Abstract
The presence of pericytes in brain regions undergoing repair is evident of the recruitment of bone marrow-derived multipotent regenerative cells to the neurovascular unit during angiogenesis. At present, post mortem sampling is the only way to identify them. Therefore, such cell typing is inadequate for preserving neural progenitor cells for any meaningful stem cell therapy. We aimed to target cerebral pericytes in vivo using dual gene transcript-targeted MRI (GT-tMRI) in male C57black6 mice after a 60-min bilateral carotid artery occlusion (BCAO). We attached superparamagnetic iron oxide nanoparticles (SPIONs) to phosphorothioate-modified micro-DNA that targets actin or nestin mRNA. Because BCAO compromises the blood-brain barrier (BBB) and induces expression of α-smooth muscle (αSM)-actin and nestin antigens by pericytes in new vessels, we delivered pericyte-specific magnetic resonance contrast agents (SPION-actin or SPION-nestin at 4 mg Fe/kg) by i.p. injection to C57black6 mice that had experienced BCAO. We demonstrated that the surge in cerebral iron content by inductively coupled plasma-mass spectrometry matched the increase in the frequency of relaxivity. We also found that SPION-nestin was colocalized in αSM- actin- and nestin-expressing pericytes in BCAO-treated C57black6 or transgenic mice [B6.Cg-Tg(CAG-mRFP1) 1F1Hadj/J, expressing red fluorescent protein by actin promoter]. We identified pericytes in the repair patch in living brains after BCAO with a voxel size of 0.03 mm3. The presence of electron-dense nanoparticles in vascular pericytes in the region of BBB injury led us to draw the conclusion that GT-tMRI can noninvasively reveal neural progenitor cells during vascularization.—Liu, C. H., Ren, J. Q., You, Z., Yang, J., Liu, C.-M., Uppal, R., Liu, P. K. Noninvasive detection of neural progenitor cells in living brains by MRI.
Keywords: angiogenesis, drug delivery, pericyte, pharmacokinetics
Approximately 250,000 Americans die each year as a result of cardiac arrest. Fewer than 10% of those who experience cardiac arrest survive >6 mo, and those who survive can develop residual neurological deficits. Currently, magnetic resonance imaging (MRI) and positron emission tomography are the only noninvasive tools available for identifying the location of damaged brain tissue and the important process of tissue repair including therapy using stem cells and the formation of cerebral neovascularization (angiogenesis). Several mesenchymal and hematopoietic lineage cells have been used to validate localized angiogenesis; these cell types include neuronal, glial, and endothelial biomarkers. Using gene transcript-targeted MRI (GT-tMRI) contrast agents specific to actin mRNA (1), we have previously detected a repair patch of new blood vessels after cerebral ischemia in mice, achieving a resolution of 0.06 mm3 using superparamagnetic iron oxide nanoparticles (SPIONs); however, single-target MRI was not sufficient in sensitivity to identify angiogenesis. We have searched for cell types that might express elevated levels of actin mRNA; corroborating our work, others have found that the presence of nestin and α-smooth muscle (SM) actin in brain regions undergoing repair is evidence of the recruitment of bone marrow-derived multipotent regenerative cells to the neurovascular unit during angiogenesis (2–5). Such multipotent regenerative cells can be differentiated as new pericytes in microvessels or as muscle cells in larger vessels.
Pericytes, also known as Rouget cells, vascular SM cells, or mural cells, are a type of multipotent perivascular cell found in small blood vessels (arterioles and capillaries) (6). Pericytes respond to angiogenic stimuli and guide sprouting tubes and exhibit macrophage-like characteristics. Important because of their contractile role in blood flow regulation, pericytes have been detected by several unique antibodies: the antibodies against desmin, αSM-actin, neuron-glia 2, and platelet-derived growth factor receptor-β (PDGFR-β) are traditionally used to identify pericytes. Astroglial cells also express neuron-glia 2 and PDGFR-β (7, 8) but do not express actin (1). Given their diverse activities, functions, and locations, pericytes can serve as markers for new blood vessel formation. We now describe a noninvasive technique to detect nestin mRNA in pericytes by delivering dual targeting agents to the same mice. With use of a 9.4-T magnetic resonance (MR) system with careful shimming, the GT-tMRI technique we have developed and applied successfully to image living mouse brains has an in-plane resolution of 90–120 μm/pixel and, ideally, can detect MR signal throughout a live brain, thus providing longitudinal data on the progression of brain repair through different stages after cerebral ischemia. The objective of the current study was to obtain evidence for additional biomarkers of angiogenesis-associated pericytes in vivo. Here, we describe the following aspects of this neurotechnology: global distribution for target-specific contrast agent after noninvasive delivery, target selectivity for a unique window of detection by MRI, and potential translational application to evaluate neural progenitor cells.
MATERIALS AND METHODS
Animals and housing
All procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult C57black6 male mice (Taconic Farms, Germantown, NY, USA) or B6.Cg-Tg(CAG-mRFP1)1F1Hadj/J mice, which express red fluorescent protein (RFP; driven by chicken β-actin promoter; The Jackson Laboratory, Bar Harbor, ME, USA), 2–3 mo of age (23±2 g body weight), were kept in cages with sawdust bedding, in a room with controlled light cycles (12-h light-dark). All animals were given free access to water and fed standard laboratory chow.
Nomenclature of MR probes
For consistency with the standard nomenclature, which uses capital letters for proteins, we use lowercase letters to denote mRNA-targeted probes (SPION-actin) and uppercase letters to denote future antigen-targeted probes. We use abbreviations for probes with no micro-DNA (SPION-NA) or DNA with random (scrambled) sequence (SPION-Ran).
SPION-sense oligodeoxynucleotide (sODN) conjugation
We attached NeutrAvidin (NA; Thermo Fisher Scientific, Waltham, MA, USA) to dextran-coated SPIONs (or Molday ION; BioPhysics Assay Laboratory, Worcester, MA, USA) to generate SPION-NA (30±15 nm in diameter); the freshly made SPION-NA (with a shelf life of 3 mo) was conjugated with biotinylated sODN-actin, sODN-fosB, sODN-nestin, or sODN-Ran to produce SPION-actin, SPION-fosB, SPION-nestin, or SPION-Ran probe complexes; for all of these probe complexes, we used a consistent ratio of 3 nmol sODN/mg Fe (1). SPION-NA and SPION-Ran, which produce null signal, were used as negative controls in these studies.
ODN complementary to nestin mRNA
Using the coding sequence 1101–1125 of the nestin mRNA (accession number BC062893) from GenBank, we designed and synthesized ODN-nestin (5′FITC-TCCCAAGGAAATGCAGCTTCTGCTT-3′ biotin). This antisense ODN was modified by phosphorothioate (yielding sODN) to protect it from endogenous nucleases. Sequence specificity was verified by RT-PCR from a total brain cDNA library in vitro, using REDExtract-N-Amp PCR reaction mix (Sigma-Aldrich, St. Louis, MO, USA). The product was resolved by agarose gel electrophoresis and stained with SYBR Green nucleic acid stain (9).
Cerebral ischemia model
The traditional cardiac arrest model, which uses potassium chloride and cardiac pulmonary resuscitation, has a mortality rate near 100% (10). For this study, we used a less traumatic model (11) wherein bilateral carotid artery occlusion (BCAO) is used to induce cerebral ischemia in male C57B6 mice (23±2 g). BCAO, known to induce apoptosis in the forebrain of C57B6 mice (12, 13), reduces cerebral blood flow by more than 85%, and because these mice have a defective circle of Willis (14), BCAO induces transient cerebral ischemia and abnormal water diffusion in a pattern similar to that of a cardiac arrest model (15). Sham operation (control, involving no occlusion) or cerebral ischemia induction was induced by BCAO of 30, 45, or 60 min (n=3 each; refs. 12, 13). Each BCAO scenario is thus referred to according to the length of time of occlusion; specifically, BCAO of 45 or 60 min is noted as BCAO-45 or BCAO-60, respectively.
Delivery route for a global distribution in vivo
To validate SPION distribution in the brain of awake mice, we implemented blood-brain barrier (BBB) bypass by cortical puncture. We used a 26-gauge syringe to pass through the dura to the ventricular space (9); by disturbing the BBB in this way, SPION probes are able to enter the brains of normal mice (n=6) after i.p. injection (16–18). We validated BBB bypass 1 wk after cortical puncture using Gd-diethylenetriaminepentaacetic (0.5 mmol/kg, 0.1 ml i.v.)-enhanced MRI, prescanning each of the mice that would receive SPION-sODN (n=3 each) to establish baseline T2*-weighted MRI brain maps (spin-echo rapid acquisition with refocusing echoes, repetition time/echo time = 3000/27 ms, in-plane resolution 0.12×0.12×0.5 mm3).
For GT-tMRI, one type of SPION probe (SPION-Ran or SPION-fosB, 4 mg of Fe/kg i.p.) was administered to each treatment group of 3 mice; T2*-weighted MRI scans were acquired every 2 h after probe delivery, until cerebral relaxivity (R2*) values returned to baseline levels. We confirmed the return to baseline with final MRI 24 h after SPION probe administration. This protocol of BBB bypass induction, noninvasive probe delivery, and MRI acquisition was repeated in another 6 mice for 2 control probes (SPION-actin and SPION-NA) to achieve a sample size for SPION-actin uptake at each time point and to avoid type II error (P=80% at α=0.05). Because R2* values above baseline are positively proportional to iron concentration (19), we compared R2* maps in all GT-tMR scans in the regions contralateral to the punctured hemisphere, where R2* is the rate of signal reduction (R2* = 1/T2* ms × 1000/s). R2* values in the striatum contralateral to the BBB bypass site were obtained 2, 4, 8, and 12 h after SPION delivery, and the values were compared with prescan R2* values using a t test (P≤0.05). R2* maps were aligned, and the percentage increase above the pre-i.p. baseline R2* maps was computed and shown as ΔR2*, i.e., (R2*post-i.p. − R2*pre-i.p.)/R2*pre-i.p. × 100%. Any R2* values above the baseline R2* at a statistically significant level were shown in a time course curve. A post hoc power analysis was used to compute the necessary sample size to avoid type II error for SPION-actin uptake at each time point (P=80% at α=0.05). The noise in the region of interest (ROI) comes from the background before contrast agent delivery; therefore, the R2* of pre-SPION is the background. The contrast/noise ratio was determined by dividing the average R2* post-SPION by the square root of the standard deviation of pre-SPION R2* values from the same ROI. The signal/noise ratio was 7 in the cortex and 10 in the striatum. We consider that ΔR2* values more than twice the baseline value (100% elevation) after administration of any given SPION-sODN will be far more than 3 sd (3 sd = 9–12/s or ≤50% of baseline mean) from the average background and will be less likely to include signal reduction due to background iron of the tissue.
Inductively coupled plasma-mass spectrometry (ICP-MS) for SPION biodistribution
To measure SPION probe biodistribution, mice were euthanized under retrograde perfusion with 5 ml of saline to remove blood pool iron at the noted time points after i.p. SPION-actin administration. Acid-soluble material was obtained, and its iron content was measured with added dysprosium as an internal tracer and correction factor in an Agilent 7500a ICP-MS system (Agilent Technologies, Santa Clara, CA, USA; see Supplemental Data).
Histology and electron microscopy
For immunohistochemical analysis, the mice were put under general anesthesia and retrograde-perfused with ice-cold saline. Whole brains were removed from perfused animals and frozen in n-butanol on dry ice, as described in previous publications (9). Frozen brain tissue sections (thickness 20 μm) were stained with FITC-anti-nestin antibodies (ab5968; Abcam, Cambridge, MA, USA), Cy3-anti-glial fibrillary acidic protein (GFAP; ab4674; Abcam), or Cy3-anti-αSM-actin (ab5694; Abcam). Vascular endothelia were stained by Cy3-Griffonia simplicifolia lectin I (GSL-I, 1:100 dilution), and nucleic acids were stained with Hoechst dye. For transmission electron microscopy (TEM), a 1-mm cube of tissue was excised under a dissection microscope and incubated in freshly prepared ice-cold 2% paraformaldehyde and 2.5% glutaraldehyde in PBS (4°C, overnight). The sample was transferred for TEM sample preparation to the Histology Core Facility of the Systems Biology Division at Massachusetts General Hospital. The preparation and data acquisition were masked with a code. To reduce background stains from osmium, we treated the samples for 5 min with 2% aqueous uranyl acetate for 5 min (20). The coded photographs were delivered to and decoded by the senior principal investigator.
Statistical analysis and goodness of fit of assay results
We assessed gene expression by comparing the SPION retention data with the gene copy number from each mRNA, using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) to analyze the data.
RESULTS
Cerebral ischemia recruits nestin-expressing pericytes
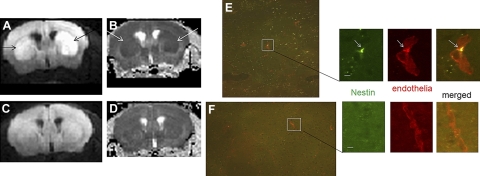
To begin detecting pericytes in vivo, we first investigated the recruitment of angiogenic pericytes (21–23). We initiated cerebral ischemia by BCAO, which induces abnormal water diffusion within 48 h after BCAO. Abnormal water diffusion is shown by hyperintense regions in diffusion-weighted images (hDWIs) in one or both hemispheres (Fig. 1A, B, arrows). Conversely, sham-operated control brains showed very few hDWIs (Fig. 1, C, D). We detected nestin-expressing cells 4 wk after BCAO in the subventricular zone (SVZ) of postmortem brain slices and in the subgranular layer of the dentate gyrus (Fig. 1E). Although nestin can be expressed in certain types of glia (24), we found that most astroglia did not express nestin after BCAO, as others have also reported (2). At this early time point after BCAO, nestin-expressing cells were scattered throughout the parenchyma; mostly they were associated within the neurovascular endothelium (Fig. 1E, insets). Mice that underwent the sham operation procedure contained only very sparse populations of nestin-expressing cells, none of which had associated itself with the neural vasculature (Fig. 1F).
Figure 1.
A, B) Abnormal water diffusion (arrows), shown by DWI-MRI, in one of 4 mice that underwent BCAO-60 on the same date: A) DWI; B) apparent diffusion coefficient (ADC). C, D) MRI of a sham-operated mouse: C) DWI; D) ADC. E, F) Postmortem nestin-expressing cells (green, arrows) in microvessels stained by Cy3-GSL-I, which appeared in the SVZ of the brain after BCAO (E), but not in sham-operated mice (F). Scale bars = 20 μm.
Global distribution of SPION-sODN in mouse brain after systemic and noninvasive delivery
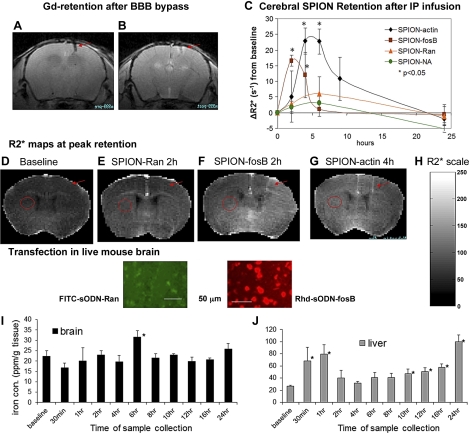
We have demonstrated that BCAO in C57black6 mice induces BBB disruption for ≥12 wk (9) and therefore provides a route for noninvasive probe delivery (1). We aimed to demonstrate that i.p. delivery would allow global distribution of SPION-sODN and a window for MRI in mouse brains with BBB disruption. We first validated a small opening of the BBB using Gd-enhanced T1-weighted MRI to detect enhanced signal surrounding the site of bypass (Fig. 2A, B). We found that the BBB remains open for 3 wk after cortical puncture. Because normal mouse brains do not express nestin, we tested the distribution of targeting SPION-fosB and SPION-actin, introduced via i.p. injection, to FosB (low abundance) and actin mRNA (high abundance) in live brains of normal mice with cortical puncture-induced BBB bypass (Fig. 2C); in addition and for comparison, we examined the distribution of 2 controls with no intracellular targets (SPION-NA and SPION-Ran). Baseline signal reduction frequency (R2*) was 32 ± 3.1/s (n>10), which represented the background before delivery of SPION-sODN, i.e., endogenous iron concentration throughout the brain (Fig. 2D). R2* was not significantly elevated in nontargeting contrast agent (SPION-Ran, Fig. 2E); this is different from what is observed with targeting contrast agents (Fig. 2F–G). We compared SPION retention in the hemisphere contralateral (Fig. 2F–G, circles) with that at the injection site to avoid bias due to cortical puncture (Fig. 2F–G, arrows). Fig. 2C shows the temporal changes (ΔR2* increases or post-contrast agent minus baseline in the ROI of the same mouse) in regional frequency of signal reduction relative to the prescan baseline in the striatum, a representative ROI contralateral to the site of bypass (Fig. 2D–G, circled in red). We observed slight and insignificant changes in the levels of nontargeting contrast agents (SPION-NA or SPION-Ran) relative to baseline levels. The temporal ΔR2* curves produced by these two nontargeting contrast agents represented the dynamics of contrast agents in transit through the brain. On the other hand, we observed statistically significant ΔR2* increases for both SPION-fosB and SPION-actin. The R2* values of SPION-fosB increased by 17/s (60% above baseline) 2 h after i.p. delivery; R2* of SPION-actin increased by 24/s (75% above baseline) at 4 and 6 h and by 12/s (38% above the baseline) at 8 h after i.p. delivery. Both targeting contrast agents returned to baseline levels by 24 h. The distribution of SPION appeared to be a rapid and dynamic process. We measured the highest R2* elevation and duration for SPION-actin, followed by SPION-fosB for fosB mRNA; the lowest measurements were those of SPION-Ran (no target mRNA). Histological striatal sections examined by post mortem optical microscopy exhibited marked differences in retention for FITC-sODN-Ran (Fig. 2E, bottom panel) and Rhd-sODN-FosB (Fig. 1F, bottom panel). The R2* was not reported for SPION-nestin under BBB bypass conditions because normal brains do not express nestin mRNA (see below). Taken together, the data in Fig. 2C–G agreed with our previous report showing that the retention and distribution profiles of both SPION-sODN and fluorophore-sODN probes are associated with mRNA activities.
Figure 2.
Global distribution of SPION-sODN and correlation with iron content after BBB bypass. A, B) Gd-enhanced MRI images acquired before (A) and after (B) administration of Gd (followed by MRI within 30 min) to show BBB bypass (arrows). C) Time course of R2* values above baseline (ΔR2*) after SPION-sODN. D–H) Representative R2* maps from baseline (D), SPION-Ran (E), SPION-fosB (F), and SPION-actin (G) at peak hours after delivery, with scale bar for R2* values (H). Histological striatal sections examined by postmortem optical microscopy showed retention for FITC-sODN-Ran (green; E, bottom panel) and Rhd-sODN-FosB (red; F, bottom panel). I, J) Iron content in the brain (I) and liver (J) at various time points (average±se of n=3/time point) after SPION-actin.
Surges in cerebral iron contents reflect changes in R2* maps
To demonstrate that SPION-sODN retention is supported by iron content, we further validated changes in iron distribution in the brain, lung, kidney, and liver. We examined the distribution of tissue iron (57Fe) at several time points after SPION-actin delivery in normal mice, using the same delivery paradigm. We found that the whole brain retained iron (micromoles per gram wet tissue) in a time-dependent manner. A statistically significant change in iron concentration was seen 6 h postinjection (Fig. 2I); the iron content returned to baseline levels and did not increase throughout the remainder of the 12-h period. There was no increase in iron concentration in the lungs and kidneys throughout the sampling time in two examinations. There was a significant elevation in iron content in the liver after 10 h (Fig. 2J). The same results were obtained in a second experiment involving 33 different mice. Hepatic iron content shows a transient but significant elevation in iron content in the liver during the first hour after delivery and a second increase after 10 h (Supplemental Fig. S1), suggesting hepatic adsorption of the degraded iron from the brain.
FITC-sODN contrast agent for nestin mRNA shows specificity in vitro and in vivo
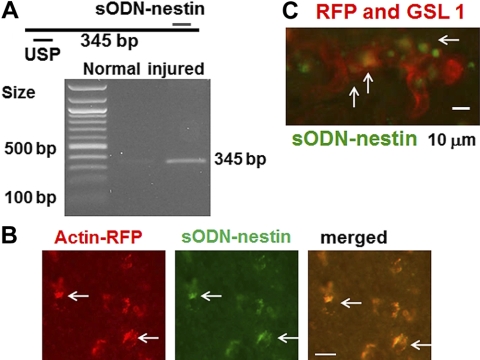
We designed a sODN-nestin probe to target nestin-expressing cells. Figure 3A shows that sODN-nestin targeted one single and unique cDNA in a total cDNA library from mouse striatum after RT-PCR, and the amplicon length matched that of the predicted cDNA segment. We observed that the amplicon signal (indicating nestin mRNA expression) was greater in the brain tissue extracted after BCAO-60 brain injury (Fig. 3A, right lane) than in normal brain (Fig. 3A, center lane); these data signified little nestin expression in normal brain. To demonstrate that SPION-nestin can label pericytes located in the microvasculature, we sought to show specificity of sODN-nestin tagged with FITC in 3 transgenic mice [B6.Cg-Tg(CAG-mRFP1)1F1Hadj/J] after BCAO-45. These mice express RFP controlled by actin. We performed diffusion-weighted imaging (DWI)/MRI within 48 h after BCAO and mapped regions of hDWIs for later reference (Supplemental Fig. S2C) to identify regions where the recruitment of pericytes was most likely to occur. We transfected FITC-sODN-nestin (12 nmol/kg i.p.) 2 (n=1) or 12 (n=2) wk after BCAO to test the specificity of sODN-nestin. We observed that FITC-sODN-nestin was selectively retained in cells expressing RFP (Supplemental Fig. S2D–F, arrows) and therefore coexpressing actin (Fig. 3B). Histology samples stained for vascular endothelia with Cy3-GSL-I revealed that the cells with FITC-sODN-nestin were located mainly in the microvascular walls (Fig. 3C, arrows). The other two transgenic mice showed the same results (Supplemental Fig. S3).
Figure 3.
A) Expected 345-bp amplicon from a mouse cDNA library; indeed, we observe one high-intensity amplicon in BCAO-treated striatal cDNA after BCAO, an observation not made in normal mouse. B) We transfected FITC-sODN-nestin (12 nmol/kg i.p.) to 3 actin-derived RFP-producing mice in wk 2 (n=1) or wk 12 (n=2) after BCAO. C) FITC-sODN-nestin overlaps with cells producing actin-derived RFP or with microvessels stained by Cy3-GSL-I (arrows). Scale bars = 20 μm (B); 10 μm (C).
SPION distribution by electron microscopy
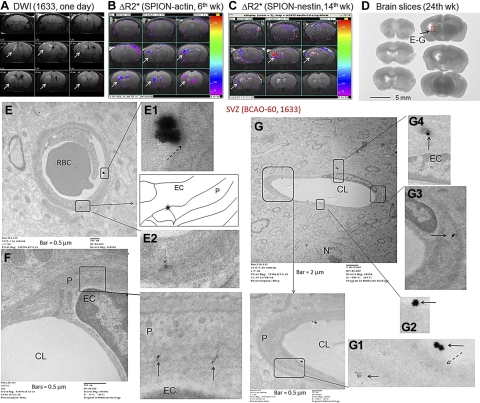
The retention of FITC-sODN-nestin by new vessels after BCAO suggests that the BBB remains leaky, thus allowing noninvasive probe delivery 12 wk after BCAO. To demonstrate that the post-BCAO leaky BBB was large enough for SPION-sODN uptake, we used hDWIs (arrows) to confirm ischemic damage in the potential ROI after BCAO-60 (n=3; Fig. 4A; one is shown). Although the ROIs observed with SPION-actin (Fig. 4B) or SPION-nestin (Fig. 4C) were somewhat variable, both probes uniformly revealed one consistent ROI in the dorsal striatum, near the SVZ region, in MR images acquired 6 and 14 wk after BCAO (Fig. 4B, C; arrows); this ROI matched that identified by hDWI on d 1 (Fig. 4A). To examine uptake and transfer of SPION-sODN, we delivered SPION-nestin (4 mg Fe/kg i.p.) and prepared tissue 4 h later. We obtained tissue from the injured site (Fig. 4D, red box), previously identified by MRI in Fig. 4A–C, and the uninjured region (contralateral to the red square) for electron microscopic examination. Figures 4E and 5G show electron-dense nanoparticles of ∼30 nm (inside diameter) in capillary lumens and suggest the points of contact. Under high magnification, the morphological features show flask-shaped invaginations of the plasma membrane (Figs. 4E, E1; 4G, G1), indicating caveolae-mediated endocytosis (25). Retained nanoparticles appeared to be migrating out of the endothelia (Fig 4E, E2; arrows), reaching the pericytes (Fig. 4F, G, G1, G3, G4, boxes) and transporting across the intercell membrane (Fig. 4E2, F, G, G4) assumed a shape similar to a caveosome (26). No such uptake was observed in uninjured or normal endothelia (Supplemental Fig. S4). The injured tissue contained far more intact SPION near the capillaries than did the normal tissue. The electron microscopic data confirm that the leaky BBB enables SPION-nestin distribution, as seen by optical microscopy.
Figure 4.
Endocytosed SPION-sODN is transported across the leaky BBB after BCAO. We delivered SPION-actin or SPION-nestin (4 mg Fe/kg or 120 nmol sODN/kg, i.p.) to mice at wk 6 and 14 after BCAO-60 to tag actin or nestin mRNA, respectively. A) Brain lesions by DWI (1 d after BCAO). B, C) ΔR2* maps of SPION-actin (B) and SPION-nestin (C); common ROIs with elevated actin and nestin expression (arrows) are approximated to ROIs by DWI-MRI. D–G) At wk 24, we again delivered SPION-nestin 4 h before sample collection for TEM. We obtained brain tissue samples (2-mm cube) from the injured region (D, red box), treated the sample with uranyl acetate (5 s) without osmium stain, and observed that SPION-nestin was being transported across the leaky BBB to pericytes (E–G). Insets (E–G) and panels E1, 2 and G1–4 show enlarged views of boxed areas. CL, capillary lumen; EC, endothelial cell; N, nucleus; P, pericytes.
Figure 5.
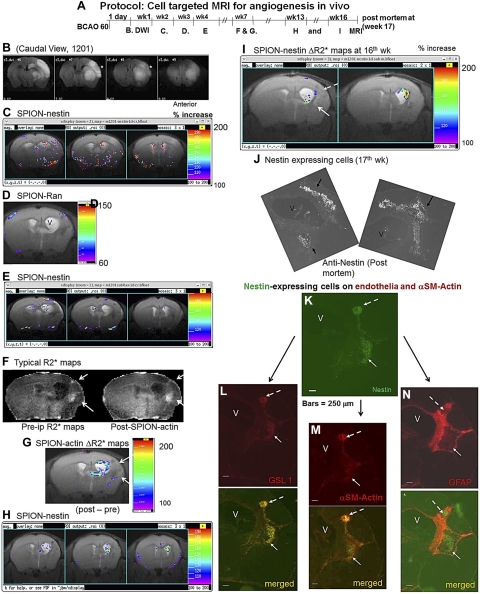
Pericyte identification by dual GT-tMRI in living mouse brains after BCAO-60. A) Protocol. B) DWI-MRI at 1 d of reperfusion (all caudal view, one of 4 surviving mice is shown). We then acquired baseline MRI data, delivered SPION-nestin, SPION-Ran, or SPION-actin and, on the next day, acquired postdelivery MRI on all mice. C–I) ROIs in ΔR2* maps from this same mouse at wk 2 (C), 3 (D), 4 (E), 7 (F, G), 13 (H), and 16 (I); color bars show 100–200% increase from baseline (ΔR2*) except D. We observed ventriculomegaly (V) by wk 3 (D). F) Typical pre- and postdelivery R2* maps, from which ΔR2* maps of the same mouse were obtained in G. G–I) Two cohorts of actin-expressing (G) and nestin-expressing cells (H, I). J–N) FITC-nestin-expressing cells (J) detected by costaining with Cy3-GSL-I for endothelia (L) and Cy3-αSM-actin for pericytes (M), but not Cy3-GFAP for astroglia (N). One of the two cohorts of pericytes has a diameter of 0.25 mm (K, broken arrow) or >3 pixels [(0.25 mm/2)2 × 3.1416/(0.117 mm)2] at the ROI. Scale bar = 250 μm (K–N).
Dual GT-tMRI identified pericytes in living brains
Throughout the course of this study, 33 mice underwent BCAO, 17 underwent BCAO-45, and 16 underwent BCAO-60. DWI validated the effect of ischemia during the first 48 h after the BCAO procedure. Fifteen of 17 mice in the BCAO-45 group (76%) survived >4 mo, but few of the mice in that group developed measurable vessels >0.045 mm3 (3 pixels; Supplemental Fig. S3A). We investigated 4 of 16 mice that survived beyond the first 3 mo after BCAO-60 (Fig. 5A). Two of these 4 mice developed unilateral hDWI within 2 d (Fig. 5B, asterisks, caudal view). We longitudinally examined multiple MRIs in both hemispheres by administering SPION-nestin 2 wk after BCAO (Fig. 5C). The mice received alternating injections of SPION-nestin, SPION-Ran, or SPION-actin between wk 2 and 13 after BCAO (Fig. 5C–I), and we then acquired MRI at 24 h after delivery of each SPION-sODN, as described previously (Fig. 2C). We generated brain ΔR2* maps for each SPION-sODN. We observed scattered hot spots after SPION-nestin delivery in wk 2 (Fig. 5C), but the ΔR2* maps showed no hot spots for nontargeting SPION-Ran (control) in wk 3, except at the surface of the brain (Fig. 5D). These data support findings on the specificity of sODN-nestin in Figs. 3 and 4. We observed ventriculomegaly at 3 wk after BCAO in the hemisphere ipsilateral to the site where hDWI were observed immediately after BCAO (Fig. 5D). By 4 wk after BCAO, more focused ROIs of nestin-expressing cells remained visible in MRI acquired with SPION-nestin (Fig. 5E). To filter potential noise in the ROIs, we delivered SPION-actin during wk 7 after BCAO. Figure 5F shows typical R2* maps before (pre-i.p.) and after (post-i.p.) SPION-actin delivery. Although two potential ROIs along the ventricular wall were present in R2* maps of SPION-actin (Fig. 5F, arrows), the subtraction ΔR2* maps did not show signal elevation in these two potential ROIs (Fig. 5G). Instead, ΔR2* elevation due to SPION-actin retention was measurable in the lateral periphery of the enlarged ventricle (Fig. 5G). These regions contained nestin-expressing cells in wk 13 (Fig. 5H), as well as in wk 16 (Fig. 5I, arrows). GT-tMRI predicted that nestin-expressing cells could be located dorsal and lateral to the enlarged ventricle.
To correlate MRI with histology, we obtained postmortem brain tissue in wk 17 after BCAO. Each slide was immunolabeled for nestin as well as for GSL-I, αSM-actin, or GFAP (control). Cells with nestin antigens were generally located dorsal and lateral to the enlarged ventricle, although some nestin-positive cells were located ventral to the ventricle (Fig. 5J). The MR signal was significantly elevated in both the lateral and dorsal ventricular regions due to SPION-nestin retention in wk 16 (Fig. 5I). Nestin colocalized in cells with endothelial markers GSL-1 and αSM-actin (Fig. 5K–M, arrows) but not with cells expressing the astroglial marker GFAP (Fig. 5N). These nestin+ cells were associated with new vasculature or angiogenesis, which appeared to be surrounded by a cohort of astroglial tubes generated by gliogenesis (Fig. 5K–N); as shown in Fig. 5L, the smaller cohort of cells had an approximate diameter of 0.25 mm, which is equivalent to the MRI observation of 4 pixels (0.014 mm2/pixel) or a voxel of 0.03 mm3 (0.12×0.12×4×0.5 mm thickness). Given the MR and histological data together, we conclude that the elevated R2* signals in the injured tissue near the SVZ are associated with SPION-nestin or SPION-actin uptake by pericytes. In summary, GT-tMR-visible agents target mRNA via the same mechanism of hybridization as that of PCR. Using dual GT-tMRI contrast agents for αSM-actin and nestin mRNA, we identified pericytes after cerebral ischemia. Knowing that pericytes are associated with angiogenesis after cerebral ischemia, our dual MR-visible contrast agents successfully detected new vessels generated during brain repair. This strategy should be applicable to in vivo cell typing in other disease models of the CNS.
DISCUSSION
Nucleic acids have been used for gene activity detection and have advanced our understanding of the molecular mechanisms of diseases in all disciplines of the biological sciences. However, gene activity studies of the brain are rarely done without the use of postmortem or biopsy samples. We have developed a novel in vivo GT-tMRI technique that brings molecular biology investigation to living brains (16–18). The original studies in this area, pioneered by Pardridge and colleagues (27), used single-peptide DNA for targeting. However, we have demonstrated that single-targeting SPION-gfap or SPION-actin (1), as well as SPION-nestin, revealed sporadically located hot spots of cells in BCAO-treated mice (Supplemental Fig S3A). We anticipated that a second SPION probe targeting αSM-actin would increase confidence for identifying true angiogenesis-associated pericytes in the brain; hence, our rationale for pursuing dual GT-tMRI to monitor pericyte recruitment.
There are 3 unique components in our targeting SPION-sODN: carrier (charges in sODN), targeting (sequence in sODN), and reporting (SPION) components. The advantages of our technique reside in the changes in sODN that enable in vivo endocytosis (28). We show that a leaky BBB after BCAO allows the transport of electron-dense nanoparticles toward the cerebral parenchyma via endothelia, which are known to play a role in endocytosis, although a large portion of SPION-sODN transiently appears in hepatic tissue (Fig. 2J). Moreover, micro-DNA-based MR contrast agents that target nestin mRNA are retained with specificity in pericytes in BCAO-treated mice. We have confirmed that the endocytosis of a tiny micro DNA facilitates the uptake of micro-DNA-based GT-tMR contrast agents in cerebral microvessels. In addition, we have demonstrated that the dual application of SPION-nestin and SPION-actin coregistered pericytes in new vessels that express actin and nestin. Indeed, we provide evidence that SPION-actin and SPION-nestin report pericytes: cerebral ischemia induces nestin- and αSM-actin-expressing pericytes in blood vessels as early as the first week after an ischemic episode in C57black6 mice; FITC-sODN-nestin is retained by cells that produce actin-derived GFP in the blood vessels; FITC-sODN-nestin and SPION-nestin are retained by pericytes, as validated by optical and electronic microscopy; and both SPION-actin and SPION-nestin, but not SPION-Ran, are retained in tissue containing pericytes. Although it is true that glia may take up nontargeting iron oxide contrast agents (29), each of our sODNs, except the one with random sequence, guides the MR contrast agent to only one specific fragment of mRNA: sODN-nestin targets nestin mRNA, as RT-PCR confirms (Fig. 3A). In addition, our TEM data did not show glial uptake of SPION-nestin at the dose we delivered; glia in gliogenesis did not retain SPION-actin (1). Although other types of neural progenitors also express nestin mRNA in the cerebral parenchyma, we have acquired substantial histological data in RFP-producing mice that support the specificity of sODN-nestin-guided reporting, and the use of additional contrast agents (SPION-actin or SPION-Ran) eliminates the reporting neural progenitors that are not associated with angiogenesis. The dual sODNs we designed guide the MR-visible contrast agent to the location of nestin- and αSM-actin-expressing pericytes with a specific ROI of 4 pixels (0.2×0.2 mm2/pixel) or a voxel of 0.02 mm3 (with 0.5 mm/MR slice), as determined in the ΔR2 maps acquired using a 9.4-T MR system. Therefore, SPION-sODN-based contrast agents exhibit specificity by preferential uptake in living brains.
We observed rapid turnover of nontargeting iron oxide in brain cells (Fig. 1). This result is consistent with pharmacokinetic studies by some (29) although not consistent with others (30). We have provided evidence from ICP-MS showing correlation between MRI and biodistribution of 57Fe of SPION-actin in the brain. Indeed, the turnover times of targeting SPION-fosB and SPION-actin are slower than that of SPION-Ran, but all are eliminated within 8 h. Most important, given that ICP-MS does not show a second wave of elevation at a later time, the brain neither reabsorbs nor retains nontargeting iron in the circulation.
In this unique longitudinal study, we have acquired pre- and post-SPION-sODN MRI data showing no elevation of endogenous iron in the vasculature, because the variable R2* signal does not appear in subtraction R2* maps (ΔR2* maps). The ΔR2* maps at each time point allow us to eliminate the background baseline signal in the same subjects for ROI identification. Many investigators have shown extended retention of iron oxide in neural cells that exhibit stem cell characteristics (30). A normal brain contains very few neural progenitors, and we should not expect significant retention (Figs. 2E and 3A). Our result indicates that SPION-sODN at the dose of 4 mg of iron (12 nmol sODN)/kg does not induce stem cell activities in live brains (Fig. 5D). Although variable R2* signals may appear before the delivery of SPION-sODNs from endogenous iron in the blood, the inability to retain significant amounts of nontargeting iron, although not yet understood, further enhances the specificity in GT-tMRI.
Some investigators have suggested that we use a stroke model. However, we determined that a middle cerebral artery occlusion stroke model (31) would be inappropriate because it can induce physical damage and recruit pericytes to brain tissue not related to ischemia. Others have suggested using a sense sequence control for GT-tMRI. Although we have observed no change in R2* values from baseline when a sense sequence is used as a control, we chose not to use it in our studies because of the possibility that two cellular targets may cause changes in the MR signal. That is, the MR signal may be affected by binding to genomic DNA if our contrast agent enters the nucleus or by micro-RNA if the binding site in our mRNA target happens to be regulated by micro RNA. By using a control with a random sequence, which has no cellular target, we therefore avoid the off-target effect that can be observed when siRNA is used. Moreover, the sODN-Ran is applicable to all species with very minor changes.
Two current limitations associated with this technology are the presence of NA in the contrast agent and the noise that may have been inherited from the external magnetic field (B0) of the 9.4-T MR system, even though we performed careful shimming before each acquisition. We used avidin-biotin linkage between SPION and sODN to reduce the cost of contrast agent preparation. This linkage can be modified to linkers when there is a need for clinical application. We envision future validation in larger animal models of angiogenesis, using lower MR field strength of 4.7 or 3 T, which may reduce inhomogeneity when we move forward with this technology.
Angiogenesis plays a central role in the establishment of new blood vessel networks that supply nutrients to embryonic tissue during development, in wound healing, and in brain repair after acute neurological insults, as well as in tumor growth and metastasis. Astrogliosis and angiogenesis tend to occur more often in patients with Alzheimer's disease (32) and hyperthermia and neurotoxicity after amphetamine exposure (33–35), whereas patients with diabetes mellitus exhibit impaired angiogenesis (36, 37). Noninvasive functional MRI was used with optogenetics technology to map the global circuitry of intact brains (38). However, our GT-tMRI involves the use of targeting probes to identify regions of altered gene expression in living normal brains affected by pathological conditions. Our analyses were extended over several months and underscored the potential for long-term in vivo monitoring of the brain recovery progress. In addition to what we have demonstrated as a new and noninvasive application of SPION-nestin and SPION-actin for tracking neural progenitor cells or stem cells during brain repair, SPION-sODN can be used as a carrier for gene-targeting delivery for theranostic purposes, to enhance brain recovery. In conclusion, we have brought molecular biology of gene imaging/targeting to applications in the live rodent brain.
Supplementary Material
Acknowledgments
The authors thank M. McKee for assistance in TEM and N. Eusemann for help with editing.
This project was supported by grants from the American Heart Association (grant 2060416) and the U.S. National Institutes of Health (grant AT004974 to J.Q.R. and grants DA026108, DA029889, and EB013768 to P.K.L.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BCAO
- bilateral carotid artery occlusion
- BBB
- blood-brain barrier
- DWI
- diffusion-weighted imaging
- GFAP
- glial fibrillary acidic protein
- GT-tMRI
- gene transcript-targeted magnetic resonance imaging
- GSL-I
- Griffonia simplicifolia lectin I
- hDWI
- hyperintense region in diffusion-weighted image
- ICP-MS
- inductively coupled plasma-mass spectrometry
- MR
- magnetic resonance
- NA
- NeutrAvidin
- ODN
- oligodeoxynucleotide
- PDGFRβ
- platelet-derived growth factor receptor-β
- RFP
- red fluorescent protein
- R2*
- relaxivity
- ΔR2*
- relaxivity above the baseline
- ROI
- region of interest
- SVZ
- subventricular zone
- SM
- smooth muscle
- sODN
- sense oligodeoxynucleotide
- SPION
- superparamagnetic iron oxide nanoparticle
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Liu C. H., You Z., Ren J. Q., Kim Y. R., Eikermann-Haerter K., Liu P. K. (2008) Noninvasive delivery of gene targeting probes to live brains for transcription MRI. FASEB J. 22, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alliot F., Rutin J., Leenan P. J., Pessac B. (1999) Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J. Neurosci. Res. 58, 367–378 [PubMed] [Google Scholar]
- 3. Abrahams J. M., Lenart C. J., Tobias M. E. (2009) Temporal variation of induction neurogenesis in a rat model of transient middle cerebral artery occlusion. Neurol. Res. 31, 528–533 [DOI] [PubMed] [Google Scholar]
- 4. Dore-Duffy P., Katychev A., Wang X., Van Buren E. (2006) CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. 26, 613–624 [DOI] [PubMed] [Google Scholar]
- 5. Kokovay E., Li L., Cunningham L. A. (2006) Angiogenic recruitment of pericytes from bone marrow after stroke. J. Cereb. Blood Flow Metab. 26, 545–555 [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Baeza A., Reina-De La Torre F., Ortega-Sanchez M., Sahuquillo-Barris J. (1998) Perivascular structures in corrosion casts of the human central nervous system: a confocal laser and scanning electron microscope study. Anat. Rec. 252, 176–184 [DOI] [PubMed] [Google Scholar]
- 7. Hughes S., Chan-Ling T. (2004) Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest. Ophthalmol. Vis. Sci. 45, 2795–2806 [DOI] [PubMed] [Google Scholar]
- 8. Bergers G., Song S. (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro. Oncol. 7, 452–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu C. H., Ren J. Q., Yang J., Liu C. M., Mandeville J. B., Rosen B. R., Bhide P. G., Yanagawa Y., Liu P. K. (2009) DNA-based MRI probes for specific detection of chronic exposure to amphetamine in living brains. J. Neurosci. 29, 10663–10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minamishima S., Bougaki M., Sips P. Y., Yu J. D., Minamishima Y. A., Elrod J. W., Lefer D. J., Bloch K. D., Ichinose F. (2009) Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120, 888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu C. H., You Z., Liu C. M., Kim Y. R., Whalen M. J., Rosen B. R., Liu P. K. (2009) Diffusion-weighted magnetic resonance imaging reversal by gene knockdown of matrix metalloproteinase-9 activities in live animal brains. J. Neurosci. 29, 3508–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu P. K., Hsu C. Y., Dizdaroglu M, Floyd R. A., Kow Y. W., Karakaya A, Rabow L. E., Cui J. K. (1996) Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J. Neurosci. 16, 6795–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang D., Shenoy A., Cui J., Huang W., Liu P. K. (2000) In situ detection of AP sites and DNA strand breaks bearing 3′-phosphate termini in ischemic mouse brain. FASEB J. 14, 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshioka H., Niizuma K., Katsu M., Okami N., Sakata H., Kim G. S., Narasimhan P., Chan P. H. (2010) NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J. Cereb. Blood Flow Metab. 31, 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minamishima S., Kida K., Tokuda K., Wang H., Sips P. Y., Kosugi S., Mandeville J. B., Buys E. S., Brouckaert P., Liu P. K., Liu C. H., Bloch K. D., Ichinose F. (2011) Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation 124, 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C. H., Kim Y. R., Ren J. Q., Eichler F., Rosen B. R., Liu P. K. (2007) Imaging cerebral gene transcripts in live animals. J. Neurosci. 27, 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C. H., Huang S., Kim Y. R., Rosen B. R., Liu P. K. (2007) Forebrain ischemia-reperfusion simulating cardiac arrest in mice induces edema and DNA fragmentation in the brain. Mol. Imaging 6, 156–170 [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C. H., Huang S., Cui J., Kim Y. R., Farrar C. T., Moskowitz M. A., Rosen B. R., Liu P. K. (2007) MR contrast probes that trace gene transcripts for cerebral ischemia in live animals. FASEB J. 21, 3004–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boxerman J. L., Hamberg L. M., Rosen B. R., Weisskoff R. M. (1995) MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 34, 555–566 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald M. L., Xavier R., Haley K. J., Welti R., Goss J. L., Brown C. E., Zhuang D. Z., Bell S. A., Lu N., McKee M., Seed B., Freeman M. W. (2007) ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J. Lipid Res. 48, 621–632 [DOI] [PubMed] [Google Scholar]
- 21. Cobbs C.S., Chen J., Greenberg D. A., Graham S. H. (1998) Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 249, 79–82 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z. G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen N., Chopp M. (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 106, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg D.A. (1998) Angiogenesis and stroke. Drug News Perspect. 11, 265–270 [DOI] [PubMed] [Google Scholar]
- 24. Fischer A. J., Scott M. A., Zelinka C., Sherwood P. (2010) A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Müller glia. Glia 58, 633–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pelkmans L., Kartenbeck J., Helenius A. (2001) Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473–483 [DOI] [PubMed] [Google Scholar]
- 27. Lee H. J., Boado R. J., Braasch D. A., Corey D. R., Pardridge W. M. (2002) Imaging gene expression in the brain in vivo in a transgenic mouse model of Huntington's disease with an antisense radiopharmaceutical and drug-targeting technology. J. Nucl. Med. 43, 948–956 [PubMed] [Google Scholar]
- 28. Beltinger C., Saragovi H. U., Smith R. M., LeSauteur L., Shah N., DeDionisio L., Christensen L., Raible A., Jarett L., Gewirtz A. M. (1995) Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J. Clin. Invest. 95, 1814–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muldoon L. L., Nilaver G., Kroll R. A., Pagel M. A., Breakefield X. O., Chiocca E. A., Davidson B. L., Weissleder R., Neuwelt E. A. (1995) Comparison of intracerebral inoculation and osmotic blood-brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am. J. Pathol. 147, 1840–1851 [PMC free article] [PubMed] [Google Scholar]
- 30. Shapiro E. M., Skrtic S., Sharer K., Hill J. M., Dunbar C. E., Koretsky A. P. (2004) MRI detection of single particles for cellular imaging. Proc. Natl. Acad. Sci. U. S. A. 101, 10901–10906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui J., Holmes E. H., Liu P. K. (1999) Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J. Neurochem. 73, 1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalaria R. N., Cohen D. L., Premkumar D. R., Nag S, LaManna J. C., Lust W. D. (1998) Vascular endothelial growth factor in Alzheimer's disease and experimental cerebral ischemia. Brain Res. Mol. Brain Res. 62, 101–105 [DOI] [PubMed] [Google Scholar]
- 33. Ricaurte G., Bryan G., Strauss L., Seiden L., Schuster C. (1985) Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science 229, 986–988 [DOI] [PubMed] [Google Scholar]
- 34. Malberg J. E., Seiden L. S. (1998) Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 18, 5086–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fornai F., Lenzi P., Frenzilli G., Gesi M., Ferrucci M., Lazzeri G., Biagioni F., Nigro M., Falleni A., Giusiani M., Pellegrini A., Blandini F., Ruggieri S., Paparelli A. (2004) DNA damage and ubiquitinated neuronal inclusions in the substantia nigra and striatum of mice following MDMA (ecstasy). Psychopharmacology (Berl.) 173, 353–363 [DOI] [PubMed] [Google Scholar]
- 36. Zhu M., Bi X., Jia Q., Shangguan S. The possible mechanism for impaired angiogenesis after transient focal ischemia in type 2 diabetic GK rats: different expressions of angiostatin and vascular endothelial growth factor. Biomed. Pharmacother. 64, 208–213 [DOI] [PubMed] [Google Scholar]
- 37. Krupinski J., Font A., Luque A., Turu M., Slevin M. (2008) Angiogenesis and inflammation in carotid atherosclerosis. Front. Biosci. 13, 6472–6482 [DOI] [PubMed] [Google Scholar]
- 38. Lee J. H., Durand R., Gradinaru V., Zhang F., Goshen I., Kim D. S., Fenno L. E., Ramakrishnan C., Deisseroth K. (2010) Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.