Abstract
Signals from the intestinal microbiota are important for normal host physiology; alteration of the microbiota (dysbiosis) is associated with multiple disease states. We determined the effect of antibiotic-induced intestinal dysbiosis on circulating cytokine levels and severity of ischemia/reperfusion injury in the heart. Treatment of Dahl S rats with a minimally absorbed antibiotic vancomycin, in the drinking water, decreased circulating leptin levels by 38%, resulted in smaller myocardial infarcts (27% reduction), and improved recovery of postischemic mechanical function (35%) as compared with untreated controls. Vancomycin altered the abundance of intestinal bacteria and fungi, measured by 16S and 18S ribosomal DNA quantity. Pretreatment with leptin (0.12 μg/kg i.v.) 24 h before ischemia/reperfusion abolished cardioprotection produced by vancomycin treatment. Dahl S rats fed the commercially available probiotic product Goodbelly, which contains the leptin-suppressing bacteria Lactobacillus plantarum 299v, also resulted in decreased circulating leptin levels by 41%, smaller myocardial infarcts (29% reduction), and greater recovery of postischemic mechanical function (23%). Pretreatment with leptin (0.12 μg/kg i.v.) abolished cardioprotection produced by Goodbelly. This proof-of-concept study is the first to identify a mechanistic link between changes in intestinal microbiota and myocardial infarction and demonstrates that a probiotic supplement can reduce myocardial infarct size.—Lam, V., Su, J., Koprowski, S., Hsu, A., Tweddell, J. S., Rafiee, P., Gross, G. J., Salzman, N. H., Baker, J. E. Intestinal microbiota determine severity of myocardial infarction in rats.
Keywords: bacteria, leptin, probiotics, biomarkers, cytokines
Humans and other animals are hosts to highly complex ecosystems of colonizing microbes. The vast majority of these microbes (tens of trillions), representing all three domains of life, live in our gastrointestinal tract. There are 1000–1500 bacterial species that colonize the human intestines, and the gene content of microbes in the human gut exceeds that of the host by 100-fold (1). Typically, the intestinal bacteria are commensal organisms that serve as a selective barrier against our greatest environmental exposure, what we eat, and play an essential role in aiding the digestion and absorption of many nutrients (2–4). Axenic (germ free) animals show developmental defects in gastrointestinal epithelial, immunological, and lymphoid tissues (5). Alteration of microbial communities (dysbiosis) in patients or animal models is associated with multiple disease states, including inflammatory bowel disease, obesity, cancer, diabetes, allergy, and response to radiation (4, 6). Intestinal microbiota have also been shown to promote cardiovascular disease, specifically atherosclerosis, by their catabolism of choline (7, 8). Myocardial ketone body metabolism is regulated by intestinal microbiota during nutrient deprivation (9). However, a proof of concept demonstration of a mechanistic link between the composition of the intestinal microbiota and susceptibility to myocardial infarction has not been reported and that is the major goal of this novel study.
Certain probiotics (live microorganisms that both inhibit and promote various species in the intestinal microbiota) may promote cardiovascular function and overall health. Lactobacillus plantarum is a probiotic supplement that confers physiological benefits (10). In animal studies, L. plantarum consumption reduced Enterobacteriaceae, which include the Escherichea and Salmonella genera, reduced bacterial translocation across the intestinal epithelium, reduced inflammatory responses, and increased antibacterial immune activity. In humans, L. plantarum reduced symptoms of irritable bowel syndrome, including abdominal bloating and pain. A study in smokers also showed that supplementation of the diet with L. plantarum reduced serum levels of leptin and fibrinogen and LDL-cholesterol, two risk factors for cardiovascular disease (11). L. plantarum 299v is found in a probiotic fruit juice marketed as ProViva in Europe and Goodbelly (NextFoods, Boulder, CO, USA) in the United States (10, 11).
The majority of the bacteria colonizing the gut are not cultivatable by current techniques. Recent studies have utilized high-throughput and sequencing technologies based on ribosomal RNA (rRNA) gene sequences to advance our understanding of this complex ecosystem (1). The sequence of the 16S and 18S rRNA gene is unique to each eubacteria and fungal species, respectively. Each species has a fixed number of genomic copy numbers for the 16S or 18S rRNA gene. Abundance of 16S or 18S copies provides an index of the relative amount of the microbe. In this study, we utilized rRNA-based polymerase chain reaction (PCR) technologies to investigate the response of this complex ecosystem to perturbation, via antibiotic treatment, and its effect on myocardial infarction. The major objectives were to determine changes in the composition of the intestinal microbiota in response to antibiotic treatment; subsequent severity of injury from myocardial ischemia/reperfusion; the functional effectors of cardioprotection; and whether L. plantarum contained in the probiotic product Goodbelly has cardioprotective effects.
MATERIALS AND METHODS
Rat handling and antibiotic treatment
Rats received humane care in compliance with the U.S National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Rat handling and use protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin (AUA1990). Rats were anesthetized by intraperitoneal injection of pentobarbital (Nembutal; 50 mg/kg) and euthanized with an overdose of intraperitoneal pentobarbital with a pneumothorax performed. While anesthetized, the rats were monitored for anesthetic depth via assessments of the pedal reflex and respiratory rate. Surgical procedures were not continued unless the pedal reflex was lost. In addition, the pedal reflex was monitored every 15–30 min during the procedure, and if detected, the rat was administered additional sodium pentobarbital. The Dahl S rat is an established model of increased susceptibility to injury from myocardial ischemia/reperfusion injury (12, 13). Male Dahl S rats (200–220 g; Charles River, Wilmington, MA, USA) were fed autoclavable laboratory rodent diet 5010 (LabDiet, St. Louis, MO, USA) and given water ad libitum before antibiotic treatment. Vancomycin, an antibiotic known to alter gastrointestinal microbiota (14), was added to the drinking water (0.5 mg/ml). Fresh fecal pellets were obtained from each rat before (d 0) and at d 6 or 7 posttreatment. Pellets were homogenized in 1 ml PBS, and 200 μl of the homogenate was used for microbial DNA isolation using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA, USA).
Fecal microbiota abundance
Isolated DNA samples were subjected to quantitative PCR using an iCycler (Bio-Rad, Hercules, CA, USA) for microbial population enumeration. The PCR reaction mixture consisted of 50% iQ SYBR Green Supermix (Bio-Rad), 0.4 μM forward and reverse primers, and 3.8% template solution in RNase/DNase-free water. Primer sets specific for the 16S and 18S rRNA of particular microbial phylum, class, genus, and species (Methanobrevibacter smithii and L. plantarum) along with reaction temperature and reference strains are detailed in Supplemental Table S1 (14–24).
Ischemia/reperfusion studies in vivo
An in vivo anesthetized rat model was used for these experiments using the general surgical protocol and determination of infarct size (IS) described previously (25). Briefly, following anesthesia, a tracheotomy for artificial ventilation was performed, with the left common carotid artery cannulated for blood pressure and heart rate measurements. A thoracotomy was performed at the fifth intercostal space, the pericardium was excised, and a silk ligature was placed distal to the left atrial appendage that spanned to the sternal portion of the left ventricle, which included the left anterior descending coronary artery. Occlusion of the area described [area at risk (AAR)] was created by placing the ends of the ligature through a polypropylene tube and fixing the snare to the epicardial surface with a hemostat. After 30 min, the hemostat was released to reperfuse the AAR. Following 2 h of reperfusion, the ligature was again occluded, and the AAR was determined by patent-blue negative staining. The heart was then excised, cross-sectioned into 4 to 5 slices, and separated into normal zone and AAR. The pieces were incubated in 1% 2,3,5-triphenyltetrazolium chloride to determine IS. The heart was then incubated overnight in 10% formaldehyde, and the infarcted tissue was dissected from the AAR. IS was expressed as a percentage of the AAR (IS/AAR). Leptin (0.12 μg/kg) was administered intravenously as a bolus at 24 and 12 h before ischemia.
Ischemia/reperfusion studies in vitro
Hearts were perfused retrogradely, as described previously (12). Briefly, hearts were perfused with modified Krebs-Henseleit buffer (120 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 1.20 mM MgSO4, 11 mM glucose, and 1.8 mM CaCl2) bubbled with 95% O2-5% CO2 for a 40-min stabilization period and subjected to 25 min of global no-flow ischemia, followed by 180 min of reperfusion. Before use, all perfusion fluids were filtered through cellulose acetate membranes with pore size of 5.0 μm to remove particulate matter. The hearts were kept in temperature-controlled chambers to maintain myocardial temperature at 37°C. A balloon connected to a pressure transducer was inserted into the left ventricle to monitor cardiac function. For some experiments, hearts were stabilized for 25 min and then perfused with vancomycin for 15 min before ischemia/reperfusion. During the initial 40-min reperfusion period, recovery of mechanical function was measured as left ventricular developed pressure (LVDP) under steady-state conditions and expressed as a percentage of preischemic LVDP. At the end of the 3 h reperfusion period, hearts were processed and stained with 2,3,5-triphenyltetrazolium chloride dye for IS determination.
Blood cytokine analysis
Blood samples were obtained for cytokine analysis before antibiotic treatment (d 0) and at d 6 of antibiotic treatment. Blood samples were kept on ice for 30 min and then centrifuged at 1000 g for 10 min at 4°C to obtain plasma. Plasma samples were then analyzed to determine the concentration of 23 cytokines (Eve Technologies, Calgary, AB, Canada).
Goodbelly supplement
Mango-flavored Goodbelly (NextFoods) juice was obtained from local supermarkets and administered 1×/d in addition to the drinking water. Each liter bottle was stored at 4°C until provided to the rats (15 ml/rat/d). Forty-five milliliters of the juice or vehicle was portioned into a small bottle with a lick spout and made available to each cage of 3 rats. The rats readily consumed the juice within 15 min and were monitored to ensure that none of the rats were excluded from drinking. Negative controls for the Goodbelly treatment included irradiated Goodbelly juice (35 kGy; Sterigenics, Gurnee, IL, USA) and sugar water (water, 92.8 mg/ml glucose, 42.2 μg/ml NaCl, 464 μg/ml KCl, and 4 mg/ml albumin) to control for the sugar, salt, and protein content of the juice. Rats were fed these negative controls in the same quantities as the active Goodbelly juice.
Statistical analysis
Bacterial densities were log10 transformed, and a paired, 2-tailed, t test was used to determine the significance of any differences. Data reported are means ± sd. Statistical analysis was performed by use of the paired, 2-tailed, t test. Significance was set at P < 0.05.
RESULTS
Vancomycin and intestinal microbiota
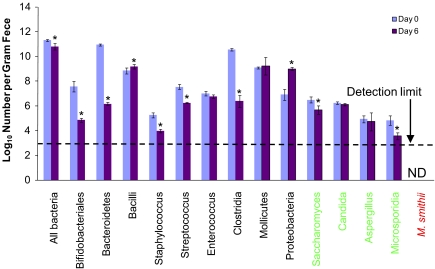
We used the minimally absorbable antibiotic vancomycin added to the drinking water as a tool to alter the composition of the intestinal microbiota. Rats received antibiotic-supplemented drinking water for up to 7 d. The microbial populations present in the feces were monitored by 16S/18S rRNA quantitative-PCR. The primers used uniquely targeted the 16S rRNA gene of each eubacteria and archaea taxon and the unique 18S rRNA gene of each fungal taxon. Vancomycin reduced total bacterial numbers but had varying effects on microbial composition that was species specific (Fig. 1).
Figure 1.
Microbial populations in the feces of vancomycin-treated rats. Vancomycin administered orally (60 mg/kg/d) by addition to the drinking water altered abundance of microbial species present in feces and reduced total microbial numbers. The x-axis labels represent 3 microbial taxa, bacteria (black), fungi (green), and archaea (red). L. plantarum is part of the Bacilli class of bacteria. ND, not detected. Data are means ± sd; n = 6/group. *P < 0.01 vs. d 0.
Vancomycin and myocardial infarction
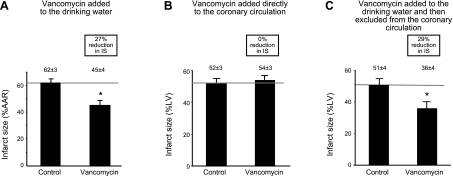
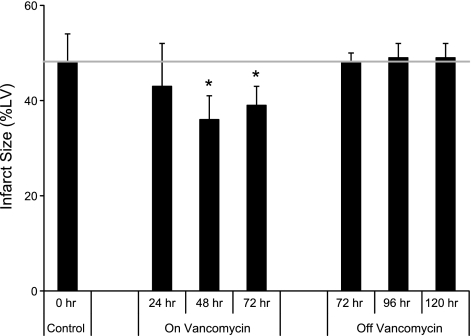
Vancomycin added to the drinking water for 7 d decreased susceptibility of the heart to injury in an in vivo model of regional myocardial ischemia/reperfusion, manifest by a reduction in myocardial IS of ∼27% (Fig. 2A). The minimum treatment time vancomycin needed to decrease IS was 48 h. We observed a return to control values for myocardial IS at 72 h following discontinuation of vancomycin (Fig. 3).
Figure 2.
Antibiotic administration and myocardial infarction. A) Vancomycin added to the drinking water (60 mg/kg/d) reduced IS in vivo. B) Antibiotic added directly to the coronary circulation of isolated hearts did not reduce IS in vitro. C) Antibiotic added to the drinking water (60 mg/kg/d) and then excluded from the coronary circulation of isolated hearts reduced IS. Data are means ± sd; n = 6/group. LV, left ventricle. Reduction in IS was similar for in vitro and in vivo studies (A, C). *P < 0.01 vs. control.
Figure 3.
Vancomycin treatment confers cardioprotection within 48 h, and the effect is lost by 72 h after cessation of treatment. Vancomycin was added to the drinking water (60 mg/kg/d) before heart excision for ischemia/reperfusion studies. Food and (vancomycin) water were fed ad libitum to all rats. Data are means ± sd; n = 4. *P < 0.05 vs. control.
To determine whether this decrease in IS was a direct effect of the antibiotic present in the coronary vasculature, we measured blood levels of vancomycin. The concentration of vancomycin in blood was below the detection limits of the assay used (1 μM). When vancomycin was added directly to the coronary perfusate at a concentration of 1 μM in an in vitro model of myocardial ischemia/reperfusion in rats, there was no reduction in IS (Fig. 2B). To determine whether the decrease in IS was indirect, vancomycin was added to the drinking water and then excluded from the coronary perfusate before ischemia/reperfusion, using an in vitro model of myocardial infarction. Vancomycin decreased IS by 29% (Fig. 2C). These studies showed vancomycin reduced IS despite the absence of the antibiotic in the coronary perfusate at the time of ischemia/reperfusion, suggesting that a direct effect of the antibiotic on the heart is not responsible for the decrease in IS. The extent of reduction in IS using in vivo and in vitro models of myocardial ischemia/reperfusion was comparable (Fig. 2A, C).
Gastrointestinal signaling and myocardial infarction
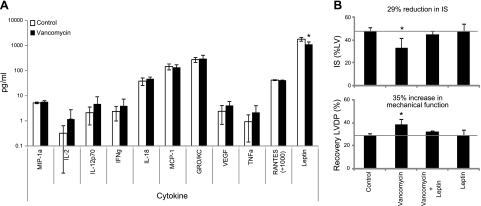
We then quantified the concentration of cytokines in the blood of rats treated with vancomycin to identify antibiotic-induced changes. The antibiotic was administered continuously via the drinking water, and the cytokines were measured before and after treatment. Twenty-three cytokines were examined. Of the 23 cytokines measured, only 11 were reliably quantified, and of these, only leptin was significantly different between the control and treatment groups (Fig. 4A). Vancomycin decreased circulating leptin by 38 ± 4%. To determine whether decreased leptin levels were associated with a reduction in IS, vancomycin-treated rats were administered leptin (0.12 μg/kg i.v.) at 24 and 12 h before ischemia/reperfusion in vitro. This dose was selected to reconstitute leptin concentrations in the circulation. Leptin abolished the decrease in IS and increase in recovery of LVDP conferred by vancomycin treatment (Fig. 4B). Leptin treatment in the absence of vancomycin pretreatment had no effect.
Figure 4.
Intestinal microbiota mediate cardioprotection via leptin. A) Quantified changes in 11 of 23 cytokines. B) Leptin reconstitution reversed cardioprotection by vancomycin. Rats were treated with leptin (0.12 μg/kg i.v.) at 24 and 12 h before myocardial ischemia/reperfusion. Data are means ± sd; n = 6/group. *P < 0.05 vs. control.
Microbiota and myocardial infarction
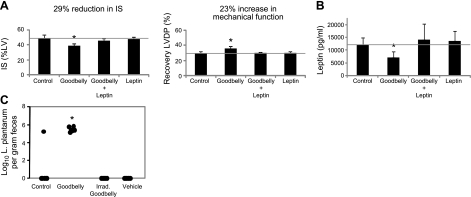
The probiotic L. plantarum lowers leptin levels by 37% in smokers (11) and in mice fed a high-fat diet by 38% (26). To determine whether Goodbelly probiotic juice, which contains 2 probiotic bacteria, L. plantarum (Lp299v) and Bifidobacterium lactis (Bi-07), can reduce leptin levels and severity of myocardial infarction, rats were fed Goodbelly for 14 d. A 29% decrease in IS was observed in Goodbelly-fed rats (Fig. 5A). Administration of leptin (0.12 μg/kg i.v.) at 24 and 12 h before ischemia/reperfusion abolished Goodbelly-induced cardioprotection (Fig. 5A). γ-Irradiated (35 kGy) Goodbelly juice, irradiated juice plus leptin, juice equivalent vehicle, and vehicle plus leptin had no effect on IS (Fig. 6A). Goodbelly treatment also decreased leptin levels in blood by 41% (Fig. 5B). Gamma irradiated (35 kGy) Goodbelly juice, irradiated juice plus leptin, juice equivalent vehicle, and vehicle plus leptin had no effect on leptin levels (Fig. 6B). L. plantarum was not detectable in the feces of rats fed control, vehicle, and irradiated juice but was present at 5.8 log10/g feces in Goodbelly-treated rats (Fig. 5C).
Figure 5.
Goodbelly juice decreased leptin and protected against myocardial infarction. Dahl S rats were treated with Goodbelly (15 ml/rat/d) for 14 d before blood leptin analysis and myocardial ischemia/reperfusion. A) Goodbelly reduced myocardial infarction that was reversed by leptin reconstitution (0.12 μg/kg at 24 and 12 h before ischemia). B) Leptin levels in blood were decreased following Goodbelly treatment. C) L. plantarum levels increased in feces of Goodbelly-treated rats using quantitative PCR of 16S rRNA. Limit of detection for L. plantarum is 3 log10/g feces. Data are means ± sd; n = 6/group. *P < 0.01 vs. control.
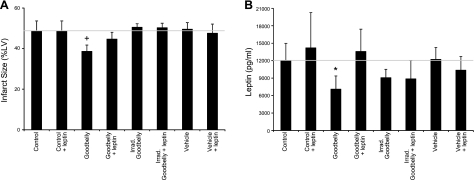
Figure 6.
Goodbelly treatment protected against myocardial infarction and decreased leptin levels. Dahl S rats were treated with either Goodbelly (15 ml/rat/d, ∼1.5×109 L. plantarum/rat/d), irradiated (35 kGy) Goodbelly, or vehicle (water, 92.8 mg/ml glucose, 42.2 μg/ml NaCl, 464 μg/ml KCl, and 4 mg albumin) for 14 d or injected with 0.12 μg/kg leptin at 24 and 12 h, or both, before heart excision for ischemia/reperfusion studies. A) IS of the hearts of treated rats. B) Blood plasma of rats in A was collected immediately before ischemia/reperfusion and analyzed for leptin levels. Data are means ± sd; n = 6. *P < 0.02, +P < 0.01 vs. control.
DISCUSSION
The results of the present study define a proof of concept and a mechanistic link between changes in intestinal microbiota and myocardial infarction. To demonstrate the role of intestinal microbiota in predicting the severity of myocardial infarction, rats were treated orally with the broad-spectrum antibiotic vancomycin to effectively reduce total microbiota numbers and alter the abundance of individual groups of intestinal microbiota. The cardioprotective effect of vancomycin is indirect as this antibiotic is minimally absorbed into the circulation and, when administered directly into the coronary circulation, had no effect on severity of myocardial infarction. When vancomycin was added to the drinking water and the heart was isolated from the circulatory system, a reduction in IS was still observed. Thus, protection was established within the myocardium by vancomycin treatment and manifest despite the absence of the antibiotic in the circulation before ischemia/reperfusion. Cardioprotection was established within 2 d of treatment and lost after treatment ceased for 3 d. We then analyzed cytokine levels in the plasma of vancomycin-treated rats to identify signaling molecules that might mediate myocardial IS. Leptin levels were decreased 38% by vancomycin treatment. The probiotic juice Goodbelly, which contains both L. plantarum (Lp299v) and B. lactis (Bi-07), also decreased circulating leptin levels similar to vancomycin and reduced myocardial IS to the same extent as vancomycin. In either case, pretreatment with leptin abolished cardioprotection by both vancomycin and Goodbelly. Even though L. plantarum is known to reduce blood leptin concentrations, our results with Goodbelly do not necessarily imply that B. lactis has no leptin-regulating activity. Future studies to investigate how individual microbiota species influence cardiovascular biology have the promise to create another dimension of personalized medicine, where the flow of information between the microbiome and the heart can lead to improved diagnostics and therapeutics.
Several studies have suggested associations between coronary disease and bacterial pathogens, such as Helicobactor pylori and Chlamydia pneumonia (27–30). Other studies have shown accelerated atherosclerosis in animal models where infectious pathogens are administered (31, 32). Recently, a direct link among intestinal microbiota, dietary phosphatidylcholine, and risk for cardiovascular disease has been elegantly demonstrated (8). In this study, broad spectrum antibiotics were employed to disrupt the intestinal microbiota and metabolomic analysis identified 3 metabolites of phosphatidylcholine linked to atherosclerosis. Oral administration of vancomycin resulted in robust and reproducible changes in the urine and fecal metabolic profiles in mice using a metabolomic approach (33). In the present study, we employed vancomycin as a tool to identify cytokines regulated by intestinal microbiota that are mechanistically linked to cardioprotection.
Little is understood of the host-microbiome interactions that influence host cardiovascular functions. Our challenge has been to show a proof of concept demonstrating that a perturbation of the intestinal microbiota manifests itself in the host's systemic metabolic phenotype that is capable of affecting severity of myocardial infarction. Metabolites synthesized by the microbiome actively influence host biology, and any dysbiosis in this virtual organ has implications for the host health. L. plantarum, a member of the bacilli taxonomy class of bacteria, is known to reduce the cardiovascular disease biomarkers fibrinogen and LDL-cholesterol in addition to regulating leptin levels in the circulation (11). Our study showed decreased blood leptin concentrations from vancomycin and Goodbelly treatment before ischemia/reperfusion resulted in increased cardioprotection. Leptin is a 16-kDa, 167-aa polypeptide synthesized and secreted into the circulation primarily by white adipocytes. The heart is also a site of leptin production and action (34). Leptin binding to its receptor activates several intracellular pathways, including JAK/STAT, MAPK, Akt, and mammalian target of rapamycin. Leptin treatment induces cardioprotection by activating JAK/STAT and Akt signaling (35). A possible cause for the apparent divergence between previous reports and the present study is the extremely high levels of leptin used previously. Leptin concentrations examined ranged from 1 to 1000 nM (35, 36) in previous studies, compared with the concentration of 0.125 nM we used to reconstitute leptin levels in the circulation.
Our results are supportive of the model of myocardial leptin resistance, which suggests that persistent high levels of leptin in the circulation desensitizes the myocardium to leptin signaling (37). Conversely, persistent reduction in the level of leptin in the circulation enhances the sensitivity of the myocardium to leptin. Taken together, these results suggest that a probiotic may be able to affect a biphasic protection of the myocardium by reducing the level of leptin in the circulation. As demonstrated in the current study, decreased leptin levels in the circulation decreases the myocardium's susceptibility to acute injury from ischemia/reperfusion while other studies have showed that reduced leptin signaling through blockade of the leptin receptor results in decreased chronic cardiac hypertrophy (38). We propose that altering the intestinal microbiota with probiotics to decrease leptin levels in the circulation may be able to mitigate or treat hypertrophy and cardiac remodeling after myocardial infarction. Considering leptin is a key metabolism regulating hormone and the intestinal microbiota contributes to nutrient digestion and absorption, we propose that the cardioprotective phenotype may be achieved via multiple and independent interactions of metabolites with signaling pathways. The present results show that the host metabolic phenotype leptin is strongly influenced by the intestinal microbiome. Little is understood about the impact of the host genome on intestinal microbiota and susceptibility to myocardial infarction. In addition, the composition of the microbiome between genetically distinct inbred rat stains is unknown. One of the authors (J.E.B.) has shown that when environmental influences are minimized, inbred rat strains exhibit different susceptibilities to injury from myocardial ischemia and reperfusion (12), suggesting an underlying genomic basis. Further studies are needed to define the impact of the host genome on intestinal microbiota, leptin signaling, and myocardial infarction.
Antibiotics have been used in patients with acute coronary syndromes in attempts to improve outcomes. The STAMINA trial with azithromycin or amoxicillin plus metronidazole plus omeprazole reported a 36% reduction in the incidence of cardiac death, unstable angina, or myocardial infarction at 12 wk, and the reduction persisted throughout the 1 yr follow-up (39). In this study, patients were enrolled after onset of myocardial infarction or unstable angina. Similarly the WIZARD trial with azithromycin (absorbed antibiotic; ref. 40), the ROXIS trial with roxithromycin (41), and a trial with gatifloxacin (absorbed antibiotic; ref. 42) have addressed the use of antibiotics in the setting of acute myocardial infarction. These clinical trials focused on antibiotics that can access the circulation and were administered after onset of myocardial infarction and did not address the impact of antibiotic treatment on the composition of the intestinal microbiota. The current study demonstrates the antibiotic vancomycin is not detectable systemically and suggests its beneficial effect on myocardial IS is exerted indirectly through signaling molecules that are released into the circulation. Our findings raise the possibility of a new approach to prevent or treat myocardial infarction: the use of probiotics to supplement the diet. If other bacterial species, in addition to L. plantarum, responsible for controlling leptin levels in the circulation are identified, their selective introduction into the diet would be therapeutically sufficient and less disruptive to the intestinal microbiota than broad-spectrum antibiotics.
The magnitude of cardioprotection afforded by vancomycin (27% reduction in IS) and Goodbelly (29% reduction in IS) in the current study is less than the protection reported with other cardioprotective strategies, such as early ischemic preconditioning (86% reduction in IS; ref. 43) and late ischemic preconditioning (66% reduction in IS; ref. 44). Ischemic preconditioning is a method of producing substantial cardioprotection using brief periods of nonlethal ischemia and reperfusion applied to the heart to confer powerful protection against a subsequent episode of sustained, lethal injury to the heart from ischemia and reperfusion. The conditioning stimulus can be applied either before (ischemic preconditioning), or after the onset of ischemia (ischemic perconditioning), or at the transition from sustained ischemia to reperfusion (ischemic postconditioning; ref. 45). Remote preconditioning (brief nonlethal ischemia and reperfusion to a limb or distant organ before sustained, lethal injury from ischemia and reperfusion to the heart) also exerts powerful cardioprotective effects (52% reduction in IS; ref. 46). However, the magnitude of cardioprotection with vancomycin and Goodbelly is comparable with that of pharmacologic preconditioning with erythropoietin (39% reduction in IS; ref. 47) and thrombopoietin (34% reduction in IS; ref. 48). Pharmacologic preconditioning involves administering a pharmacologic agent, either before, during, or at the onset of reperfusion following sustained ischemia, to confer cardioprotection.
An estimated 1.4 million people in the United States will have a new or recurrent acute myocardial infarction every year, with many survivors experiencing lasting morbidity, progression to heart failure, and death (49). Our discovery of a proof-of-concept relationship between intestinal microbiota-derived metabolites and myocardial infarction provides opportunities for both novel diagnostic tests (fecal microbiota and/or microbial metabolites in feces and/or blood as biomarkers of susceptibility to myocardial infarction) and therapeutic approaches (probiotics, nonabsorbable antimicrobials, and/or microbial metabolites) for the treatment and prevention of myocardial infarction and hypertrophy.
Supplementary Material
Acknowledgments
The authors thank Ravinder Singh (Mayo Laboratories, Rochester, MN, USA) for determining vancomycin levels in blood; Michael Hayward (Medical College of Wisconsin) for guidance with the PCR determinations; and Dr. Jeffrey I. Gordon (Washington University School of Medicine, St. Louis, MO, USA) for providing genomic DNA from M. smithii.
This work was supported by U.S. National Institutes of Health grants HL-54075 and AI-080363 (to J.E.B.), AI-057757 (to N.H.S.), and HL-074317 (to G.J.G.) and by the Foundation for Heart Science (to V.L.). J.E.B. has filed a provisional patent application based on the findings of this study. J.E.B. holds an equity interest in Microbiota Diagnostics, a limited-liability corporation formed to develop the findings of this study.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AAR
- area at risk
- IS
- infarct size
- LVDP
- left ventricular developed pressure
- rRNA
- ribosomal RNA
- PCR
- polymerase chain reaction.
REFERENCES
- 1. Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D. R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.-M., Hansen T., Le Paslier D., Linneberg A., Nielsen H. B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Dore J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Bork P., Ehrlich S. D., Wang J. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005) Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 3. Li M., Wang B., Zhang M., Rantalainen M., Wang S., Zhou H., Zhang Y., Shen J., Pang X., Zhang M., Wei H., Chen Y., Lu H., Zuo J., Su M., Qiu Y., Jia W., Xiao C., Smith L. M., Yang S., Holmes E., Tang H., Zhao G., Nicholson J. K., Li L., Zhao L. (2008) Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. U. S. A. 105, 2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholson J. K., Holmes E., Wilson I. D. (2005) Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. 3, 431–438 [DOI] [PubMed] [Google Scholar]
- 5. Round J. L., Mazmanian S. K. (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 7. Loscalzo J. (2011) Lipid metabolism by gut microbes and atherosclerosis. Circ. Res. 109, 127–129 [DOI] [PubMed] [Google Scholar]
- 8. Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., Wu Y., Schauer P., Smith J. D., Allayee H., Tang W. H., DiDonato J. A., Lusis A. J., Hazen S. L. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford P. A., Crowley J. R., Sambandam N., Muegge B. D., Costello E. K., Hamady M., Knight R., Gordon J. I. (2009) Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. U. S. A. 106, 11276–11281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molin G. (2001) Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73, 380S–385S [DOI] [PubMed] [Google Scholar]
- 11. Naruszewicz M., Johansson M. L., Zapolska-Downar D., Bukowska H. (2002) Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 76, 1249–1255 [DOI] [PubMed] [Google Scholar]
- 12. Baker J. E., Konorev E. A., Gross G. J., Chilian W. M., Jacob H. J. (2000) Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am. J. Physiol. Heart Circ. Physiol. 278, H1395–H1400 [DOI] [PubMed] [Google Scholar]
- 13. Shi Y., Hutchins W., Ogawa H., Chang C. C., Pritchard K. A., Jr., Zhang C., Khampang P., Lazar J., Jacob H. J., Rafiee P., Baker J. E. (2005) Increased resistance to myocardial ischemia in the Brown Norway vs. Dahl S rat: role of nitric oxide synthase and Hsp90. J. Mol. Cell. Cardiol. 38, 625–635 [DOI] [PubMed] [Google Scholar]
- 14. Croswell A., Amir E., Teggatz P., Barman M., Salzman N. H. (2009) Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 77, 2741–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad S., Khan Z., Mustafa A. S., Khan Z. U. (2002) Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. J. Clin. Microbiol. 40, 2483–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. (2009) Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS One 4, e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang H. W., Nam Y. D., Sung Y., Kim K. H., Roh S. W., Yoon J. H., An K. G., Bae J. W. (2007) Quantitative real time PCR assays for the enumeration of Saccharomyces cerevisiae and the Saccharomyces sensu stricto complex in human feces. J. Microbiol. Methods 71, 191–201 [DOI] [PubMed] [Google Scholar]
- 18. Costa C., Costa J. M., Desterke C., Botterel F., Cordonnier C., Bretagne S. (2002) Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 40, 2224–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubota H., Tsuji H., Matsuda K., Kurakawa T., Asahara T., Nomoto K. Detection of human intestinal catalase-negative, gram-positive cocci by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 76, 5440–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuda K., Tsuji H., Asahara T., Matsumoto K., Takada T., Nomoto K. (2009) Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules. Appl. Environ. Microbiol. 75, 1961–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller A., Stellermann K., Hartmann P., Schrappe M., Fatkenheuer G., Salzberger B., Diehl V., Franzen C. (1999) A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clin. Diagn. Lab. Immunol. 6, 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ombrouck C., Ciceron L., Biligui S., Brown S., Marechal P., van Gool T., Datry A., Danis M., Desportes-Livage I. (1997) Specific PCR assay for direct detection of intestinal microsporidia Enterocytozoon bieneusi and Encephalitozoon intestinalis in fecal specimens from human immunodeficiency virus-infected patients. J. Clin. Microbiol. 35, 652–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song Y., Kato N., Liu C., Matsumiya Y., Kato H., Watanabe K. (2000) Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 187, 167–173 [DOI] [PubMed] [Google Scholar]
- 24. Van Kuppeveld F. J., van der Logt J. T., Angulo A. F., van Zoest M. J., Quint W. G., Niesters H. G., Galama J. M., Melchers W. J. (1992) Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 58, 2606–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross E. R., Peart J. N., Hsu A. K., Grover G. J., Gross G. J. (2003) K(ATP) opener-induced delayed cardioprotection: involvement of sarcolemmal and mitochondrial K(ATP) channels, free radicals and MEK1/2. J. Mol. Cell. Cardiol. 35, 985–992 [DOI] [PubMed] [Google Scholar]
- 26. Takemura N., Okubo T., Sonoyama K. (2010) Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med. 235, 849–856 [DOI] [PubMed] [Google Scholar]
- 27. Danesh J., Collins R., Peto R. (1997) Chronic infections and coronary heart disease: is there a link? Lancet 350, 430–436 [DOI] [PubMed] [Google Scholar]
- 28. Epstein S. E., Speir E., Zhou Y. F., Guetta E., Leon M., Finkel T. (1996) The role of infection in restenosis and atherosclerosis: focus on cytomegalovirus. Lancet 348(Suppl. 1), s13–s17 [DOI] [PubMed] [Google Scholar]
- 29. Patel P., Mendall M. A., Carrington D., Strachan D. P., Leatham E., Molineaux N., Levy J., Blakeston C., Seymour C. A., Camm A. J., Northfield T. C. (1995) Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ 311, 711–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saikku P., Leinonen M., Mattila K., Ekman M. R., Nieminen M. S., Makela P. H., Huttunen J. K., Valtonen V. (1988) Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 2, 983–986 [DOI] [PubMed] [Google Scholar]
- 31. Madan M., Bishayi B., Hoge M., Messas E., Amar S. (2007) Doxycycline affects diet- and bacteria-associated atherosclerosis in an ApoE heterozygote murine model: cytokine profiling implications. Atherosclerosis 190, 62–72 [DOI] [PubMed] [Google Scholar]
- 32. Muhlestein J. B., Anderson J. L., Hammond E. H., Zhao L., Trehan S., Schwobe E. P., Carlquist J. F. (1998) Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97, 633–636 [DOI] [PubMed] [Google Scholar]
- 33. Yap I. K., Li J. V., Saric J., Martin F. P., Davies H., Wang Y., Wilson I. D., Nicholson J. K., Utzinger J., Marchesi J. R., Holmes E. (2008) Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J. Proteome Res. 7, 3718–3728 [DOI] [PubMed] [Google Scholar]
- 34. Purdham D. M., Zou M. X., Rajapurohitam V., Karmazyn M. (2004) Rat heart is a site of leptin production and action. Am. J. Physiol. Heart Circ. Physiol. 287, H2877–H2884 [DOI] [PubMed] [Google Scholar]
- 35. Smith C. C., Dixon R. A., Wynne A. M., Theodorou L., Ong S. G., Subrayan S., Davidson S. M., Hausenloy D. J., Yellon D. M. (2010) Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am. J. Physiol. Heart Circ. Physiol. 299, H1265–H1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith C. C., Yellon D. M. (2011) Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol. Ther. 129, 206–219 [DOI] [PubMed] [Google Scholar]
- 37. Ren J., Zhu B. H., Relling D. P., Esberg L. B., Ceylan-Isik A. F. (2008) High-fat diet-induced obesity leads to resistance to leptin-induced cardiomyocyte contractile response. Obesity 16, 2417–2423 [DOI] [PubMed] [Google Scholar]
- 38. Purdham D. M., Rajapurohitam V., Zeidan A., Huang C., Gross G. J., Karmazyn M. (2008) A neutralizing leptin receptor antibody mitigates hypertrophy and hemodynamic dysfunction in the postinfarcted rat heart. Am. J. Physiol. Heart Circ. Physiol. 295, H441–H446 [DOI] [PubMed] [Google Scholar]
- 39. Stone A. F., Mendall M. A., Kaski J. C., Edger T. M., Risley P., Poloniecki J., Camm A. J., Northfield T. C. (2002) Effect of treatment for Chlamydia pneumoniae and Helicobacter pylori on markers of inflammation and cardiac events in patients with acute coronary syndromes: South Thames Trial of Antibiotics in Myocardial Infarction and Unstable Angina (STAMINA). Circulation 106, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 40. O'Connor C. M., Dunne M. W., Pfeffer M. A., Muhlestein J. B., Yao L., Gupta S., Benner R. J., Fisher M. R., Cook T. D. (2003) Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 290, 1459–1466 [DOI] [PubMed] [Google Scholar]
- 41. Gurfinkel E., Bozovich G., Beck E., Testa E., Livellara B., Mautner B. (1999) Treatment with the antibiotic roxithromycin in patients with acute non-Q-wave coronary syndromes. The final report of the ROXIS Study. Eur. Heart J. 20, 121–127 [DOI] [PubMed] [Google Scholar]
- 42. Cannon C. P., Braunwald E., McCabe C. H., Grayston J. T., Muhlestein B., Giugliano R. P., Cairns R., Skene A. M. (2005) Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N. Engl. J. Med. 352, 1646–1654 [DOI] [PubMed] [Google Scholar]
- 43. Fryer R. M., Pratt P. F., Hsu A. K., Gross G. J. (2001) Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J. Pharmacol. Exp. Ther. 296, 642–649 [PubMed] [Google Scholar]
- 44. Rajesh K. G., Sasaguri S., Zhitian Z., Suzuki R., Asakai R., Maeda H. (2003) Second window of ischemic preconditioning regulates mitochondrial permeability transition pore by enhancing Bcl-2 expression. Cardiovasc. Res. 59, 297–307 [DOI] [PubMed] [Google Scholar]
- 45. Hausenloy D. J., Yellon D. M. (2011) The therapeutic potential of ischemic conditioning: an update. Nat. Rev. Cardiol. 8, 619–629 [DOI] [PubMed] [Google Scholar]
- 46. Wolfrum S., Schneider K., Heidbreder M., Nienstedt J., Dominiak P., Dendorfer A. (2002) Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc. Res. 55, 583–589 [DOI] [PubMed] [Google Scholar]
- 47. Baker J. E., Kozik D., Hsu A. K., Fu X., Tweddell J. S., Gross G. J. (2007) Darbepoetin alfa protects the rat heart against infarction: dose-response, phase of action, and mechanisms. J. Cardiovasc. Pharmacol. 49, 337–345 [DOI] [PubMed] [Google Scholar]
- 48. Baker J. E., Su J., Hsu A., Shi Y., Zhao M., Strande J. L., Fu X., Xu H., Eis A., Komorowski R., Jensen E. S., Tweddell J. S., Rafiee P., Gross G. J. (2008) Human thrombopoietin reduces myocardial infarct size, apoptosis, and stunning following ischaemia/reperfusion in rats. Cardiovasc. Res. 77, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roger V. L., Go A. S., Lloyd-Jones D. M., Adams R. J., Berry J. D., Brown T. M., Carnethon M. R., Dai S., de Simone G., Ford E. S., Fox C. S., Fullerton H. J., Gillespie C., Greenlund K. J., Hailpern S. M., Heit J. A., Ho P. M., Howard V. J., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Makuc D. M., Marcus G. M., Marelli A., Matchar D. B., McDermott M. M., Meigs J. B., Moy C. S., Mozaffarian D., Mussolino M. E., Nichol G., Paynter N. P., Rosamond W. D., Sorlie P. D., Stafford R. S., Turan T. N., Turner M. B., Wong N. D., Wylie-Rosett J. (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123, e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.