Abstract
Periodontitis is the most common lytic bone disease and one of the first clinical manifestations of diabetes. Diabetes increases the risk of periodontitis. The aim of the present study was to examine mechanisms by which diabetes aggravates periodontitis. Ligature-induced periodontitis was examined in Goto-Kakizaki rats with type 2 diabetes. A tumor necrosis factor (TNF)-specific-inhibitor, pegsunercept, was applied to diabetic rats after the onset of periodontal disease. Interferon-γ (IFN-γ), TNF-α, interleukin-1 β (IL-1β), fibroblast growth factor-2 (FGF-2), transforming growth factor beta-1 (TGFβ-1), bone morphogenetic protein-2 (BMP-2), and BMP-6 were measured by real-time RT-PCR, and histological sections were examined for leukocyte infiltration and several parameters related to bone resorption and formation. Inflammation was prolonged in diabetic rats and was reversed by the TNF inhibitor, which reduced cytokine mRNA levels, leukocyte infiltration, and osteoclasts. In contrast, new bone and osteoid formation and osteoblast numbers were increased significantly vs. untreated diabetic animals. TNF inhibition in diabetic animals also reduced apoptosis, increased proliferation of bone-lining cells, and increased mRNA levels of FGF-2, TGFβ-1, BMP-2, and BMP-6. Thus, diabetes prolongs inflammation and osteoclastogenesis in periodontitis and through TNF limits the normal reparative process by negatively modulating factors that regulate bone.—Pacios, S., Kang, J., Galicia, J., Gluck, K., Patel, H., Ovaydi-Mandel, A., Petrov, S., Alawi, F., Graves, D. T. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation.
Keywords: bone formation, cytokine, growth factor, inhibitor
Periodontitis is the most common lytic disease of bone and a frequent complication of diabetes (1, 2). Individuals with diabetes have increased risk and severity of periodontal disease (1, 2). Moreover, it has been reported that periodontitis is one of the first clinical manifestations of diabetes (2). Periodontitis involves the loss of supporting structure for the tooth consisting of connective tissue attachment and bone. The control of this disease is important in maintaining the integrity and function of the oral cavity.
Periodontal disease is initiated by bacteria that induce an inflammatory response that causes tissue destruction. More than 700 bacterial species can be found in the oral cavity, yet only a small percentage of these are thought to initiate periodontal disease (3). The process of periodontitis involves colonization of the tooth surface, penetration of the connective tissue by bacteria or their products, and stimulation of inflammation that induces periodontal destruction and might also limit the repair process (4). The inflammatory response, rather than the direct pathological effects of the bacteria, is thought to cause the tissue destruction of periodontal disease, as shown by inhibiting prostaglandins and cytokines or through the action of anti-inflammatory lipid mediators (5–8).
Periodontal disease in humans and experimental animal models is linked to both the innate and adaptive immune response. Several studies have reported that individuals with periodontitis exhibit increased levels of interleukin-1 (IL-1), tumor necrosis factor α (TNF-α), and interleukin-6 (IL-6) in gingiva and crevicular fluid, which is a gingival exudate (9). Both genetic deletion and specific inhibition of these cytokines have been found to reduce periodontal disease progression (6, 9–11). Similarly, mediators of the adaptive immune response are elevated in individuals with periodontal disease, and inhibition or genetic deletion of mediators, such as receptor activator of nuclear factor κ-B ligand (RANKL) and interferon-γ (IFN-γ), result in reduced periodontal disease progression (10, 12, 13).
Several studies demonstrate that the prevalence and severity of periodontal disease are greater in patients with diabetes mellitus (DM) and in animal models of diabetes (1, 2). Diabetes may affect periodontal disease through a number of different avenues, including an impaired antibacterial defense. However, studies investigating whether diabetes causes a change in oral flora have had inconsistent results. An alternative explanation is that diabetes alters the inflammatory response to periodontal pathogens. This finding is supported by studies examining the response to a well-defined inoculum of periodopathic bacteria (14). Under normal circumstances, inflammation normally resolves through an active process regulated by cellular signals (15). However, resolution of inflammation is impaired in the diabetic animals. A greater inflammatory response explains the loss of periodontal tissues, since inflammatory mediators stimulate the production of lytic enzymes that break down connective tissue and formation of osteoclasts that resorb bone, both of which are hallmarks of periodontal disease.
One of the inflammatory mediators that has been implicated in the tendency of diabetics to have greater periodontal disease is TNF-α. Cell cultures studies have shown that lipopolysaccharide (LPS)-stimulated monocytes from individuals with type 1 diabetes produce a greater amount of TNF-α than monocytes from nondiabetic individuals (16). Moreover, TNF-α has been shown to mediate the destructive events induced by exposure to periodontal pathogens (17, 18). In addition, higher levels of TNF-α have been noted in a type 1 diabetes mouse model of periodontal disease that was linked to greater receptor for advanced glycation end-product (RAGE) signaling (19). However, it is not known whether elevated TNF-α contributes to the diabetic complication of enhanced periodontitis.
In the study described here, we determined the degree to which enhanced TNF levels in animals with type 2 diabetes contribute to compromised resolution of periodontal disease. The results demonstrate that diabetes-enhanced TNF plays an important role in the prolonged inflammation and osteoclastogenesis observed in type 2 diabetes in animals and that the enhanced inflammation through TNF significantly reduces the normal capacity to repair resorbed bone by suppressing the expression of growth factors that are needed to simulate proliferation and differentiation and inhibit apoptosis of osteoblasts or their precursors. These studies, for the first time, suggest a molecular basis by which type 2 diabetes could negatively affect bone in a number of pathological conditions by suppressing the expression of mediators needed for bone formation through enhanced and prolonged inflammation.
MATERIALS AND METHODS
Induction of periodontal bone loss and preparation of specimens
Male type 2 diabetes model Goto-Kakizaki (GK) and normoglycemic Wistar rats (8 wk old) were purchased from Charles River Laboratories (Wilmington, MA, USA). GK rats typically become diabetic at 8 wk of age, and experiments were started at 12 wk. Periodontitis was induced by tying silk ligatures around the maxillary second molars. Placement of ligatures induces periodontal disease by facilitating bacterial invasion of gingiva (20). After 7 d, ligatures were removed to initiate resolution of periodontal inflammation and a repair process. Rats were euthanized on d 0, 7 (ligature removal), 11, 15, and 22, based on a previous study (21). The maxillary and mandibular jaws were fixed in 4% paraformaldehyde for 3 d at 4°C and decalcified in Immunocal (Decal Chemical Corp., Tallman, NY, USA) at 4°C for 12 d. Sagittal paraffin sections were prepared at 5 μm; the area between the first and second molar was examined. Measurements in the gingival connective tissue were performed from the epithelial attachment to the crest of bone; measurements associated with bone were carried out from the crest of bone to a depth of 1 mm. A given data set was obtained by counting immunopostive cells or by histomorphometric analysis by one examiner who was calibrated by a second examiner. A diagram of the periodontal tissues is shown in Supplemental Fig. S1. All animal procedures were approved by the University of Medicine and Dentistry of New Jersey Institutional Animal Care and Use Committee.
Inhibition of TNF-α
Diabetic animals were treated with a TNF-α specific inhibitor, pegsunercept, that was generously provided by Amgen (Thousand Oaks, CA, USA). It was administered by intraperitoneal injection (4 mg/kg body weight) at the time of ligature removal and every 4 d thereafter in order to focus on the resolution of periodontal inflammation. Control animals were treated with vehicle alone following the same schedule. Serum glucose levels for vehicle- and pegsunercept-treated diabetic animals were similar, >400 mg/dl.
Detection of number and depth of polymorphonuclear (PMN) and mononuclear leukocytes
The number of mononuclear and PMN leukocytes and the depth of PMN cells in gingival connective tissue were counted in hematoxylin and eosin (H&E) stained sections examined at ×1000 view. The identification of these cells was confirmed by a pathologist.
Osteoclasts, osteoblasts, and osteoid and new bone formation
Osteoclasts were identified and counted as multinucleated tartrate-resistant acid phosphatase (TRAP)-positive bone-lining cells from the bone crest to a depth of 1.0 mm, as we have previously described (21). Osteoblasts were counted as cuboidal bone-lining cells in areas of bone remodeling, and their identity was confirmed by immunohistochemistry using an antibody specific for osteocalcin (data not shown). New bone formation was identified in TRAP-stained sections using the reversal line as a guide, as we have previously described (21). The area of osteoid formation was measured as the unmineralized bone matrix between osteoblasts and the mineralized bone surface evident in H&E-stained sections.
Detection of apoptotic bone-lining cells
Apoptotic cells were detected by an in situ transferase-mediated dUTP nick-end labeling (TUNEL) assay by means of the DeadEnd Fluorometric TUNEL System kit purchased from Promega (Madison, WI, USA) with rTdT enzyme, following the manufacturer's instructions. The number of apoptotic cells was counted from the bone crest to a depth of 1 mm by computer-assisted analysis (Nikon, Melville, NY, USA) from images captured at ×200 view on an immunofluorescence microscope.
Immunohistochemistry
The numbers of cells expressing proliferating cell nuclear antigen (PCNA), bone morphogenetic protein-2 (BMP-2), and fibroblast growth factor-2 (FGF-2) either lining bone or adjacent to bone in the periodontal ligament (PDL) were counted. Sections were stained by immunohistochemistry using paraffin sections with an antibody specific for PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), BMP-2 (NovusBiologicals, Littleton, CO, USA), or FGF-2 (Santa Cruz Biotechnology). Primary antibody was detected by avidin-biotin horseradish peroxidase complex using a biotinylated secondary antibody. To enhance the signal to noise ratio, citrate (pH 6) antigen retrieval was used along with tyramide signal amplification that enhances the chromogenic signal (PerkinElmer, Waltham, MA, USA). Sections were examined at ×600 view.
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from periodontum (tooth, gingival, and alveolar bone around second and third molars) of vehicle- and pegsunercept-treated diabetic rats and assessed for mRNA of rat IFN-γ, TNF-α, IL-1β, FGF-2, transforming growth factor β-1 (TGFβ-1), BMP-2, and BMP-6 by real-time PCR using Taq-Man primers and probe sets (Applied Biosystems, Foster City, CA, USA). Results were normalized to a housekeeping gene, ribosomal protein L32. The experiments were carried out with 4 to 5 animals/group with triplicate samples and performed 2 to 3 times with similar results.
Statistical analysis
One-way analysis of variance was used to determine the differences between groups at a given time point and to compare difference from baseline values within a group. The significance level was set at P < 0.05.
RESULTS
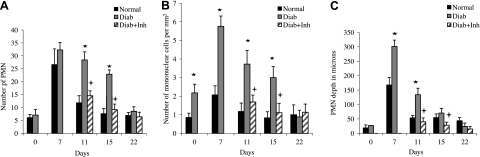
To characterize the inflammatory response at the onset of periodontitis and during its resolution, the number of PMN cells was examined (Fig. 1A and Supplemental Fig. S2). On d 0 in the normoglycemic group, the number of PMN cells was low and increased 4.4-fold after the initiation of periodontitis (d 7; P<0.05). At later time points after ligatures were removed, the number of PMN cells decreased substantially and was close to baseline 8 d later (on d 15). The diabetic group at baseline was similar to the normoglycemic group and also increased 4.5-fold after the initiation of periodontitis. However, on d 11 and 15, the number of PMN cells was still high, so that the values were 2.4- and 3-fold higher in the diabetic group than in the normoglycemic group (P<0.05). By d 15, the numbers were similar to baseline values. Treatment of diabetic animals with a TNF inhibitor reduced the number of PMN cells to that of the normoglycemic animals. The number of mononuclear leukocytes followed a similar pattern, with high levels persisting in the diabetic group following removal of etiologic factors, which was normalized by treatment with a TNF-inhibitor (Fig. 1B).
Figure 1.
Diabetes prolongs an inflammatory infiltrate. Destructive periodontitis was initiated in rats by placement of ligatures around the molar teeth, which facilitates bacterial invasion of gingiva. Resolution of periodontal inflammation was induced by removal of ligatures. TNF was inhibited in diabetic rats by injection with pegsunercept when ligatures were removed and every 4 d thereafter (diab+inh group). Control rats received vehicle alone. Rats were euthanized before placing the ligature (0 d), simultaneously with ligature removal (7 d), and after ligature removal (11, 15, and 22 d). Numbers of PMN cells (A) and mononuclear cells (B) and depth of PMN cells (C) were examined in the gingival connective tissue from the epithelium adjacent to the tooth to distance of 1 mm toward alveolar bone, assessed in H&E-stained sections. *P < 0.05 vs. normoglycemic (normal) group; +P < 0.05 vs. diabetic (diab) group.
Periodontal bone resorption has been linked to the transition of an inflammatory infiltrate from the subepithelial space to deeper areas of connective tissue. At baseline, PMN cells in both normoglycemic and diabetic groups were found only in proximity to the epithelium (Fig. 1C). The inflammatory infiltrate moved deeper within the connective tissue in both normoglycemic and diabetic groups with the onset of periodontitis, although considerably more so in the diabetic group, on d 7. On d 11, the depth of the infiltrate was reduced in the normoglycemic animals, while it remained substantially deeper in the diabetic group (P<0.05). Inhibition of TNF resulted in rapid reversal, so that the inflammatory infiltrate was restored to the subepithelial connective tissue area.
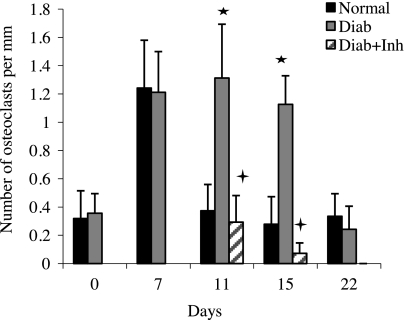
To establish how type 2 diabetes may affect the cessation of bone loss, osteoclast numbers were measured (Fig. 2). At baseline, the number of osteoclasts per millimeter of bone length was low in diabetic and normoglycemic groups. It increased 4-fold on d 7 in the normoglycemic group (P<0.05) and quickly returned to baseline levels within 4 d after the etiologic factor was removed (on d 11). Osteoclast numbers also increased in the diabetic rats with the initiation of periodontal disease but were 2.7- and 4-fold higher in the diabetic group vs. the normoglycemic group on d 11 and 15 (P<0.05). When treated with a TNF inhibitor, the number of osteoclasts in the diabetic group returned to normoglycemic levels.
Figure 2.
Diabetes increases osteoclast formation, which is due to prolonged inflammation. Induction and resolution of periodontal disease and application of TNF inhibitor were performed as in Fig. 1. Osteoclasts were counted in TRAP-stained sections as described in Materials and Methods. *P < 0.05 vs. normoglycemic group; +P < 0.05 vs. diabetic group.
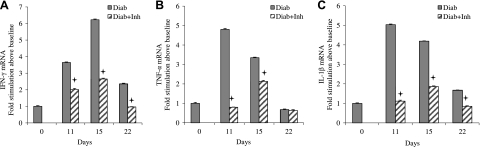
Both the adaptive and innate immune responses are thought to contribute to periodontitis. To assess the effect of the TNF inhibitor on inflammatory cytokine mRNA levels in the periodontal tissue of diabetic rats, IFN-γ (Fig. 3A), TNF-α (Fig. 3B), and IL-1β (Fig. 3C) were measured. IFN-γ mRNA levels in the diabetic animals were substantially higher during the resolution phase of periodontal inflammation compared to baseline. Similarly, IL-1 and TNF-α levels in the diabetic group were higher, and both were decreased by inhibition of TNF. The results indicate that the overall mRNA levels of inflammatory cytokines during the resolution phase in the diabetic animals were reversed by inhibiting TNF.
Figure 3.
Diabetes prolongs mRNA levels of inflammatory cytokines. Induction and resolution of periodontitis and application of TNF inhibitor were performed as in Fig. 1. IFN-γ (A), TNF-α (B), and IL-1β (C) mRNA levels were measured in RNA extracted from rat periodontium by RT-PCR. +P < 0.05 vs. diabetic group.
Bone is programmed to repair, a process called coupled bone formation, which limits net bone loss following an episode of bone resorption. We examined the effect of type 2 diabetes on formation of osteoid, an immature bone matrix (Fig. 4A), new bone formation (Fig. 4B), and the number of osteoblasts capable of forming bone matrix (Fig. 4C). The amounts of osteoid and new bone formation were low in both normoglycemic and diabetic groups at baseline and during the initiation of periodontal inflammation. When periodontal disease ceased, new osteoid formation increased 4-fold and bone formation >10-fold in the normoglycemic group, peaking 8 d after ligatures were removed (on d 15; P<0.05). The diabetic group showed a different pattern, without a significant burst of osteoid or bone formation. Thus, the formation of bone and osteoid was significantly less in the diabetic compared to normoglycemic animals (P<0.05). When treated with a TNF inhibitor, both osteoid and new bone formation in the diabetic group increased to a level equivalent to that of the normoglycemic group, which indicated that diabetes exerted its negative effect on bone formation largely through its effect on inflammation involving TNF.
Figure 4.
Diabetes reduces periodontal bone formation and osteoblast numbers, and this decrease is reversed in diabetic animals by TNF inhibition. New osteoid formation (A) and bone formation (B) were measured in histological sections; osteoblasts were counted per millimeter of bone length (C) in H&E-stained sections. Osteoblast counts were confirmed using osteocalcin-specific immunostaining. *P < 0.05 vs. normoglycemic group; +P < 0.05 vs. diabetic group.
A potential mechanism by which the amount of osteoid and new bone formation is limited by type 2 diabetes is the number of osteoblastic cells that can form bone matrix. This value increased >2.7-fold in the normoglycemic group after periodontal inflammation was reduced during the resolution of inflammation (P<0.05; Fig. 4C). The diabetic group had significantly fewer numbers of osteoblasts at each of these time points (P<0.05). The decrease in osteoblasts in the diabetic group was directly attributable to inflammation, as the number increased to normoglycemic levels when diabetic rats were treated with a TNF inhibitor.
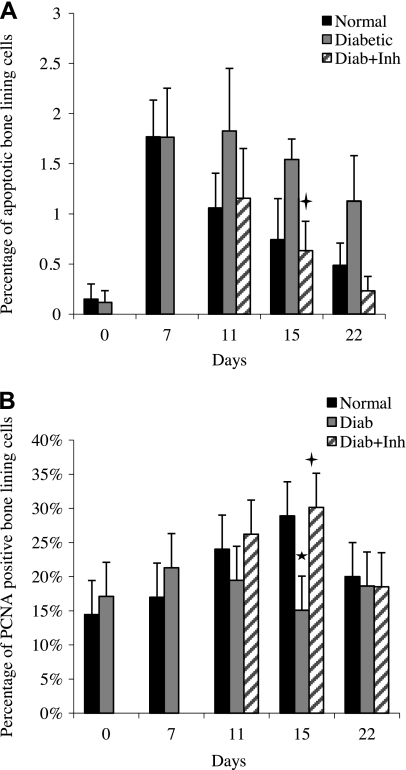
Potential mechanisms by which the number of osteoblasts is limited in animals with type 2 diabetes include increased apoptosis (Fig. 5A) and decreased proliferation of bone-lining cells (Fig. 5B). The number of apoptotic cells in the normoglycemic group increased 10-fold with the induction of periodontal disease and decreased during resolution of periodontal inflammation. The diabetic group exhibited a different pattern, so that the number of apoptotic bone-lining cells was typically 2-fold higher in the diabetic group compared to normoglycemic animals during the resolution phase. When treated with a TNF inhibitor, the higher level of apoptosis was reversed in diabetic animals and followed the same pattern as normoglycemic animals. Proliferation of bone-lining cells was also examined by immunohistochemistry for expression of PCNA (Supplemental Fig. S3). The level of proliferating bone-lining cells significantly increased during the resolution phase in normoglycemic but not in diabetic rats. When TNF was inhibited in diabetic animals, the number of cells expressing PCNA was restored to normoglycemic levels. PCNA-immunopositive cells in proximity to bone were predominantly fibroblastic (Table 1). However, the proportion of fibroblasts and bone-lining cells that were immunopositive for PCNA were similar.
Figure 5.
Diabetic rats have increased apoptosis and decreased proliferation of bone-lining cells, which is reversed by TNF inhibition. Induction and resolution of periodontal disease and application of TNF inhibitor were performed as in Fig. 1. A) Apoptotic bone-lining cells in histological sections were measured by the TUNEL assay. B) Number of PCNA-immunopositive cells was measured by immunohistochemistry. *P < 0.05 vs. normoglycemic group; +P < 0.05 vs. diabetic group.
Table 1.
Immunopositive cells that expressed PCNA, BMP-2, and FGF-2
| Expression | Relative ranking |
|
|---|---|---|
| Based on total number of cells | Based on proportion | |
| PCNA | Fibroblasts > bone-lining cells > endothelial cells | Fibroblasts = bone-lining cells > endothelial cells |
| BMP-2 | Fibroblasts > bone-lining cells > endothelial cells | Bone-lining cells > fibroblasts > endothelial cells |
| FGF-2 | Fibroblasts > bone-lining cells > endothelial cells | Fibroblasts = bone-lining cells > endothelial cells |
Immunopositive cells in proximity to bone were assessed as bone-lining, fibroblastic, or endothelial cells that were part of blood vessels.
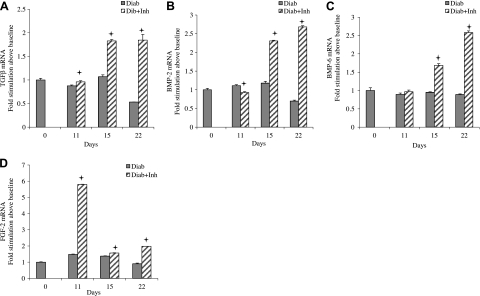
Apoptosis and proliferation of bone-lining cells may be modulated by growth factors that stimulate osteoblasts or their precursors. We examined mRNA levels of a number of factors that affect bone, including TGFβ-1 (Fig. 6A), BMP-2 (Fig. 6B), BMP-6 (Fig. 6C), and FGF-2 (Fig. 6D), focusing on the period of resolution, when apoptosis was elevated and proliferation reduced in the diabetic group. During this period in diabetic animals, little or no increase was found in mRNA levels of these growth factors compared to baseline. However, when TNF was inhibited, mRNA for each of these growth factors increased, with peaks ranging from 2- to 6-fold.
Figure 6.
Diabetes reduces growth factor mRNA levels during resolution of periodontal inflammation, which is reversed by TNF inhibition. Induction and resolution of periodontal disease and application of TNF inhibitor were performed as in Fig. 1. RNA was isolated from periodontal tissue, and mRNA levels of TGFβ (A), BMP-2 (B), BMP-6 (C), and FGF-2 (D) were measured by real-time PCR. +P < 0.05 vs. diabetic group.
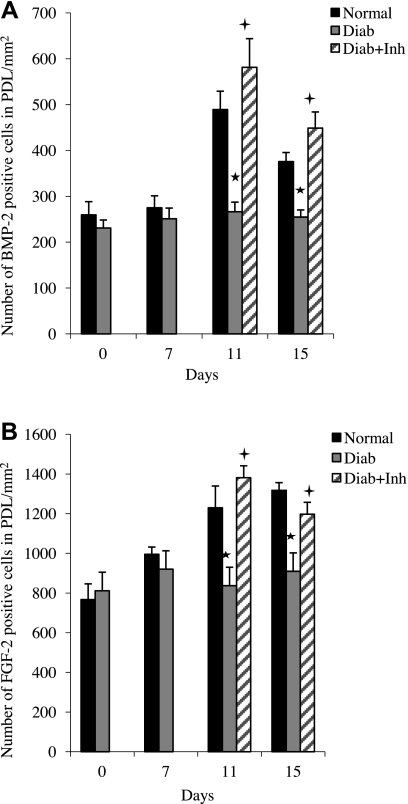
To determine whether there was increased expression of growth factors in proximity to bone, immunohistochemistry was performed, measuring the number of BMP-2-immunopositive cells (Fig. 7A and Supplemental Fig. S4) and FGF-2-immunopositive cells (Fig. 7B) in the periodontal ligament adjacent to bone. At baseline and at the onset of periodontal disease, the number of BMP-2-immunopositive cells was low and similar in the normoglycemic and diabetic groups. BMP-2 expression increased almost 2-fold in the normoglycemic group when periodontal disease was halted but exhibited little increase in the diabetic group. However, when TNF was inhibited, a 2-fold increase was found in the number of BMP-2-immunopositive cells (P<0.05). A similar pattern was observed for FGF-2. In the normoglycemic group, the number of FGF-2-positive cells increased after ligature removal, but relatively little change was found in the diabetic animals. When diabetic animals were treated with TNF inhibitor, a significant 1.7-fold increase was found in the number of FGF-2-expressing cells. BMP-2- and FGF-2-immunopositive cells in proximity to bone were primarily fibroblasts (Table 1). Other cells that prominently expressed BMP-2 and FGF-2 were bone-lining and endothelial cells.
Figure 7.
TNF inhibition enhances BMP-2 and FGF-2 production in the periodontium in diabetic specimens. Induction and resolution of periodontal disease and application of TNF inhibitor were performed as in Fig. 1. Numbers of BMP-2- and FGF-2-expressing cells in proximity to bone (within the periodontal ligament) were measured by immunohistochemistry using antibodies specific for BMP2 (A) and FGF-2 (B). *P < 0.05 vs. normoglycemic group; +P < 0.05 vs. diabetic group.
DISCUSSION
Results described here provide new insight as to how type 2 diabetes can affect bone by its effect on the resolution of inflammation. It is well known that the inflammatory response, rather than the direct pathological effects of bacteria, is pivotal in stimulating periodontal disease (4). Bacteria stimulate an inflammatory response that induces a series of changes that can be damaging to the periodontal tissue, including the destruction of connective tissue matrix and resorption of bone. We found that the formation of a PMN or mononuclear cell inflammatory infiltrate in the type 2 diabetes model rats peaked on d 7 after the initiation of periodontitis and that this inflammation was prolonged compared to the normoglycemic rats, which was normalized by treatment with a TNF inhibitor. Inhibition of TNF also reversed the expression of proinflammatory cytokines induced by initiation of periodontitis. The TNF inhibitor caused a general reduction in cytokine levels; cytokines associated with both the innate and adaptive immune response were reduced significantly when TNF was inhibited. The consequence of these inflammatory changes was a prolonged period of high osteoclast numbers, which was reversed when TNF was blocked. TNF antagonists can directly or indirectly regulate differentiation and activation of osteoclasts, thus reducing bone destruction under pathological conditions (22). Thus, a consequence of prolonged inflammation in the diabetic periodontium is a difficulty in turning off osteoclasts and bone resorption, leading to a longer period of periodontal destruction.
Bone resorption is followed by a period of bone formation, a coupling process that limits the amount of net bone loss, which occurs during the resolution of inflammation in the periodontium (4). We found that type 2 diabetes model GK rats do not generate a burst of bone formation during resolution of inflammation, agreeing with results previously shown in a different type 2 diabetes model, the Zucker diabetic fatty rat (21). Although it has been known that decreased bone formation occurs in diabetic animals, the relationship between inflammation and diminished bone formation has not previously been established. Results presented here demonstrate that the reversal of inflammation increases the capacity of the animal with type 2 diabetes to form new bone. The molecular mechanisms for this reversal were investigated by examining the effect of type 2 diabetes on the production of growth factors that control proliferation, differentiation, or apoptosis of osteoblasts or their precursors. The expression of these factors was blunted in animals with type 2 diabetes but was significantly reversed when inflammation was resolved by inhibition of TNF. The results thus directly link prolonged inflammation in the diabetic group with impaired expression of bone-regulating growth factors. It is also striking that when inflammation was resolved in the diabetic animals by treatment with pegsunercept, substantial improvement was found in the cellular events regulated by these factors, including an increase in proliferation and formation of osteoblasts and a decrease in apoptosis of bone-lining cells.
Previous studies have examined the effect of TNF-specific inhibitors on bone formation. Humans treated with infliximab, a monoclonal antibody against TNF-α, had increased osteocalcin, a marker of bone formation in rheumatoid arthritis, osteoporosis, and other conditions (23). Moreover, infliximab therapy in Crohn's disease influences bone metabolism by enhancing bone formation and decreasing bone resorption. TNF impairs the function of bone-forming osteoblasts by suppressing mature osteoblast function, such as the production of a matrix that is competent for mineralization and by blocking the differentiation of osteoblasts where inflammation is thought to be present (24). This finding is consistent with reports that TNF inhibits differentiation of osteoblasts in vitro (25, 26) and also interferes with bone morphogenetic protein signaling (27). Although it has been reported that inflammation limits bone formation in osteoporosis by reducing Fra-1, we did not observe changes in Fra-1 mRNA levels (data not shown and ref. 28). It has also been reported that the anti-inflammatory mediator, resolvin E1, promotes regeneration of periodontal tissue, which may be related to its anti-inflammatory properties (8, 29). The experiments presented in the present study represent proof of principle that excessive production of inflammatory mediators such as TNF-α limits bone coupling in periodontitis. However, the experimental strategy may not necessarily be extrapolated directly to human studies due to the importance of TNF in up-regulating antibacterial defenses (30, 31).
In sumary, diabetes has an important effect on the periodontium. Both type 1 and type 2 diabetes model animals exhibit an increase in TNF-α in response to a bacterial stimulus when compared to normoglycemic controls (14, 32).We examined the effect of diabetes during the resolution of periodontal inflammation and found that type 2 diabetes prolongs enhanced inflammation. The ramifications of this prolonged inflammation were studied by examining the impact on bone. The diabetic condition reduced coupled bone formation that occurred in the normoglycemic group during resolution of periodontal inflammation. This finding was linked directly to inflammation, as the amounts of new bone and osteoid formed were reversed by inhibiting TNF. The effect was not due to the changes in hyperglycemia, as serum glucose levels did not significantly change after treatment with pegsunercept. The mechanism by which inflammation affects bone was studied by examining factors that regulate bone cells. The production of these factors was enhanced significantly when inflammation was reduced in diabetic animals and explains the increased proliferation and reduced apoptosis of bone cells, as well as the increased numbers of osteoblasts. These results provide a mechanistic basis for how diabetes can negatively affect bone through the effects of enhanced inflammation on the expression of critical factors needed to stimulate bone formation.
Supplementary Material
Acknowledgments
The authors thank Tesfahun Desta (University of Medicine and Dentistry of New Jersey) for help in the experiments and Sunitha Batchu (University of Pennsylvania School of Dental Medicine) for help in preparing the manuscript.
This work was supported by the U.S. National Institute of Dental and Craniofacial Research, grants DE018307 and DE0DE17732.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMP
- bone morphogenetic protein
- DM
- diabetes mellitus
- FGF-2
- fibroblast growth factor-2
- GK
- Goto-Kakizaki
- H&E
- hematoxylin and eosin
- LPS
- lipopolysaccharide
- PCNA
- proliferating cell nuclear antigen
- PDL
- periodontal ligament
- PMN
- polymorphonuclear
- RAGE
- receptor for advanced glycation end-product
- RANKL
- receptor activator of nuclear factor κ-B ligand
- TGFβ-1
- transforming growth factor β-1
- TRAP
- tartrate-resistant acid phosphatase.
REFERENCES
- 1. Graves D. T., Kayal R. A. (2008) Diabetic complications and dysregulated innate immunity. Front. Biosci. 13, 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamster I. B., Lalla E., Borgnakke W. S., Taylor G. W. (2008) The relationship between oral health and diabetes mellitus. J. Am. Dent. Assoc. 139(Suppl.), 19S–24S [DOI] [PubMed] [Google Scholar]
- 3. Kumar P. S., Leys E. J., Bryk J. M., Martinez F. J., Moeschberger M. L., Griffen A. L. (2006) Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J. Clin. Microbiol. 44, 3665–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graves D. T., Li J., Cochran D. L. (2010) Inflammation and uncoupling as mechanisms of periodontal bone loss. J. Dent. Res. 90, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams R., Jeffcoat M., Kaplan M., Goldhaber P., Johnson H., Wechter W. (1985) Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science 227, 640–642 [DOI] [PubMed] [Google Scholar]
- 6. Assuma R., Oates T., Cochran D., Amar S., Graves D. (1998) IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immun. 160, 403–409 [PubMed] [Google Scholar]
- 7. Yu H., Li Q., Herbert B., Zinna R., Martin K., Junior C. R., Kirkwood K. L. (2011) Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 18, 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N. A., Levy B. D., Serhan C. N., Van Dyke T. E. (2006) RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 20, 401–403 [DOI] [PubMed] [Google Scholar]
- 9. Garlet G. P. (2010) Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 89, 1349–1363 [DOI] [PubMed] [Google Scholar]
- 10. Baker P., Dixon M., Evans R., Dufour L., Johnson E., Roopenian D. (1999) CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67, 2804–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delima A., Spyros K., Amar S., Graves D. T. (2002) Inflammation and tissue loss caused by periodontal pathogens is reduced by IL-1 antagonists. J. Infect. Dis. 186, 511–516 [DOI] [PubMed] [Google Scholar]
- 12. Teng Y., Nguyen H., Gao X., Kong Y., Gorczynski R., Singh B., Ellen R., Penninger J. (2000) Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Invest. 106, R59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han X., Kawai T., Eastcott J. W., Taubman M. A. (2006) Bacterial-responsive B lymphocytes induce periodontal bone resorption. J. Immunol. 176, 625–631 [DOI] [PubMed] [Google Scholar]
- 14. Naguib G., Al-Mashat H., Desta T., Graves D. (2004) Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J. Invest. Dermatol. 123, 87–92 [DOI] [PubMed] [Google Scholar]
- 15. Spite M., Serhan C. N. (2010) Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ. Res. 107, 1170–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salvi G., Collins J., Yalda B., Arnold R., Lang N., Offenbacher S. (1997) Monocytic TNFalpha secretion patterns in IDDM patients with periodontal diseases. J. Clin. Periodontol. 24, 8–16 [DOI] [PubMed] [Google Scholar]
- 17. Graves D., Oskoui M., Volejnikova S., Naguib G., Cai S., Desta T., Kakouras A., Jiang Y. (2001) Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 80, 1875–1879 [DOI] [PubMed] [Google Scholar]
- 18. Garlet G. P., Cardoso C. R., Campanelli A. P., Ferreira B. R., Avila-Campos M. J., Cunha F. Q., Silva J. S. (2007) The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin. Exp. Immunol. 147, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalla E., Lamster I. B., Feit M., Huang L., Spessot A., Qu W., Kislinger T., Lu Y., Stern D. M., Schmidt A. M. (2000) Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Invest. 105, 1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rovin S., Costich E. R., Gordon H. A. (1966) The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J. Periodontal Res. 1, 193–204 [DOI] [PubMed] [Google Scholar]
- 21. Liu R., Bal H. S., Desta T., Krothapalli N., Alyassi M., Luan Q., Graves D. T. (2006) Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J. Dent. Res. 85, 510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fantuzzi F., Del Giglio M., Gisondi P., Girolomoni G. (2008) Targeting tumor necrosis factor alpha in psoriasis and psoriatic arthritis. Expert Opin. Ther. Targets 12, 1085–1096 [DOI] [PubMed] [Google Scholar]
- 23. Miheller P., Muzes G., Racz K., Blazovits A., Lakatos P., Herszenyi L., Tulassay Z. (2007) Changes of OPG and RANKL concentrations in Crohn's disease after infliximab therapy. Inflamm. Bowel Dis. 13, 1379–1384 [DOI] [PubMed] [Google Scholar]
- 24. Pacifici R. (2010) The immune system and bone. Arch. Biochem. Biophys. 503, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilbert L., He X., Farmer P., Boden S., Kozlowski M., Rubin J., Nanes M. S. (2000) Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 141, 3956–3964 [DOI] [PubMed] [Google Scholar]
- 26. Lencel P., Delplace S., Hardouin P., Magne D. (2011) TNF-alpha stimulates alkaline phosphatase and mineralization through PPARgamma inhibition in human osteoblasts. Bone 48, 242–249 [DOI] [PubMed] [Google Scholar]
- 27. Guo R., Yamashita M., Zhang Q., Zhou Q., Chen D., Reynolds D. G., Awad H. A., Yanoso L., Zhao L., Schwarz E. M., Zhang Y. E., Boyce B. F., Xing L. (2008) Ubiquitin ligase Smurf1 mediates tumor necrosis factor-induced systemic bone loss by promoting proteasomal degradation of bone morphogenetic signaling proteins. J. Biol. Chem. 283, 23084–23092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang J., Wang Z., Tang E., Fan Z., McCauley L., Franceschi R., Guan K., Krebsbach P. H., Wang C. Y. (2009) Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 15, 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasturk H., Kantarci A., Goguet-Surmenian E., Blackwood A., Andry C., Serhan C. N., Van Dyke T. E. (2007) Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 179, 7021–7029 [DOI] [PubMed] [Google Scholar]
- 30. Havell E. A. (1989) Evidence that tumor necrosis factor has an important role in antibacterial resistance. J. Immunol. 143, 2894–2899 [PubMed] [Google Scholar]
- 31. Kim E. Y., Priatel J. J., Teh S. J., Teh H. S. (2006) TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J. Immunol. 176, 1026–1035 [DOI] [PubMed] [Google Scholar]
- 32. Graves D. T., Naguib G., Lu H., Leone C., Hsue H., Krall E. (2005) Inflammation is more persistent in Type 1 diabetic mice. J. Dent. Res. 84, 324–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.