant change was found in collagen type II expression, such that mechanical compression produced mean fold changes of 0.76 and −0.66 in WT and ORPK chondrocytes, respectively. In WT cells, cyclic compression induced a significant 12% increase in sGAG synthesis (P<0.05; Fig. 2B). This response was completely abolished in ORPK cells, indicating that cilia are required for mechanosensitive proteoglycan synthesis (Fig. 2B). No significant difference was found in cell proliferation, as indicated by negligible changes in DNA content with loading of 0.5 and 0.7% in WT and ORPK chondrocytes, respectively.
Mechanical loading activates ATP release in both WT and ORPK chondrocytes
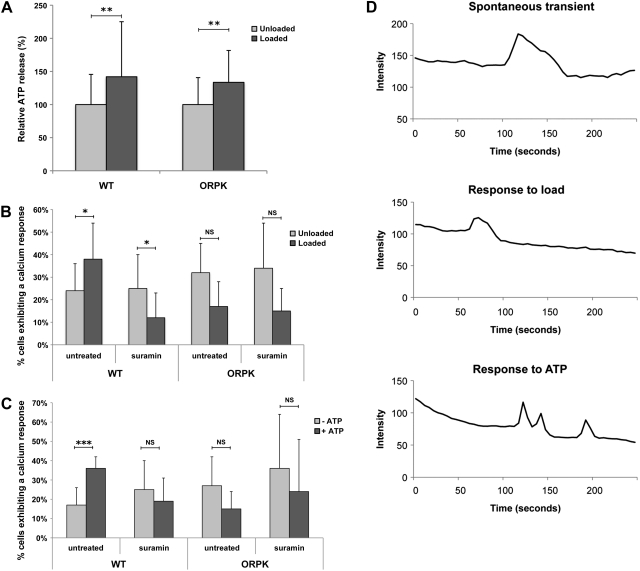
Our previous studies have shown that primary bovine chondrocytes release ATP through unopposed connexin hemichannels when subjected to compressive loading (35), which, in turn, triggers mechanically induced up-regulation of proteoglycan synthesis (3). Therefore, we next examined whether this response was cilia dependent by measuring cumulative ATP release over a 1-h period from agarose constructs seeded with either WT or ORPK chondrocytes. Mean ATP release into the medium for unloaded WT and ORPK cells was 4.63 ± 0.65 and 3.15 ± 0.26 nM/construct, respectively, with no significant difference between the two cell types. Compressive loading induced statistically significant increases in ATP release in both WT and ORPK cells (P<0.01). No statistically significant difference was found in the magnitude of the increase between WT and ORPK cells, with mean increases of 42 and 34%, respectively. This finding suggests that the mechanosensitive ATP release mechanism was not affected by loss of cilia (Fig. 3A).
Figure 3.
Primary cilia are not required for mechanosensitive ATP release but are required for subsequent ATP-induced Ca2+ signaling. A) One hour of cyclic compression (1 Hz, 0–15%) up-regulates ATP release in both WT and ORPK chondrocytes (n=36 constructs), B) Compression induces a significant increase in the percentage of cells exhibiting Ca2+ transients in WT cells but not in ORPK cells (n=6 from a total of 150 cells/group). Mechanically induced Ca2+ signaling was inhibited by the P2 receptor antagonist suramin in WT cells, indicating that the Ca2+ response is due to mechanosensitive ATP release. C) WT cells responded to exogenous application of 100 μM ATP with increased Ca2+ signaling, whereas ORPK cells were completely unresponsive (n=3 from a total of 150 cells/group). D) Three representative Ca2+ traces from an unstimulated WT cell and cells exposed to either load or ATP. Transients in response to load or ATP were significantly shorter, with reduced rise and fall times when compared to spontaneous transients. Bars represent means ± sd. NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Mechanically induced Ca2+ signaling is abolished in ORPK cells
Previous studies have shown that chondrocytes exhibit mechanosensitive Ca2+ signaling (32, 33), which is mediated by ATP release (36). We therefore examined whether these events involved the primary cilium. In unloaded agarose constructs, spontaneous intracellular Ca2+ transients occurred in 15–25% of both WT and ORPK cells, with no significant difference between the two cell types. Compressive loading significantly increased the percentage of WT cells exhibiting Ca2+ transients to 30–40%, (P<0.05; Fig. 3B). However, the number of Ca2+ transients in ORPK loaded cells was significantly lower than both unloaded ORPK controls (P<0.05) and WT loaded cells (P<0.001; Fig. 3B). Temporally, transients observed following load were significantly shorter with a reduction in both transient rise and fall time. These data indicate that cilia are essential for mechanosensitive activation of Ca2+ in response to compression.
Loss of ATP-mediated Ca2+ signaling in ORPK cells
Given that both ciliated and nonciliated cells release ATP in response to load, we then investigated whether the Ca2+ response to load was mediated by ATP release and subsequent P2 receptor activation by subjecting WT and ORPK cells to compression in the presence of suramin, a nonspecific P2 receptor antagonist. We found that suramin reversed the mechanosensitive Ca2+ response in WT cells (P<0.05) but had no significant effect on ORPK cells (Fig. 3B). We next examined whether WT and ORPK could elicit purinergic-induced Ca2+ signaling and found that neither cell type showed any response to the addition of 1 μM ATP. However, WT cells showed a robust Ca2+ response to exogenous 100 μM ATP, which was inhibited by suramin. However, ORPK cells did not respond to 100 μM ATP (Fig. 3C), which suggests that loss of chondrocyte primary cilia prevents P2 receptor-activated Ca2+ signaling.
ORPK cells show normal P2 receptor expression but disrupted polycystin expression
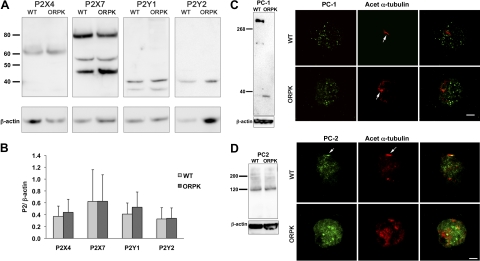
To investigate the abnormal purine reception in ORPK cells, we examined the expression profiles of human and bovine P2 receptors in chondrocytes (38, 39). Western blots showed that both WT and ORPK expressed similar levels of P2X4, P2X7, P2Y1 and P2Y2 (Fig. 4A, B). P2X2 was also examined but was not detectable in either cell type. To investigate the role of the cilium in P2-mediated calcium signaling further, we also examined the expression profiles of PC1 and PC2, which have been linked functionally to purinergic Ca2+ signaling (40). Western blots showed that WT cells expressed the full-length PC1 protein at ∼350 kDa, which was completely absent in ORPK cells. In contrast, ORPK cells expressed a smaller band at ∼35 kDa, most likely to represent the C-terminal tail (Fig. 4C). Immunofluorescence showed that PC1 was located on the cell membrane and within the cytoplasm as puncta in both WT and ORPK chondrocytes (Fig. 4C). However, unlike in epithelial cells, PC1 was not detected on WT primary cilia. This was verified in both monolayer and 3D cultures. Western blots showed that PC2 was expressed in WT and ORPK cells at similar levels (Fig. 4D) and was located on the cell membrane and throughout the cytoplasm in both cell types and on primary cilia of WT cells.
Figure 4.
P2, PC1, and PC2 expression in WT and ORPK cells. A) Western blots of P2 receptors, P2X4, P2X7, P2Y1, and P2Y2. B) Quantification of P2 receptor expression reveals no significant differences between WT and ORPK chondrocytes. Data were normalized to β-actin. Bars represent means ± sd (n=3). C) Western blot shows full-length PC1 in WT cells, but only a small (∼35 kDa) cleaved product is present in ORPK cells. Confocal extended focus images show that PC1 (green) is present as small bright puncta within the cystoplasm and on the cell membrane in both WT and ORPK cells and is not detectable on any WT primary cilia (arrow). D) Western blot shows that PC2 expression is unaffected by ORPK mutation. Immunofluorescent labeling of PC2 (green) shows expression throughout the cell and localization to the primary cilium in WT cells (yellow). In ORPK cells, PC1 and PC2 were detected within the cytoplasm only. In both cases, primary cilia were labeled with anti-acetylated α-tubulin (arrow, red).
DISCUSSION
A wide variety of cell types across evolution exhibit mechanosensitive behavior, and increasing evidence indicates that primary cilia are key mechanosensory organelles (41). In articular cartilage, mechanical loading is essential for development, health, and homeostasis through the control of ECM synthesis and catabolism. Here we show that the chondrocyte primary cilium is a fundamental part of this mechanotransduction mechanism, regulating matrix synthesis in response to the physiological compressive strain.
Chondrocytes derived from Tg737/IFT88 (ORPK) mice provided an appropriate model to investigate the role of chondrocyte primary cilia in response to compressive loading. These chondrocytes exhibit stunted or no cilia, with elevated and disorganized levels of acetylated α-tubulin, as seen in situ (16, 42). By contrast, WT cells exhibited primary cilia with a frequency of 20% in agarose (37). The 3D agarose culture system maintained chondrocytic phenotype in a rounded morphology with expression of aggrecan and collagen type II. Interestingly, the ORPK cells showed a significantly greater level of aggrecan gene expression compared to WT cells, but this was not translated into an increase in sGAG synthesis, which was reduced compared to WT cells. It is unclear how loss of IFT should down-regulate proteoglycan synthesis, although previous studies report a similar loss of cartilage proteoglycan content in Bardet-Biedl syndrome mice that have reduced cilia expression (43). In addition, given the known tubulin defects within ORPK cells (16, 42, 44), it may be due to defects in secretion via the tubulin network (45). The agarose model also enabled the application of cellular compressive strain in the physiological range reported for articular chondrocytes (46, 47). Due to the absence of a fully developed negatively charged ECM in this model, it is unlikely that compression induced changes in osmotic pressure, electrical streaming potentials, or pH, all of which have been linked to cartilage mechanotransduction (1, 2). Similarly, the agarose model does not include the direct linkage between the primary cilium and the pericellular matrix, as shown in situ where integrins are expressed on the cilium (25). Nevertheless, gross cyclic compressive loading, 1 Hz and 0–15% strain, was shown here to stimulate synthesis of proteoglycan in WT cells (Fig. 2B), in agreement with numerous previous studies using primary bovine chondrocytes (3, 4, 30). This response was completely abrogated in ORPK cells, indicating that the primary cilium is required for chondrocyte mechanotransduction.
Using identical cyclic loading of primary chondrocytes, we, and others, have shown that the mechanosensitive stimulation of proteoglycan synthesis is mediated by mechanically induced release of ATP (3, 5, 35, 48). ATP release is an early mechanotransduction event, whereby ATP binds to P2 receptors activating intracellular Ca2+ signaling (36). When ATP is not utilized by the cell, it is quickly hydrolyzed to pyrophosphate (48). In the present study, we also show mechanically induced ATP release (Fig. 3A). This purinergic-Ca2+ mechanotransduction pathway is similar to that reported for kidney epithelial cells subjected to fluid shear, where the ATP release is mediated by deflection of the primary cilium (22, 49, 50). However, in contrast to epithelial cells, the present study shows that mechanosensitive release of ATP occurs to a similar extent in both WT and ORPK chondrocytes, suggesting that primary cilia are not required for this part of the chondrocyte mechanotransduction pathway. It is clear that ATP-mediated Ca2+ signaling occurs within seconds of applied loading; however, the lack of sensitivity of the ATP assay required measurements to be made from medium collected after a 1-h period to allow for cumulative release and diffusion. Therefore, the local concentrations of pericellular ATP in response to load are likely to be much greater that the nanomolar concentrations measured in the surrounding media. This is further supported by the finding that Ca2+ signaling in WT cells was activated by 100 μM ATP but 1 μM ATP had no effect.
Primary cilia were necessary, however, to respond to extracellular ATP. Either compression or addition of 100 μM ATP activated Ca2+ signaling in WT but not in ORPK chondrocytes. The role of the calcium and the cilium in chondrocytes is in contrast to studies in bone cells, where the cilium is critical to mechanotransduction independently of intracellular Ca2+ (51). The involvement of P2 receptors was confirmed by treatment with the P2 receptor inhibitor suramin, which abolished the Ca2+ signaling responses to both compression and ATP in WT cells (Fig. 3B, C). Indeed, both apyrase and suramin have previously been shown to abolish downstream mechanically induced proteoglycan synthesis in chondrocyte-agarose constructs (35). The characteristics of both load-induced and ATP-induced Ca2+ transients were similar to those reported in previous studies (36). Furthermore, the nature of the load-induced transients has been shown previously to involve both extracellular and intracellular sources of Ca2+ (36). This finding suggests involvement of both P2X and P2Y receptors, which facilitate extracellular Ca2+ entry and release of intracellular Ca2+ stores, respectively. However, considerable crosstalk occurs between Ca2+ pathways with the involvement of mechanisms such as calcium-induced calcium release and store-operated calcium channels. Interestingly, WT and ORPK cells expressed the range of P2 receptors (P2X4, P2X7, P2Y1, and P2Y2) found in cartilage (33). Taken together, these data suggest that the primary cilium is necessary for ATP reception but does not modulate the expression of these receptors.
In other cell types, heterologous expression of the cilia protein PC1 in both its full and cleaved forms has also been found to influence Ca2+ release (52–55), including ATP-induced Ca2+ signaling (40). Moreover, PC1 and PC2 are thought to interact and reciprocally influence cellular and ciliary localization (56, 57). In the present study, we show that while ATP receptors are unaffected, full-size PC1 expression is disrupted in ORPK cells, leaving only a C-terminal cleavage product. Although not examined in this study, this finding may explain the lack of response to ATP, which is of additional significance given the roles for the polycystins in mechanostransduction and cilia-mediated Ca2+ signaling in other cells types (52, 58, 59).
The present study therefore demonstrates for the first time that primary cilia are essential for chondrocyte mechanotransduction and the control of ECM secretion in response to physiological compressive strain. In particular, the chondrocyte primary cilium is required for ATP-induced Ca2+ signaling. Thus, in summary, although the mechanosensory role of this highly conserved organelle appears to be ubiquitous, the signaling pathway differs significantly from that found in epithelial cells and osteoblasts exposed to pure fluid shear. This finding suggests that primary cilia mechanotransduction is specialized to the tissue-specific biomechanical environment.
Acknowledgments
This work was supported by a Wellcome Trust project grant (A.W. and N.Z.). Additional support was provided by a Royal Society of New Zealand Marsden Fast Start grant, Arthritis New Zealand, and the Auckland Medical Research Foundation (N.Z., S.R.M., and C.A.P.), and by the U.S. National Institutes of Health, National Center for Research Resources (NIH/NCRR 5P20RR017696; C.J.H.). The authors are grateful to Devina Jethwa (Queen Mary University of London, London, UK) for support in analyzing ATP-induced calcium transients.
Footnotes
- ECM
- extracellular matrix
- IFT
- intraflagellar transport
- ORPK
- Oak Ridge polycystic kidney
- PC1
- polycystin-1
- PC2
- polycystin-2
- WT
- wild-type.
REFERENCES
- 1. Urban J. P. (1994) The chondrocyte: a cell under pressure. Br. J. Rheumatol. 33, 901–908 [DOI] [PubMed] [Google Scholar]
- 2. Grodzinsky A. J., Levenston M. E., Jin M., Frank E. H. (2000) Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2, 691–713 [DOI] [PubMed] [Google Scholar]
- 3. Chowdhury T. T., Knight M. M. (2006) Purinergic pathway suppresses the release of. NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J. Cell. Physiol. 209, 845–853 [DOI] [PubMed] [Google Scholar]
- 4. Lee D. A., Bader D. L. (1997) Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J. Orthop. Res. 15, 181–188 [DOI] [PubMed] [Google Scholar]
- 5. Millward-Sadler S. J., Wright M. O., Flatman P. W., Salter D. M. (2004) ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 41, 567–575 [PubMed] [Google Scholar]
- 6. Palmer G. D., Chao Ph P. H., Raia F., Mauck R. L., Valhmu W. B., Hung C. T. (2001) Time-dependent aggrecan gene expression of articular chondrocytes in response to hyperosmotic loading. Osteoarthritis Cartil. 9, 761–770 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter J. A., Martin J. A., Brown T. D. (2006) Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43, 603–609 [PubMed] [Google Scholar]
- 8. Wheatley D. N., Bowser S. S. (2000) Length control of primary cilia: analysis of monociliate and multiciliate PtK1 cells. Biol. Cell 92, 573–582 [DOI] [PubMed] [Google Scholar]
- 9. Poole C. A., Flint M. H., Beaumont B. W. (1985) Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 5, 175–193 [DOI] [PubMed] [Google Scholar]
- 10. Poole C. A., Jensen C. G., Snyder J. A., Gray C. G., Hermanutz V. L., Wheatley D. N. (1997) Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol. Int. 21, 483–494 [DOI] [PubMed] [Google Scholar]
- 11. Poole C. A., Zhang Z. J., Ross J. M. (2001) The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J. Anat. 199, 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedersen L. B., Veland I. R., Schroder J. M., Christensen S. T. (2008) Assembly of primary cilia. Dev. Dyn. 237, 1993–2006 [DOI] [PubMed] [Google Scholar]
- 13. Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murcia N. S., Richards W. G., Yoder B. K., Mucenski M. L., Dunlap J. R., Woychik R. P. (2000) The Oak Ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 15. Yoder B. K., Tousson A., Millican L., Wu J. H., Bugg C. E., Jr., Schafer J. A., Balkovetz D. F. (2002) Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Renal Physiol. 282, F541–F552 [DOI] [PubMed] [Google Scholar]
- 16. McGlashan S. R., Haycraft C. J., Jensen C. G., Yoder B. K., Poole C. A. (2007) Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 26, 234–246 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Q., Murcia N. S., Chittenden L. R., Richards W. G., Michaud E. J., Woychik R. P., Yoder B. K. (2003) Loss of the Tg737 protein results in skeletal patterning defects. Dev. Dyn. 227, 78–90 [DOI] [PubMed] [Google Scholar]
- 18. Liu W., Murcia N. S., Duan Y., Weinbaum S., Yoder B. K., Schwiebert E., Satlin L. M. (2005) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am. J. Physiol. Renal Physiol. 289, F978–F988 [DOI] [PubMed] [Google Scholar]
- 19. Wang S., Zhang J., Nauli S. M., Li X., Starremans P. G., Luo Y., Roberts K. A., Zhou J. (2007) Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell. Biol. 27, 3241–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nauli S. M., Kawanabe Y., Kaminski J. J., Pearce W. J., Ingber D. E., Zhou J. (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iomini C., Tejada K., Mo W., Vaananen H., Piperno G. (2004) Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hovater M. B., Olteanu D., Hanson E. L., Cheng N. L., Siroky B., Fintha A., Komlosi P., Liu W., Satlin L. M., Bell P. D., Yoder B. K., Schwiebert E. M. (2008) Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Sig. 4, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masyuk A. I., Masyuk T. V., Splinter P. L., Huang B. Q., Stroope A. J., LaRusso N. F. (2006) Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen C. G., Poole C. A., McGlashan S. R., Marko M., Issa Z. I., Vujcich K. V., Bowser S. S. (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol. Int. 28, 101–110 [DOI] [PubMed] [Google Scholar]
- 25. McGlashan S. R., Jensen C. G., Poole C. A. (2006) Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 54, 1005–1014 [DOI] [PubMed] [Google Scholar]
- 26. Moyer J. H., Lee-Tischler M. J., Kwon H. Y., Schrick J. J., Avner E. D., Sweeney W. E., Godfrey V. L., Cacheiro N. L., Wilkinson J. E., Woychik R. P. (1994) Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264, 1329–1333 [DOI] [PubMed] [Google Scholar]
- 27. Yoder B. K., Richards W. G., Sommardahl C., Sweeney W. E., Michaud E. J., Wilkinson J. E., Avner E. D., Woychik R. P. (1997) Differential rescue of the renal and hepatic disease in an autosomal recessive polycystic kidney disease mouse mutant. A new model to study the liver lesion. Am. J. Pathol. 150, 2231–2241 [PMC free article] [PubMed] [Google Scholar]
- 28. Jat P. S., Noble M. D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. (1991) Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. U. S. A. 88, 5096–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee D. A., Bader D. L. (1995) The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell. Dev. Biol. Anim. 31, 828–835 [DOI] [PubMed] [Google Scholar]
- 30. Mauck R. L., Seyhan S. L., Ateshian G. A., Hung C. T. (2002) Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann. Biomed. Eng. 30, 1046–1056 [DOI] [PubMed] [Google Scholar]
- 31. Chowdhury T. T., Bader D. L., Lee D. A. (2006) Dynamic compression counteracts IL-1beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology 43, 413–429 [PubMed] [Google Scholar]
- 32. Roberts S. R., Knight M. M., Lee D. A., Bader D. L. (2001) Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. J. Appl. Physiol. 90, 1385–1391 [DOI] [PubMed] [Google Scholar]
- 33. Pingguan-Murphy B., Lee D. A., Bader D. L., Knight M. M. (2005) Activation of chondrocyte calcium signalling by dynamic compression is independent of number of cycles. Arch. Biochem. Biophys. 444, 45–51 [DOI] [PubMed] [Google Scholar]
- 34. Farndale R. W., Sayers C. A., Barrett A. J. (1982) A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 9, 247–248 [DOI] [PubMed] [Google Scholar]
- 35. Garcia M., Knight M. M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 28, 510–515 [DOI] [PubMed] [Google Scholar]
- 36. Pingguan-Murphy B., El-Azzeh M., Bader D. L., Knight M. M. (2006) Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J. Cell. Physiol. 209, 389–397 [DOI] [PubMed] [Google Scholar]
- 37. McGlashan S. R., Knight M. M., Chowdhury T. T., Joshi P., Jensen C. G., Kennedy S., Poole C. A. (2010) Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol. Int. 34, 441–446 [DOI] [PubMed] [Google Scholar]
- 38. Koolpe M., Benton H. P. (1997) Calcium-mobilizing purine receptors on the surface of mammalian articular chondrocytes. J. Orthop. Res. 15, 204–212 [DOI] [PubMed] [Google Scholar]
- 39. Knight M. M., McGlashan S. R., Garcia M., Jensen C. G., Poole C. A. (2009) Articular chondrocytes express connexin 43 hemichannels and P2 receptors—a putative mechanoreceptor complex involving the primary cilium? J. Anat. 214, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hooper K. M., Boletta A., Germino G. G., Hu Q., Ziegelstein R. C., Sutters M. (2005) Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am. J. Physiol. Renal Physiol. 289, F521–F530 [DOI] [PubMed] [Google Scholar]
- 41. Satir P., Christensen S. T. (2007) Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 [DOI] [PubMed] [Google Scholar]
- 42. Sharma N., Kosan Z. A., Stallworth J. E., Berbari N. F., Yoder B. K. (2011) Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell 22, 806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaushik A. P., Martin J. A., Zhang Q., Sheffield V. C., Morcuende J. A. (2009) Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J. Orthop. Res. 27, 1093–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song B., Haycraft C. J., Seo H. S., Yoder B. K., Serra R. (2007) Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 305, 202–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moskalewski S., Thyberg J., Lohmander S., Friberg U. (1975) Influence of colchicine and vinblastine on the golgi complex and matrix deposition in chondrocyte aggregates. An ultrastructural study. Exp. Cell Res. 95, 440–454 [DOI] [PubMed] [Google Scholar]
- 46. Guilak F., Ratcliffe A., Mow V. C. (1995) Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J. Orthop. Res. 13, 410–421 [DOI] [PubMed] [Google Scholar]
- 47. Knight M. M., Lee D. A., Bader D. L. (1998) The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim. Biophys. Acta 1405, 67–77 [DOI] [PubMed] [Google Scholar]
- 48. Graff R. D., Lazarowski E. R., Banes A. J., Lee G. M. (2000) ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 43, 1571–1579 [DOI] [PubMed] [Google Scholar]
- 49. Praetorius H. A., Leipziger J. (2009) Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol. (Oxf.) 197, 241–251 [DOI] [PubMed] [Google Scholar]
- 50. Praetorius H. A., Leipziger J. (2009) ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malone A. M., Anderson C. T., Tummala P., Kwon R. Y., Johnston T. R., Stearns T., Jacobs C. R. (2007) Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. U. S. A. 104, 13325–13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chauvet V., Tian X., Husson H., Grimm D. H., Wang T., Hiesberger T., Igarashi P., Bennett A. M., Ibraghimov-Beskrovnaya O., Somlo S., Caplan M. J. (2004) Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 114, 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woodward O. M., Li Y., Yu S., Greenwell P., Wodarczyk C., Boletta A., Guggino W. B., Qian F. (2011) Identification of a polycystin-1 cleavage product, P100, that regulates store operated Ca entry through interactions with STIM1. PLoS One 5, e12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weber K. H., Lee E. K., Basavanna U., Lindley S., Ziegelstein R. C., Germino G. G., Sutters M. (2008) Heterologous expression of polycystin-1 inhibits endoplasmic reticulum calcium leak in stably transfected MDCK cells. Am. J. Physiol. Renal Physiol. 294, F1279–F1286 [DOI] [PubMed] [Google Scholar]
- 55. Wildman S. S., Hooper K. M., Turner C. M., Sham J. S., Lakatta E. G., King B. F., Unwin R. J., Sutters M. (2003) The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl- conductance through increased Ca2+ entry. Am. J. Physiol. Renal Physiol. 285, F1168–F1178 [DOI] [PubMed] [Google Scholar]
- 56. Chapin H. C., Rajendran V., Caplan M. J. (2010) Polycystin-1 surface localization is stimulated by polycystin-2 and cleavage at the G protein-coupled receptor proteolytic site. Mol. Biol. Cell 21, 4338–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delmas P., Nomura H., Li X., Lakkis M., Luo Y., Segal Y., Fernandez-Fernandez J. M., Harris P., Frischauf A. M., Brown D. A., Zhou J. (2002) Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 277, 11276–11283 [DOI] [PubMed] [Google Scholar]
- 58. Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 59. Egorova A. D., Khedoe P. P., Goumans M. J., Yoder B. K., Nauli S. M., ten Dijke P., Poelmann R. E., Hierck B. P. (2011) Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ. Res. 108, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]