Abstract
Bone diseases such as postmenopausal osteoporosis are primarily caused by excessive formation and activity of osteoclasts (OCLs). Receptor activator of nuclear factor-κB ligand (RANKL) is a key initiating cytokine for OCL differentiation and function. RANKL induces calcium (Ca2+) oscillations, resulting in selective and robust induction of nuclear factor of activated T cells c1 (NFATc1), a Ca2+-responsive transcription factor that drives osteoclastogenesis. Store-operated Ca2+ entry (SOCE) is a major Ca2+ influx pathway in most nonexcitable cell types and is activated by any stimulus that depletes Ca2+ stores in the endoplasmic reticulum. Although the role of Orai1, a SOCE channel in the plasma membrane, in maintaining Ca2+ oscillations and transactivation of NFAT in other cell types is well known, its contribution to osteoclastogenesis remains unclear. We show here that silencing of the Orai1 gene with viral delivery of shRNA reduces SOCE and inhibits RANKL-induced osteoclastogenesis of RAW264.7 cells, a murine monocyte/macrophage cell line, by suppressing the induction of NFATc1. This was accompanied by defective induction of OCL-specific genes, such as tartrate-resistant acid phosphatase and immunoreceptor OCL-associated receptor, which are known to be direct transcriptional targets of NFATc1 during osteoclastogenesis. In addition, maturation of OCLs was abrogated by defective cell fusion of pre-OCLs depleted of Orai1, consistent with defective RANKL-mediated induction of d2 isoform of vacuolar ATPase Vo domain that is involved in cell fusion of pre-OCLs. We found that the functional bone resorbing capacity was severely impaired in OCLs depleted of Orai1, potentially related to the observed decrease in the induction of cathepsin K, a major bone matrix degrading protease. Our results indicate that Orai1 plays a critical role in the differentiation and function of OCLs, suggesting that Orai1 might be a potential therapeutic target for the treatment or prevention of bone loss caused by OCLs.—Hwang, S.-Y., Putney, J. W. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1.
Keywords: store-operated channels, bone remodeling, osteoporosis, receptor activator of nuclear factor κB ligand, tartrate-resistant acid phosphatase
Osteoporosis is the most common type of bone disease. Approximately 10 million Americans and 200 million people worldwide are affected by osteoporosis (1, 2). This disease is characterized by low bone mass and deterioration of bone microarchitecture, with increased risk of bone fractures. Despite its rigid and inert appearance, bone is a metabolically active and dynamic tissue that is continuously renewed throughout life by a process of bone remodeling (3). In a healthy individual, this bone remodeling results from the balanced actions of 2 types of cells: bone resorption by osteoclasts (OCLs), followed by bone formation by osteoblasts (3, 4). However, an imbalance in this process in favor of bone resorption is known to cause a number of skeletal disorders, including osteoporosis in postmenopausal women (4, 5). It has been shown that excessive formation and function of OCLs are the major causative factor of bone loss in these pathological conditions (6, 7). OCLs are unique bone resorbing (removing) cells differentiated from hematopoietic progenitors of monocyte/macrophage lineage and become mature by cell fusion of the progenitors (4, 8). Receptor activator of nuclear factor-κB ligand (RANKL) is a key initiating cytokine that drives OCL differentiation (9–11). In an attempt to identify specific genes that are induced during RANKL-mediated OCL differentiation, Takayanagi et al. (12) found that RANKL induces Ca2+ oscillations (repetitive cycling of intracellular Ca2+), resulting in high and selective induction of nuclear factor of activated T cells c1 (NFATc1), one of the 5 NFAT family members, NFATc1 to c4 and NFAT5 (13). NFATc1-deficient OCL precursors failed to differentiate into mature OCLs, and ectopic expression of NFATc1 induced osteoclastogenesis even in the absence of RANKL (12). Although Ca2+ signaling appears critical for RANKL-mediated signaling pathways, the Ca2+ channels involved in Ca2+ signaling and the resultant NFAT activation in osteoclastogenesis are largely unknown. We considered that Orai1, a store-operated Ca2+ entry (SOCE) channel subunit, might play a role in OCL differentiation based on the following observations. First, Orai1 Ca2+ channels provide a major Ca2+ influx pathway in hematopoietic cells from which OCLs are differentiated. Second, SOCE mediated by Orai1 channels plays a critical role in maintenance of Ca2+ oscillations (14). Third, SOCE mediated by Orai1 is required for transactivation of NFAT in other hematopoietic cells, such as T cells (15). Furthermore, Kar et al. (16) recently showed that the nuclear translocation of NFAT is mediated by Ca2+ signal generated from near SOCE channels. Clinically, a severe combined immune deficiency syndrome was shown to be attributed to mutations in the Orai1 gene. T cells from patients with severe combined immune deficiency show a marked reduction in SOCE, resulting in impairment of transactivation of NFAT (15, 17). In the current study, we have used a RNAi gene-knockdown strategy to examine the role of Orai1-mediated SOCE in OCL differentiation and function. By use of viral delivery of Orai1-specific shRNA, we show that silencing of Orai1 gene expression leads to a decreased number and defective resorptive function of OCLs. We found that multinucleation of OCLs was defective in OCLs depleted of Orai1. We also provide evidence that Orai1 is necessary for transactivation of NFATc1 during RANKL-mediated osteoclastogenesis of RAW264.7 cells (hereafter referred to as RAW cells). Our results suggest that Orai1 provides a critical link between Ca2+ signaling and OCL differentiation by controlling activation of NFATc1.
MATERIALS AND METHODS
Reagents and antibodies
Recombinant mouse soluble RANKL was obtained from PeproTech EC (London, UK). pLKO.1-scrambled shRNA and pLKO.1-mouse Orai1 shRNA lentiviral plasmid were purchased from Addgene (plasmid 1864; Addgene, Cambridge, MA, USA) and Sigma (St. Louis, MO, USA; sequence of mouse Orai1 shRNA: CCGGCACAACCTCAACTCGGTCAAACTCGAGTTTGACCGAGTTGAGGTTGTGTTTTTG), respectively.
Anti-NFATc1 (7A6), and anti-lamin B (M-20) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-ATP6v0d2 antibody (ab87059) was purchased from Abcam (Cambridge, MA, USA).
Cell culture
RAW cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and were maintained in Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen), 100 U/ml penicillin G, and 100 μg/ml streptomycin (Invitrogen) at 37°C with 5% CO2.
Lentivirus packaging and infection of RAW cells
Recombinant lentiviruses were produced by cotransfecting the pLKO.1-scramble or pLKO.1-mouse Orai1 shRNA plasmids with psPAX2 and pMD2.G into HEK293T/17 cells (ATCC) using Lipofectamine Plus (Invitrogen). The medium was replaced after 24 h, and viral supernatants were harvested 2 d post-transfection and stored at −80°C. For infection, RAW cells were seeded at a density of 250,000 cells/well of a 6-well plate and infected with 100–200 multiplicity of infection of viral supernatant containing 5 μg/ml polybrene. The medium was replaced next day. Cells were cultured for an additional 24 h and replated for further experiments.
In vitro osteoclastogenesis
Infected RAW cells were plated on a 48-well plate at a density of 5000 cells/well and cultured in the presence of 100 ng/ml RANKL for 4 d. The culture medium was replaced every 2 d. The cultured cells were subjected to tartrate-resistant acid phosphatase (TRAP) staining using an acid phosphatase leukocyte kit (387A; Sigma). TRAP staining was carried out in triplicate wells for each condition according to the manufacturer's instruction. The number and size of TRAP-positive multinuclear cells (TRAP+ MNCs; >3 nuclei) were measured. For size measurement of OCLs, regions of interest (ROIs) were drawn for each OCL cell. The size of each ROI was analyzed by MetaMorph imaging software (Molecular Devices Corp., Sunnyvale, CA, USA). For measurement of secreted TRAP into medium during osteoclastogenesis, cells were seeded on a 96-well plate at a density of 3000 cells/well and cultured in the presence or absence of RANKL for 4 d (medium was replaced on d 2). On d 4, medium was collected, and 25 μl of the medium was transferred to a 96-well plate containing 75 μl of TRAP solution (acid phosphatase leukocyte kit 387A; Sigma). The mixture was incubated at 37°C for 2 h, and the absorbance was read at 540 nm (adapted from a protocol of TRAP staining kit; B-Bridge International, Cupertino, CA, USA).
In vitro pit formation assay
Infected RAW cells were plated at a density of 3000 cells/well using 16-well BD BioCoat Osteologic calcium phosphate-coated slides (BD Biosciences, San Jose, CA, USA) and cultured in the presence of 100 ng/ml RANKL for 6 d. The medium was replaced every 2 d. Cells were detached with sodium hypochlorite solution (Sigma) and rinsed with distilled water. The plates were stained with 5% silver nitrate for better resolution, as instructed in BD Biosciences Technical Bulletin 444 (http://www.bdbiosciences.com/external_files/dl/doc/tech_bulletin/live/web_enabled/TB444.pdf). The number of pits per well was enumerated in quadruplicate wells. Pit size was measured using MetaMorph imaging software.
Optical imaging of intracellular Ca2+ concentrations
RAW cells on the coverslip were loaded with 5 μM fura-2/AM (Invitrogen) for 45 min in the presence of 500 μM sulfinpyrazone at room temperature. Changes in intracellular Ca2+ concentrations ([Ca2+]i) were recorded as the ratio of fluorescence emitted when cells were excited alternately at 340- and 380-nm wavelengths of light. Typically, peak ratio values of fura-2-loaded RAW cells in response to thapsigargin (TG; 2 μM) were <1.5, while Rmax, determined in situ, averaged ∼6, indicating that the indicator was less than half-saturated and thus yielded ratios essentially proportional to Ca2+concentrations.
RNA isolation and real-time quantitative RT-PCR (qRT-PCR)
Infected RAW cells were seeded at a density of 250,000 cells/well of a 6-well plate. After culture for 24 h, cells were treated with 100 ng/ml RANKL for 2 d, and total RNA was extracted using RNeasy mini kit (Qiagen, Valenica, CA, USA). Total RNA (1 μg) was reverse transcribed to cDNA using the Omniscript RT kit (Qiagen) according to the manufacturer's protocol. qRT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) with the ABI Prism 7000 Instrument (Applied Biosystems). The mRNA levels were normalized to GAPDH. The following sequences of primers were used: NFATc1, sense 5′-CTCGAAAGACAGCACTGGAGCAT-3′ and antisense 5′-CGGCTGCCTTCCGTCTCATAG-3′; dendritic cell-specific transmembrane protein (DC-STAMP), sense 5′-TGGAAGTTCACTTGAAACTACGTG-3′ and antisense 5′-CTCGGTTTCCCGTCAGCCTCTCTC-3′; cathepsin K, sense 5′-ACGGAGGCATTGACTCTGAAGATG-3′ and antisense 5′-GTTGTTCTTATTCCGAGCCAAGAG-3′; TRAP, sense 5′-CTGGAGTGCACGATGCCAGCGACA-3′ and antisense 5′-TCCGTGCTCGGCGATGGACCAGA-3′; d2 isoform of vacuolar ATPase Vo domain (ATP6v0d2), sense 5′-TCAGATCTCTTCAAGGCTGTGCTG-3′ and antisense 5′-GTGCCAAATGAGTTCAGAGTGATG-3′ (preceding primer sequences were all originally described by Kim et al., ref. 18); osteoclast-associated receptor (OSCAR), sense 5′-TGGCGGTTTGCACTCTTCA-3′ and antisense 5′-GATCCGTTACCAGCAGTTCCAGA-3′ (19); and GAPDH, sense 5′-AACTTTGGCATTGTGGAAGG-3′ and antisense 5′-GGAGACAACCTGGTCCTCAG-3′.
Subcellular fractionation and Western blot analysis
The RAW cells infected with either scrambled or Orai1 shRNA lentivirus were seeded on a 6-well plate at a density of 250,000 cells/well and cultured in the presence or absence of RANKL for 2 d. Cytoplasmic and nuclear fractionation was performed using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's protocol. Cytoplasmic proteins (30 μg) and nuclear proteins (4 μg) were separated on 4–20% gradient SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to a PVDF membrane. The membrane was incubated with primary antibodies at 4°C overnight, incubated with a horseradish peroxidase-conjugated secondary antibody for 45 min, and developed using an enhanced chemiluminescence system. Densitometric analysis was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Cell viability
Cell viability was determined using a cell proliferation reagent, WST-1 (Roche Appliced Science, Indianapolis, IN, USA), according to the manufacturer's protocol.
Statistical analyses
Statistical significance was determined by use of ANOVA or Student's t test. All statistical analyses were carried out using GraphPad Prism software (GraphPad, La Jolla, CA, USA).
RESULTS
Knockdown of Orai1 impairs RANKL-mediated osteoclastogenesis of RAW cells
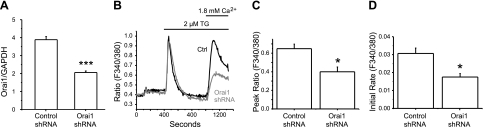
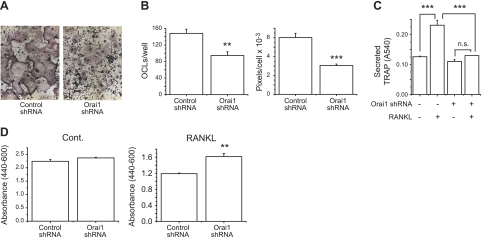
RAW264.7 cells are a mouse monocyte/macrophage cell lineage known to undergo osteoclastogenesis in the presence of RANKL (20–22). To investigate the role of Orai1 in RANKL-induced osteoclastogenesis, we developed an RNAi strategy of viral expression of shRNA directed against mouse Orai1 message. We infected RAW cells with lentivirus expressing either scrambled or mouse Orai1 shRNA. After 2 d, cells were replated and cultured for an additional 48 h. We confirmed the efficiency of lentiviral expression of Orai1 shRNA by qRT-PCR. As shown in Fig. 1A, the level of Orai1 mRNA from RAW cells infected with Orai1 shRNA virus was reduced by ∼50% compared with the control cells (3.9±0.17 vs. 2.1±0.08; P<0.001). We have so far been unable to detect mouse Orai1 protein by Western blot. However, further experiments using Ca2+ imaging analysis showed that the reduced levels of Orai1 message were consistent with reduction in functional Orai1 protein (Fig. 1B). TG (2 μM), an inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase, was used to deplete Ca2+ stores in the endoplasmic reticulum (ER), leading to activation of SOCE. The TG-induced SOCE was assessed by restoration of extracellular 1.8 mM Ca2+ after ER Ca2+ store depletion by TG (Fig. 1B and ref. 23). As shown in Fig. 1B, silencing of the Orai1 gene resulted in reduction of SOCE compared with the cells infected with scrambled shRNA. The peak value of [Ca2+]i from cells infected with Orai1 shRNA virus was significantly lower compared with the control (Fig. 1C). Also, the initial rate of rise in [Ca2+]i was significantly reduced in cells infected with Orai1 shRNA virus (Fig. 1D). Low concentrations of gadolinium (2.5 μM), a SOCE channel blocker, further reduced the calcium entry in Orai1 shRNA-infected cells, suggesting either incomplete knockdown of Orai1 or participation of other Orai isoforms, Orai2 or 3 (Supplemental Fig. S4). We next investigated the role of Orai1 in OCL differentiation induced by RANKL. RAW cells infected with virus encoding control (scrambled) or Orai1 shRNA were cultured in the presence of RANKL (100 ng/ml) for 4 d. The mature OCLs were visualized by TRAP (an OCL biomarker, stained in dark purple, Fig. 2A), and TRAP+ MNCs were counted. As shown in Fig. 2B (left panel), the number of TRAP+ MNCs was significantly decreased in the group derived from RAW cells infected with Orai1 shRNA [Orai1-knockdown (KD) OCLs] compared with that with scrambled shRNA (control OCLs). The TRAP protein secreted into the medium during osteoclastogenesis was also measured. As shown in Fig. 2C, the amount of secreted TRAP protein was also markedly reduced from Orai1-KD OCLs compared with control, consistent with the defective induction of TRAP protein. In addition to the defect in the formation of TRAP+ MNCs, we noticed that the size of individual TRAP+ MNC appears to be reduced in Orai1-KD OCLs (Fig. 2B, right panel). The size of each TRAP+ MNC was measured as described in Materials and Methods. The average size of TRAP+ MNCs differentiated from RAW cells depleted of Orai1 was significantly smaller than that from the control (Fig. 2B, right panel, and Supplemental Fig. S2). These results suggest that cell fusion of pre-OCLs is impaired in Orai1-KD OCLs. This observation is consistent with the previous report that inhibition of Orai1 resulted in suppressed multinucleation of OCLs generated from human monocytes (24). Mature OCLs are formed by cell-cell fusion of precursor cells, and multinucleation is a prerequisite for maturation and efficient resorption activity of OCLs (6, 25, 26). These data suggest that knockdown of Orai1 abrogates RANKL-induced osteoclastogenesis in vitro by inhibiting the cell fusion of pre-OCLs. We investigated whether knockdown of Orai1 affects cell proliferation or viability by using the WST-1 reagent. The cell proliferation rate in the absence of RANKL is similar in both groups (Fig. 2D, left panel). In the presence of RANKL, the cell proliferation in Orai1-KD OCLs was actually higher compared with the control (Fig. 2D, right panel), indicating that the inhibition of OCL formation in Orai1-KD OCLs is not caused by inhibition of cell proliferation or survival.
Figure 1.
Knockdown of Orai1 reduces TG-induced SOCE in RAW264.7 cells. A) RNA was isolated from RAW cells infected with lentivirus expressing either scrambled or Orai1 shRNA for 5 d. Expression levels of mouse Orai1 message were measured by qRT-PCR. Statistical significance was evaluated by Student's t test. Data represent 2 independent experiments with similar results. Error bars denote se between samples performed in triplicate. B) Ca2+ imaging of RAW cells infected with the scrambled or Orai1 shRNA lentivirus. Cells were loaded with 5 μM fura-2-AM dye, and SOCE was revealed by readdition of 1.8 mM Ca2+ after TG-induced Ca2+ release from ER and measured by optical imaging of intracellular Ca2+ concentration. C, D) Peak value (C) and initial rates (D) of SOCE increase from B were measured. Statistical significance was evaluated by Student's t test. All data are means ± se from 3 independent experiments (30–40 cells/experiment analyzed). *P < 0.05, ***P < 0.001.
Figure 2.
Knockdown of Orai1 inhibits RANKL-mediated osteoclastogenesis of RAW264.7 cells. A) Effect of Orai1 knockdown on TRAP staining in OCLs induced by RANKL. RAW cells infected with either control (scrambled) or Orai1 shRNA were cultured with RANKL (100 ng/ml) for 4 d and were stained for TRAP. B) Both number (left panel) and average size (right panel) of TRAP+ MNCs were measured. Note that the size of OCLs generated from RAW cells depleted of Orai1 is markedly smaller than that of control (panel A and Supplemental Fig. S2). C) TRAP secreted in the medium during osteoclastogenesis was measured at d 4. D) WST-1 cell proliferation assay of OCLs cultured with (right panel) or without RANKL (left panel) for 4 d. Data are means ± se of triplicate samples from 3 (B, C) or 2 (D) independent experiments. n.s., not significant. **P < 0.01, ***P < 0.001; ANOVA followed by Newman-Keuls multiple comparison test.
Knockdown of Orai1 leads to defective bone resorption in vitro
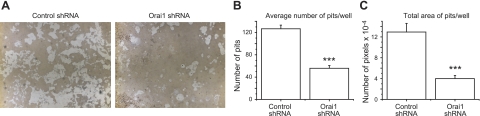
Since we found that OCL formation was impaired in Orai1-KD OCLs (Fig. 2), we next investigated the role of Orai1 in the resorbing capacity of OCLs using Ca2+-phosphate-coated plates. RAW cells infected with either control or Orai1 shRNA lentivirus were grown on the plate for 6 d in the presence of RANKL. The resorption pit formation in vitro was significantly reduced for Orai1-KD OCLs compared with those for control cells (Fig. 3A). Consistent with the decreased number of TRAP+ MNCs generated from RAW cells depleted of Orai1 as shown in Fig. 2B, the average number of pits formed on the Ca2+-phosphate-coated plate was markedly lower in Orai1-KD OCLs (Fig. 3B). Furthermore, the total area of pits was substantially diminished in Orai1-KD OCLs compared with control (Fig. 3C), consistent with the observation that maturation of OCL via cell fusion is impaired in pre-OCLs depleted of Orai1 (Fig. 2B, right panel). These results indicate that Orai1-mediated Ca2+ influx is important for proper differentiation of OCL and thus for their resorbing function of OCLs in vitro.
Figure 3.
Knockdown of Orai1 inhibits the resorption activity of OCLs. A) RAW cells infected with either control (left panel) or Orai1 shRNA (right panel) lentivirus were grown on the Ca2+-phosphate-coated plate in the presence of 100 ng/ml RANKL for 6 d. B, C) Both average number (B) and total area of pits (C) were measured as described in Materials and Methods. Data are means ± se of quadruplicate samples from 3 independent experiments. ***P < 0.001.
Knockdown of Orai1 inhibits induction of NFATc1 and other genes downstream of NFATc1 in osteoclastogenesis
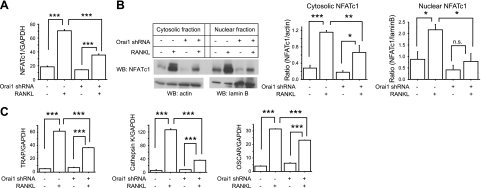
Takayanagi et al. (12) found that RANKL induces sustained Ca2+ oscillations, which are critical for robust induction of NFATc1. Thus, it appears that Ca2+ oscillations provide a sustained Ca2+ signal necessary to activate calcineurin, which is required for activation and translocation of NFATc1. To test the hypothesis that SOCE mediated by Orai1 channels provides Ca2+ signals for the activation of NFATc1 during osteoclastogenesis, RAW cells infected with control or Orai1 shRNA lentiviruses were cultured in the presence or absence of RANKL (100 ng/ml) for 2 d, and cell lysates were prepared for both qRT-PCR and Western blot analysis. Since it has been reported that the levels of autoamplified NFATc1 in RAW cells were highest at 2 d after RANKL treatment (ref. 22 and Supplemental Fig. S1), total RNA and proteins were isolated at d 2. As shown in Fig. 4A, we confirmed by qRT-PCR that mRNA levels of NFATc1 in RAW cells infected with scrambled shRNA were substantially up-regulated 2 d after treatment of RANKL compared with the control cells cultured in the absence of RANKL (Fig. 4A). Notably, the up-regulation of NFATc1 induced by RANKL was significantly blunted in Orai1-KD OCLs (Fig. 4A). We next carried out Western blot analyses to examine whether knockdown of Orai1 also affects induction and nuclear translocation of NFATc1 proteins in RANKL-mediated osteoclastogenesis. Both cytoplasmic and nuclear protein fractions were prepared from control and Orai1 shRNA-infected RAW cells cultured with or without RANKL for 2 d. Consistent with previous studies with RAW cells (22, 27), expression of NFATc1 protein was dramatically up-regulated in control RAW OCL cells in response to RANKL treatment for 2 d compared with controls cultured in the absence of RANKL (Fig. 4B). Consistent with the qRT-PCR data shown in Fig. 4A, we found that RANKL induction of cytosolic NFATc1 protein was significantly reduced in lysates prepared from Orai1-KD OCLs (Fig. 4B, left and middle panels). We also investigated protein levels of nuclear NFATc1 since nuclear translocation is a key step for up-regulation of NFATc1 via autoamplification in osteoclastogenesis and constitutes direct evidence of activation of calcineurin by Ca2+ signals (13, 28). As shown in Fig. 4B (left and right panels), we confirmed that RANKL treatment resulted in greater nuclear levels of NFATc1 proteins compared with the controls cultured without RANKL (18, 29). As expected from the change in cytosolic NFATc1 protein, protein levels of nuclear NFATc1 were markedly decreased in Orai1-KD OCLs compared with those of control (Fig. 4B, left and right panels). These results support the hypothesis that SOCE mediated by Orai1 channels plays a key role in osteoclastogenesis, presumably by providing a sustained Ca2+ signal that activates calcineurin, resulting in activation, nuclear translocation, and induction of NFATc1 during RANKL-mediated osteoclastogenesis.
Figure 4.
Knockdown of Orai1 blunts induction of cytosolic and nuclear NFATc1 during RANKL-mediated osteoclastogenesis. A) Infected RAW cells were cultured with or without RANKL for 2 d, and qRT-PCR analysis of NFATc1 message level was performed. NFATc1 was normalized to GAPDH. Statistical significance was evaluated by an ANOVA followed by Newman-Keuls multiple comparison test. Data are means ± se of triplicate samples from 3 independent experiments with similar results. B) Left panel: Western blot (WB) analysis of cytosolic and nuclear fractionation prepared from infected cells cultured with or without RANKL for 2 d. Actin and lamin B were used as markers of cytosolic and nuclear fractions, respectively. Middle panel: densitometric analysis of cytosolic (middle panel) protein levels. Right panel: densitometric analysis of nuclear NFATc1 protein levels. Data are means ± se of 3 independent experiements with similar results. C) qRT-PCR analysis of the expression of osteoclastic genes known to be a direct transcriptional target of NFATc1 in osteoclastogenesis. Data are means ± se of triplicate samples from 3 independent experiments with similar results. *P < 0.05, **P < 0.01, ***P < 0.001.
Robust induction of NFATc1 by RANKL during osteoclastogenesis is essential for the transcription of many downstream genes whose protein products play important roles in OCL differentiation and function. A number of OCL-specific genes, such as TRAP, cathepsin K, and OSCAR (12, 30, 31) are highly up-regulated during RANKL-induced osteoclastogenesis, and promoter binding assays have indicated that these genes are directly regulated by NFATc1. Thus, we next investigated by qRT-PCR the regulation of these key genes downstream of NFATc1 in OCLs depleted of Orai1. We confirmed that all 3 genes are highly up-regulated in control cells in response to RANKL for 2 d (Fig. 4C). However, consistent with the reduced NFATc1 expression, induction of TRAP gene by RANKL was significantly diminished in Orai1KD-OCLs compared with controls (Fig. 4C, left panel). The reduced induction of the TRAP gene is consistent with the observed defective formation of TRAP+-MNC OCLs and reduced secretion of TRAP in the medium, as described earlier (Fig. 2A, C). We also found that RANKL-mediated induction of the cathepsin K gene was markedly inhibited in Orai1-KD OCLs compared with the control OCLs (Fig. 4C, middle panel). Given that cathepsin K is highly expressed in OCLs and is a major protease responsible for bone resorption (30, 32), the loss of cathepsin K may be at least partially responsible for the observed decrease in the resorbing activity of Orai1-KD OCLs (Fig. 3). In addition, we found that induction of OSCAR by RANKL is significantly reduced in Orai1-KD OCLs (Fig. 4C, right panel).
These findings suggest that Orai1-mediated Ca2+ entry contributes significantly to the induction of OCL-specific genes by regulating transcription of NFATc1, a master regulator of osteoclastogenesis.
Orai1 plays a key role in induction of genes involved in cell fusion of OCL progenitors
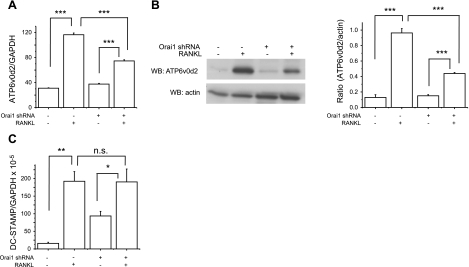
The fusion of mononuclear OCL precursors is essential for differentiation to multinucleated mature OCLs (6, 25). Although Zhou et al. (24) and our data suggest that Orai1-mediated Ca2+ entry plays a critical role in multinucleation of OCLs (Fig. 2B, right panel), the mechanism underlying the defect is unknown. To address the mechanism of the defective cell fusion present in Orai1-KD OCLs, we assessed the expression of two key genes regulating fusion of pre-OCLs: one encoding the d2 isoform of vacuolar ATPase Vo domain (ATP6v0d2) and the other encoding dendritic cell-specific transmembrane protein (DC-STAMP). Both genes are highly induced in osteoclastogenesis activated by RANKL and are essential for cell fusion of OCLs (26, 33). More relevant to our study, it is known that NFATc1 directly binds to the promoter regions of these two genes, leading to strong induction of the two molecules during RANKL-mediated osteoclastogenesis (18). Thus, we reasoned that the reduced induction NFATc1 as well as reduced cell size seen in Orai1-KD OCLs may indicate reduced induction of these two fusion-related genes. Consistent with previous reports (18), these two genes are substantially induced during RANKL-mediated osteoclastogenesis (Fig. 5A, C). Notably, induction of ATP6v0d2 mRNA was dramatically blunted in Orai1-KD OCLs compared with control OCLs (Fig. 5A). Western blot analysis consistently indicated that expression of ATP6v0d2 protein induced by RANKL was markedly reduced in Orai1-KD OCLs compared with that in control OCLs (Fig. 5B). However, we failed to see any difference in RANKL induction of DC-STAMP mRNA between Orai1-KD-OCL and control-OCL groups (Fig. 5C). These results suggest that the induction of the ATP6v0d2 gene is impaired in Orai1-KD OCLs, potentially leading to the observed defect in cell fusion of Orai1-KD OCLs. The defective induction of the ATP6v0d2 may be the consequence of the observed impairment of NFATc1 induction in Orai1-KD OCLs, as demonstrated in Fig. 4A, B.
Figure 5.
Regulator of cell fusion is suppressed in OCLs depleted of Orai1. A) Infected RAW cells were cultured with or without RANKL for 2 d, and qRT-PCR analysis was performed to examine the expression of ATP6v0d2 induced by RANKL. Statistical significance was evaluated by an ANOVA. Data represent 3 independent experiments with similar results. B) Left panel: Western blot analysis of ATP6v0d2 from RAW cells cultured with or without RANKL for 2 d. Right panel: densitometric analyses of ATP6v0d2 protein levels were performed from 3 independent experiments. Data represent means ± se. C) qRT-PCR analysis was performed to examine the expression of DC-STAMP. *P < 0.05, **P < 0.01, ***P<0.001.
DISCUSSION
Excessive formation and resorbing activity of OCLs are a major cause of various skeletal disorders, such as postmenopausal osteoporosis and bone destruction in rheumatoid arthritis (5, 34–36). Therefore, considerable effort has been focused on understanding the signaling pathways involved in OCL differentiation and function. As one such signaling pathway, Ca2+ signaling is known to be involved in OCL differentiation and functions (37–39). Notably, Takayanagi et al. (12) showed that RANKL induces Ca2+ oscillations, resulting in robust induction of NFATc1, an essential transcription factor that drives osteoclastogenesis. However, the Ca2+ channels involved in this Ca2+ signaling are largely unknown. Zhou et al. (24) previously demonstrated that pharmacological inhibition or knockdown of Orai1 by siRNA suppresses osteoclastogenesis of human monocytes. However, the mechanism for this effect was not addressed. In the present study, we confirmed in studies utilizing a mouse monocyte cell line that Orai1 plays a critical role in the RANKL-mediated differentiation of OCLs. We found a decrease in the number of OCLs formed from RAW cells depleted of Orai1. Since SOCE mediated by Orai1 channels is critical for transactivation of NFAT in lymphocytes (13, 15), we investigated the role of Orai1 in the RANKL-mediated induction of NFATc1 in osteoclastogenesis. Here we provide evidence that Orai1 contributes to the activation of NFATc1 induced by RANKL. The induction of NFATc1 by RANKL was substantially reduced in Orai1-KD OCLs compared with control OCLs, suggesting a role of Orai1 in the autoamplification mechanism of NFATc1 in osteoclastogenesis. As a possible mechanism, we found that the translocation of NFATc1 was impaired in OCLs depleted of Orai1, indicating a critical role of Orai1 in the activation of calcineurin, a Ca2+/calmodulin-dependent phosphatase. This suggests that SOCE mediated by Orai1 channels provides a sustained Ca2+ signaling to keep calcineurin active, leading to dephosphorylation of NFATc1 that can move to the nucleus. While we observed a decrease in expression and secretion of TRAP in Orai1-KD OCLs, a previous study (24) reported no such effect of Orai1 inhibition on TRAP. This discrepancy may result from differences in species (human vs. mouse) and/or the gene silencing method used [transient siRNA for Zhou et al. (24) vs. viral shRNA infection in the current study] potentially leading to different levels of residual Orai1 proteins in OCLs. There is also a possibility that different levels of other isoforms of Orai, Orai2 and 3, are present in cells used in both groups; in neither study was there complete loss of SOCE following knockdown of Orai1. Multinucleation of mononuclear pre-OCLs by cell fusion is important for differentiation and maturation of OCLs (6, 25, 40). Consistent with the report of Zhou et al. (24), we also observed that cell fusion of pre-OCLs is impaired in Orai1-KD OCLs. In addition, we found, as a possible mechanism for this impairment, that induction of ATP6v0d2 is considerably decreased in Orai1-KD OCLs compared with control OCLs. ATP6v0d2 is known to be an essential player for cell fusion of OCLs (33) and to be a transcriptional target of NFATc1 (18). However, there was no effect of Orai1 knockdown on the induction of DC-STAMP, another important gene involved in cell fusion of OCLs (26). This suggests that factors other than NFATc1 can regulate DC-STAMP expression in OCLs. It is also possible that the levels of NFATc1 present in Orai1-KD OCLs might be sufficient for full activation of the promoter region of the DC-STAMP gene but not for ATP6v0d2. In addition, we examined other fusion genes involved in OCL differentiation, including E-cadherin and integrins. We found by qRT-PCR that the expression of E-cadherin is largely undetectable in OCLs derived from RAW cells (data not shown). The expression of αν-integrin induced by RANKL was slightly but significantly reduced in cells depleted of Orai1 (Supplemental Fig. S3). However, interestingly, the expression levels of β3-integrin are higher in OCLs depleted of Orai1 compared with those in control OCLs (Supplemental Fig. S3). Also, we demonstrated that the defective differentiation of OCLs results in diminished functional (resorptive) activity, which is consistent with the observed decrease in the induction of cathepsin K in Orai1-KD OCLs. Interestingly, we also observed impaired induction of OSCAR, a costimulatory receptor linked to the adaptor protein FcRγ, containing an ITAM motif. During osteoclastogenesis induced by RANKL, these receptors recruit the spleen tyrosine kinase, which activates phospholipase C, leading to Ca2+ release, presumably leading to Ca2+ influx (41–43). Reduction of OSCAR expression could reduce Ca2+ signaling, thereby contributing to the decreased activation of NFATc1 as observed in the current study.
Taken together, the current study provides evidence that SOCE mediated by Orai1 channels contributes to osteoclastogenesis by regulating the RANKL-mediated induction of NFATc1 and thus a number of key downstream genes that are essential for osteoclastogenesis. It is likely that SOCE is one component of a complex signaling mechanism, possibly involving other Ca2+ channels as well (44–46). Orai1 might be a potential therapeutic target to inhibit aberrant activity of OCLs in postmenopausal osteoporosis. However, since Orai1 plays a major role in immune responses, such as T-cell activation (47), direct blockade of Orai1 channels might have adverse effects on the immune system, as seen in patients carrying null mutations in the Orai1 gene. Therefore, it may be necessary to find more specific targets downstream of Ca2+ influx through Orai1 channels in osteoclastogenesis. In addition, in order to elucidate the in vivo role of Orai1 in bone homeostasis, it will be helpful to examine and characterize the bone phenotype in mouse models deficient in SOCE. Given the fact that NFATc1 is also important for osteoblast differentiation and bone formation (48), understanding the role played by SOCE in the differentiation of osteoblasts may provide a more complete picture of how Ca2+ signaling is involved in bone homeostasis.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS). The authors thank Dr. Xiaoling Li and Dr. Robert Oakley for helpful comments. The authors thank Dr. Gary Bird for technical advice and helpful discussion. The authors also thank Dr. Charles Romeo and Dr. Negin Martin (Viral Vector Core, NIEHS) for lentivirus preparations.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATP6v0d2
- d2 isoform of vacuolar ATPase Vo domain
- DC-STAMP
- dendritic cell-specific transmembrane protein
- ER
- endoplasmic reticulum
- NFAT
- nuclear factor of activated T cells
- OCL
- osteoclast
- Orai1-KD OCL
- Orai1-knockdown osteoclast
- OSCAR
- osteoclast-associated receptor
- RANKL
- receptor activator of nuclear factor-κB ligand
- RAW cells
- RAW264.7 cells
- SOCE
- store-operated calcium entry
- TG
- thapsigargin
- TRAP
- tartrate resistant acid phosphatase
- TRAP+ MNC
- TRAP-positive multinucleated cell.
REFERENCES
- 1. Lin J. T., Lane J. M. (2004) Osteoporosis: a review. Clin. Orthop. Relat. Res. 126–134 [PubMed] [Google Scholar]
- 2. Lane N. E. (2006) Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 194, S3–11 [DOI] [PubMed] [Google Scholar]
- 3. Zaidi M. (2007) Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 [DOI] [PubMed] [Google Scholar]
- 4. Novack D. V., Teitelbaum S. L. (2008) The osteoclast: friend or foe? Annu. Rev. Pathol. 3, 457–484 [DOI] [PubMed] [Google Scholar]
- 5. Raisz L. G. (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 7. Rodan G. A., Martin T. J. (2000) Therapeutic approaches to bone diseases. Science 289, 1508–1514 [DOI] [PubMed] [Google Scholar]
- 8. Baron R. (1989) Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec. 224, 317–324 [DOI] [PubMed] [Google Scholar]
- 9. Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 [DOI] [PubMed] [Google Scholar]
- 10. Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 11. Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U. S. A. 95, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E. F., Mak T. W., Kodama T., Taniguchi T. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 3, 889–901 [DOI] [PubMed] [Google Scholar]
- 13. Hogan P. G., Chen L., Nardone J., Rao A. (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 14. Wedel B., Boyles R. R., Putney J. W., Bird G. S. (2007) Role of the store-operated calcium entry proteins, Stim1 and Orai1, in muscarinic-cholinergic receptor stimulated calcium oscillations in human embryonic kidney cells. J. Physiol. 579, 679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 16. Kar P., Nelson C., Parekh A. B. (2011) Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 286, 14795–14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feske S. (2007) Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 18. Kim K., Lee S. H., Ha K. J., Choi Y., Kim N. (2008) NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 22, 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim Y., Sato K., Asagiri M., Morita I., Soma K., Takayanagi H. (2005) Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J. Biol. Chem. 280, 32905–32913 [DOI] [PubMed] [Google Scholar]
- 20. Hsu H., Lacey D. L., Dunstan C. R., Solovyev I., Colombero A., Timms E., Tan H. L., Elliott G., Kelley M. J., Sarosi I., Wang L., Xia X. Z., Elliott R., Chiu L., Black T., Scully S., Capparelli C., Morony S., Shimamoto G., Bass M. B., Boyle W. J. (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. U. S. A. 96, 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumoto M., Sudo T., Saito T., Osada H., Tsujimoto M. (2000) Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J. Biol. Chem. 275, 31155–31161 [DOI] [PubMed] [Google Scholar]
- 22. Ishida N., Hayashi K., Hoshijima M., Ogawa T., Koga S., Miyatake Y., Kumegawa M., Kimura T., Takeya T. (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 277, 41147–41156 [DOI] [PubMed] [Google Scholar]
- 23. Takemura H., Hughes A. R., Thastrup O., Putney J. W. (1989) Activation of calcium entry by the tumor promoter, thapsigargin, in parotid acinar cells. Evidence that an intracellular calcium pool, and not an inositol phosphate, regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 264, 12266–12271 [PubMed] [Google Scholar]
- 24. Zhou Y., Lewis T. L., Robinson L. J., Brundage K. M., Schafer R., Martin K. H., Blair H. C., Soboloff J., Barnett J. B. (2011) The role of calcium release activated calcium channels in osteoclast differentiation. J. Cell. Physiol. 226, 1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fallon M. D., Teitelbaum S. L., Kahn A. J. (1983) Multinucleation enhances macrophage-mediated bone resorption. Lab. Invest. 49, 159–164 [PubMed] [Google Scholar]
- 26. Yagi M., Miyamoto T., Sawatani Y., Iwamoto K., Hosogane N., Fujita N., Morita K., Ninomiya K., Suzuki T., Miyamoto K., Oike Y., Takeya M., Toyama Y., Suda T. (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirotani H., Tuohy N. A., Woo J. T., Stern P. H., Clipstone N. A. (2004) The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 279, 13984–13992 [DOI] [PubMed] [Google Scholar]
- 28. Asagiri M., Sato K., Usami T., Ochi S., Nishina H., Yoshida H., Morita I., Wagner E. F., Mak T. W., Serfling E., Takayanagi H. (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamel Mohamed S. G., Sugiyama E., Shinoda K., Hounoki H., Taki H., Maruyama M., Miyahara T., Kobayashi M. (2005) Interleukin-4 inhibits RANKL-induced expression of NFATc1 and c-Fos: a possible mechanism for downregulation of osteoclastogenesis. Biochem. Biophys. Res. Commun. 329, 839–845 [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto M., Kogawa M., Wada S., Takayanagi H., Tsujimoto M., Katayama S., Hisatake K., Nogi Y. (2004) Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU. 1. J. Biol. Chem. 279, 45969–45979 [DOI] [PubMed] [Google Scholar]
- 31. Kim K., Kim J. H., Lee J., Jin H. M., Lee S. H., Fisher D. E., Kook H., Kim K. K., Choi Y., Kim N. (2005) Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 280, 35209–35216 [DOI] [PubMed] [Google Scholar]
- 32. Gelb B. D., Moissoglu K., Zhang J., Martignetti J. A., Bromme D., Desnick R. J. (1996) Cathepsin K: isolation and characterization of the murine cDNA and genomic sequence, the homologue of the human pycnodysostosis gene. Biochem. Mol. Med. 59, 200–206 [DOI] [PubMed] [Google Scholar]
- 33. Lee S. H., Rho J., Jeong D., Sul J. Y., Kim T., Kim N., Kang J. S., Miyamoto T., Suda T., Lee S. K., Pignolo R. J., Koczon-Jaremko B., Lorenzo J., Choi Y. (2006) v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 12, 1403–1409 [DOI] [PubMed] [Google Scholar]
- 34. Romas E., Bakharevski O., Hards D. K., Kartsogiannis V., Quinn J. M., Ryan P. F., Martin T. J., Gillespie M. T. (2000) Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 43, 821–826 [DOI] [PubMed] [Google Scholar]
- 35. Romas E., Gillespie M. T., Martin T. J. (2002) Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 30, 340–346 [DOI] [PubMed] [Google Scholar]
- 36. Fujikawa Y., Shingu M., Torisu T., Itonaga I., Masumi S. (1996) Bone resorption by tartrate-resistant acid phosphatase-positive multinuclear cells isolated from rheumatoid synovium. Br. J. Rheumatol. 35, 213–217 [DOI] [PubMed] [Google Scholar]
- 37. Mentaverri R., Kamel S., Brazier M. (2003) Involvement of capacitive calcium entry and calcium store refilling in osteoclastic survival and bone resorption process. Cell Calcium 34, 169–175 [DOI] [PubMed] [Google Scholar]
- 38. Blair H. C., Schlesinger P. H., Huang C. L., Zaidi M. (2007) Calcium signalling and calcium transport in bone disease. Subcell. Biochem. 45, 539–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hwang S. Y., Putney J. W., Jr. (2011) Calcium signaling in osteoclasts. Biochim. Biophys. Acta 1813, 979–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teitelbaum S. L. (2000) Bone resorption by osteoclasts. Science 289, 1504–1508 [DOI] [PubMed] [Google Scholar]
- 41. Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., Iwata T., Ohnishi H., Matozaki T., Kodama T., Taniguchi T., Takayanagi H., Takai T. (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 [DOI] [PubMed] [Google Scholar]
- 42. Mocsai A., Humphrey M. B., Van Ziffle J. A., Hu Y., Burghardt A., Spusta S. C., Majumdar S., Lanier L. L., Lowell C. A., Nakamura M. C. (2004) The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. U. S. A. 101, 6158–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takayanagi H. (2007) The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 1116, 227–237 [DOI] [PubMed] [Google Scholar]
- 44. Van der Eerden B. C., Hoenderop J. G., de Vries T. J., Schoenmaker T., Buurman C. J., Uitterlinden A. G., Pols H. A., Bindels R. J., van Leeuwen J. P. (2005) The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. U. S. A. 102, 17507–17512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoenderop J. G., van Leeuwen J. P., van der Eerden B. C., Kersten F. F., van der Kemp A. W., Merillat A. M., Waarsing J. H., Rossier B. C., Vallon V., Hummler E., Bindels R. J. (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masuyama R., Vriens J., Voets T., Karashima Y., Owsianik G., Vennekens R., Lieben L., Torrekens S., Moermans K., Vanden B. A., Bouillon R., Nilius B., Carmeliet G. (2008) TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell. Metab. 8, 257–265 [DOI] [PubMed] [Google Scholar]
- 47. Hogan P. G., Lewis R. S., Rao A. (2010) Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koga T., Matsui Y., Asagiri M., Kodama T., de C. B., Nakashima K., Takayanagi H. (2005) NFAT and Osterix cooperatively regulate bone formation. Nat. Med. 11, 880–885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.