Abstract
Accumulation of the amyloid β (Aβ) peptide derived from the amyloid precursor protein (APP) plays a central role in the pathogenesis of Alzheimer's disease (AD). We previously reported that the scaffolding protein RanBP9 is markedly increased in AD brains and promotes Aβ generation by scaffolding APP/BACE1/LRP complexes together and accelerating APP endocytosis. Because APP, LRP, and RanBP9 all physically interact with β-integrins, we investigated whether RanBP9 alters integrin-dependent cell adhesion and focal adhesion signaling. Here, we show that RanBP9 overexpression dramatically disrupts integrin-dependent cell attachment and spreading in NIH3T3 and hippocampus-derived HT22 cells, concomitant with strongly decreased Pyk2/paxillin signaling and talin/vinculin localization in focal adhesion complexes. Conversely, RanBP9 knockdown robustly promotes cell attachment, spreading, and focal adhesion signaling and assembly. Cell surface biotinylation and endocytosis assays reveal that RanBP9 overexpression and RanBP9 siRNA potently reduces and increases surface β1-integrin and LRP by accelerating and inhibiting their endocytosis, respectively. Primary hippocampal neurons derived from RanBP9-transgenic mice also demonstrate severely reduced levels of surface β1-integrin, LRP, and APP, as well as neurite arborization. Therefore, these data indicate that RanBP9 simultaneously inhibits cell-adhesive processes and enhances Aβ generation by accelerating APP, LRP, and β1-integrin endocytosis.—Woo, J. A., Roh, S.-E., Lakshmana, M. K., Kang, D. E. Pivotal role of RanBP9 in integrin-dependent focal adhesion signaling and assembly.
Keywords: APP, LRP, RanBPM, integrin, adhesion
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by accumulations of the amyloid β (Aβ) peptide and hyperphosphorylated tau in senile plaques and neurofibrillary tangles, respectively. Aβ is a neurotoxic peptide derived from β- and γ-secretase cleavages of the amyloid precursor protein (APP). The vast majority of APP is constitutively cleaved in the middle of the Aβ sequence by α-secretase [a disintegrin and metalloproteinase (ADAM) 10/17] in the nonamyloidogenic pathway, thereby abrogating the generation of an intact Aβ peptide. However, a small proportion of APP is cleaved in the amyloidogenic pathway, leading to the secretion of Aβ peptides (37–42 aa) via 2 proteolytic enzymes, β-secretase and γ-secretase, known as β-site APP-cleaving enzyme 1 (BACE1) and presenilin, respectively (1). The proteolytic processing of APP to generate Aβ requires endocytosis from the cell surface and localization to cholesterol-rich membrane rafts, where both BACE1 and the presenilin complex are enriched (2, 3).
APP regulates cell adhesion, cell proliferation, neurite outgrowth, and neuronal migration, at least in part, via its interactions with integrins (4, 5). The integrin family mediates both cell-cell and cell-substratum adhesion by binding their extracellular ligands, such as laminin and fibronectin. Integrins consist of an α and β subunit, and each subunit has a large extracellular portion, a single transmembrane segment, and a short cytoplasmic tail (6). The cytoplasmic tails of β-integrins bind to intracellular ligands to mediate dynamic cellular responses, such as cell adhesion, migration, neurogenesis, apoptosis, and synaptic stability. Hence, integrins provide a transmembrane link for the bidirectional transmission of signals across the plasma membrane by binding both extracellular and intracellular ligands (6). The ability to connect to the actin cytoskeleton is an important part of the adhesive function of the integrin family. Within focal adhesions, structural proteins, such as vinculin and talin, anchor β-integrins to the actin cytoskeleton, while signaling proteins, such as focal adhesion kinase (FAK), Pyk2, paxillin, and Src, mediate downstream signaling events in a transient and controlled manner (6).
β-Integrins interact not only with APP but also with the low-density lipoprotein receptor-related protein (LRP; refs. 4, 7, 8). Indeed, LRP functions to mediate APP and β1-integrin trafficking to the cell surface, as well as their endocytosis, resulting in changes in Aβ generation and cell adhesion and motility, respectively (9, 10). Recently, we demonstrated that the scaffolding protein Ran-binding protein 9 (RanBP9) interacts with the cytoplasmic tails of LRP, APP, and BACE1, and functions as a scaffold on which APP is brought together with BACE1 and LRP. Such interactions of RanBP9 promote the endocytosis of APP and strongly increase BACE1 cleavage of APP to generate Aβ (11). RanBP9 is also associated with membrane raft microdomains, consistent with its activity to promote APP localization to rafts (11). Conversely, siRNA knockdown of RanBP9 reduces BACE1 cleavage of APP and Aβ generation, indicating that endogenous RanBP9 normally functions in this capacity (11). In addition, a 60-kDa proteolytic fragment of RanBP9 is robustly increased in brains of patients with AD, and this fragment strongly potentiates Aβ generation via BACE1 processing of APP (12). Interestingly, it has been shown that RanBP9 also physically interacts with β1- and β2-integrin (13), although the functional significance of this interaction is unknown. Given that RanBP9 not only interacts with β-integrins but also with APP and LRP (11, 13), all of which are critical for integrin-mediated signaling, we hypothesized that RanBP9 alters integrin trafficking and/or downstream signaling events. In this study, we found that RanBP9 potently interferes with cell adhesion, as well as focal adhesion signaling and assembly by accelerating the endocytosis of β1-integrin and LRP in a manner analogous to the control of APP endocytosis resulting in enhanced Aβ generation.
MATERIALS AND METHODS
Generation of RanBP9-transgenic mice
N-terminally Flag-tagged human full-length RanBP9 was inserted into the mouse Thy-1 expression cassette (pTSC21K plasmid generously provided by Prof. Jon W. Gordon, Mount Sinai School of Medicine, New York, NY, USA) at the unique SalI site introduced through the linker. Fertilized embryos (pronuclei) from superovulated CB6 mice were microinjected with Thy-1-RanBP9 expression construct and implanted into the oviduct of pseudopregnant recipient mice. Several lines of RanBP9-transgenic mice were identified (including lines 528, 599, and 629). To maintain the lines, the transgenic progeny were crossed with C57BL6 mice. Identification of transgenic mice was achieved by PCR genotyping from genomic DNA extracted from tail using forward primer 5′-GCCACGCATCCAATACCAGCA-3′ and reverse primer 5′-TGCCTGGATTTTGGTTCTCCAC-3′, yielding a PCR product with the size of 443 bp only in RanBP9-transgenic mice. The mouse monoclonal RanBP9 antibody (generously provided by Dr. Elisabetta Bianchi, Pasteur Institute, Paris, France) and Flag M2 antibody (Sigma, St. Louis, MO, USA) were used to determine endogenous and transgenic RanBP9 expression from mouse brain lysates and cryostat sections.
Plasmids and cell adhesion assays
Plasmids pLHCX Flag-RanBP9, p3X Flag-RanBP9, pEGFP-RanBP9, and pEYFP-N1 constructs have previously been described (11, 12). For cell attachment assays, NIH3T3 or HT22 cells were cultured in DMEM supplemented with 10% FBS in fibronectin coated 6-well plates. Transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol for 24 h. Cells (4×105) were added to each well in 96-well plates and incubated in a CO2 incubator at 37°C for 15, 30, or 60 min. Plates were shaken at 300 rpm for 1 min and then washed with washing buffer 3 times. Remaining cells were fixed in 4% paraformaldehyde for 15 min at room temperature, washed 3 times again, stained with crystal violet for 10 min, and lysed in 2% SDS for 30 min. Plates were read at 550 nm using a microplate reader. For spreading assays, images were acquired with a phase-contrast light microscope (Nikon, Tokyo, Japan) every 30 min after replating on fibronectin-coated plates. Spread cells were categorized as those whose nucleus could be clearly distinguished from the cytoplasm. Transient plasmid and siRNA transfections were performed using conditions and sequences previously described (11).
Sholl analysis
Primary hippocampal neurons were transfected with pEYFP plasmid at day in vitro 10 (DIV10). At DIV12, images were acquired with a Nikon Eclipse Ti with YFP channel (492 to >535; ×200). Sholl analysis was performed on the basal neuritic trees using spheres in radius increments of 10 μm. The number of neuritic intersections at each circle was counted.
Surface biotinylation and internalization assay
For surface biotinylation, confluent cells in 6-well plates were washed 3 times in phosphate-buffered saline (PBS) and treated with 2.0 mg/ml sulfo-NHS-LC-biotin in PBS (pH 8.0) under gentle shaking for 1 h on ice. The cells were then washed 3 times in PBS and lysed in 1% Nonidet P-40 lysis buffer. Biotinylated proteins were isolated by pulldown with antibiotin antibody together with anti-mouse agarose beads. Biotin internalization assay was performed using previously described methods (11). Briefly, cells were grown on 6-well plate dishes and incubated twice for 20 min at 4°C with 2 mg/ml of the non-membrane-permeating, cleavable biotin derivative sulfosuccinimidyl 2-(biotinamido)ethyl-1,3′-dithiopropionate (Pierce, Rockford, IL, USA). Cells were then washed with cold PBS containing 0.1 M glycine, followed by several washes with cold PBS. Cells were incubated with culture medium for 0 or 3 min at 37°C to allow internalization of the labeled proteins. Internalization was stopped by rapid cooling on ice. To cleave biotin exposed at the cell surface, cells were incubated 3 times for 20 min at 4°C with 50 mM 2-mercaptoethanesulfonic acid (Sigma) in 50 mM Tris-HCl (pH 8.7), 100 mM NaCl, and 2.5 mM CaCl2. After thorough rinsing with PBS containing 20 mM HEPES, cells were lysed in 1% Nonidet P-40 buffer, and internalized biotinylated proteins were immunoprecipitated with antibiotin antibody and subjected to immunoblotting for LRP or β1-integrin.
Immunnofluorescence
Primary hippocampal neurons isolated from postnatal day 0 (P0) RanBP9 transgenic and wild-type littermate controls were grown on coverslips until DIV14. Neurons were then fixed in 4% paraformaldehyde for 20 min at room temperature and subjected to immunofluorescence for extracellular epitopes of β1-integrin (sc-8978, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), LRP (sc-25469, 1:250; Santa Cruz Biotechnology), or APP (63 d, 1:500) in 3% normal goat serum (NGS) in PBS overnight at 4°C without permeabilization. Then intracellular Flag-RanBP9 was detected using the M2 antibody (Sigma; 1:500) in 3% NGS/PBS containing 0.2% Triton X-100, followed by sequential secondary antibody incubations, Alexa Fluor 488 anti-rabbit IgG and Alexa Fluor 594 anti-mouse IgG (Invitrogen) for 30 min each, and DAPI staining. For vinculin staining in transfected HT22 cells, cells were similarly fixed in 4% paraformaldehyde, followed by detection of primary antibodies (vinculin, V9131, 1:500; Sigma; and RanBP9, PAB16671, 1:500; Abnova, Walnut, CA, USA), secondary antibodies (Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 594 anti-rabbit IgG), and DAPI staining. Fluorescence images were visualized using the Nikon Eclipse Ti microscope.
Statistical analysis
Data were analyzed by Instat3 software (GraphPad Software, San Diego, CA, USA) using either Student's t test or 1-way ANOVA followed by a Neuman-Keuls post hoc test. Data are expressed as means ± se. Differences were deemed significant when P < 0.05. The signal intensity from immunoblots was quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
RESULTS
RanBP9 potently impairs cell adhesion
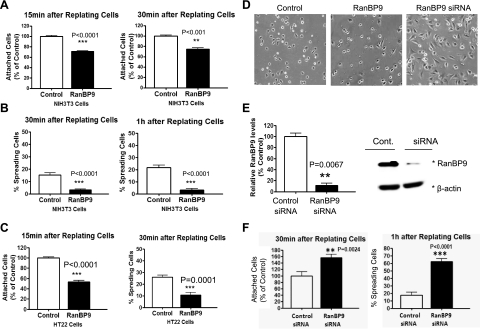
RanBP9 interacts with β-integrins, APP, and LRP (11, 13), all of which are involved in integrin trafficking and signaling. Thus, we tested whether RanBP9 might alter cell attachment and spreading (cell adhesion) in mouse hippocampal HT22 cells and NIH3T3 cells. Vector control or RanBP9-transfected cells were trypsinized and replated onto fibronectin-coated plates for various times, after which unattached cells were removed by shaking and tapping, and the amount of attached cells was quantitated by cresyl violet colorimetric reading. RanBP9 significantly delayed the rate of cell attachment after 15 and 30 min of plating on fibronectin in both NIH3T3 (Fig. 1A) and HT22 cells (Fig. 1C, left panel). Next, we investigated whether RanBP9 impairs cell spreading. We categorized and counted spread cells as those whose nuclei could be clearly distinguished from the cytoplasm. As expected, cell spreading on fibronectin-coated plates was also significantly delayed by RanBP9 in both NIH3T3 (Fig. 1B, D) and HT22 cells (Fig. 1C, right panel). Transfection efficiencies, as measured by FACS analysis of EGFP-transfected NIH3T3 and HT22 cells, were typically ∼90% for both cell lines (Supplemental Fig. S1). To determine whether endogenous RanBP9 is normally involved in the inhibition of cell adhesion, we used siRNA duplexes to silence the expression of endogenous RanBP9 in NIH3T3 cells. Transient transfections of the RanBP9 siRNA reduced endogenous RanBP9 by ∼90% compared to control siRNA-transfected cells without altering β-actin levels (Fig. 1E). As expected, knockdown of RanBP9 via siRNA significantly promoted cell attachment after 30 min of replating on fibronectin-coated plates (Fig. 1F, left panel). Moreover, cell spreading was dramatically enhanced by RanBP9 knockdown (Fig. 1D, F, right panels). Therefore, endogenous RanBP9 normally functions to inhibit cell adhesion.
Figure 1.
RanBP9 strongly impairs cell adhesion. A, C) NIH3T3 (A) and HT22 (C, left panel) cells were transiently transfected for 24 h, and cells were replated onto fibronectin-coated 96-well plates and incubated at 37°C for 15 or 30 min, after which unattached cells were removed by shaking and tapping, and the amounts of attached cells were quantitated by cresyl violet colorimetric reading. Note that RanBP9 transfection delays cell adhesion to fibronectin-coated plates (A; C, left panel). Values are normalized to vector control-transfected plates. B, C) Quantitation of spreading assays demonstrates markedly reduced cell spreading in RanBP9-transfected NIH3T3 (B) and HT22 (C, right panel) cells; y axis represents the actual percentage of cells spreading after 30 and 60 min. D–F) After RanBP9 siRNA transfection (E), cell attachment and spreading assays were performed (F). Note that knockdown of RanBP9 robustly enhances cell attachment and spreading (F). For spreading assays, images were acquired with a phase-contrast light microscope (Nikon) every 30 min after replating on fibronectin-coated plates (D). Representative images at 1 h after replating are shown. Asterisks at right of immunoblots (E, right panel) indicate protein band positions. Error bars = se. **P < 0.01; ***P < 0.001.
RanBP9 disrupts focal adhesion signaling and complex assembly
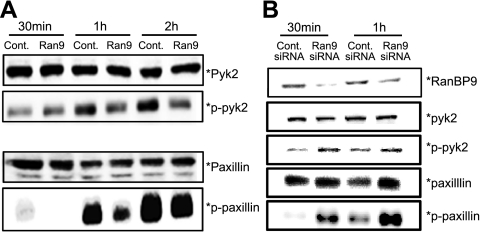
The phosphorylation and activation of paxillin and pyk2 are two signaling components of focal adhesions. Consistent with the delay in cell attachment and spreading in RanBP9-transfected cells, phospho-paxillin and phospho-pyk2 were markedly reduced by RanBP9 after 1 and 2 h of plating on fibronectin, whereas no changes were seen with total paxillin and pyk2 (Fig. 2A). Knockdown of endogenous RanBP9 by siRNA transfection, on the other hand, dramatically increased phospho-pyk2 and phospho-paxillin after 30 and 60 min of plating on fibronectin, consistent with the faster rate of cell adhesion (Fig. 2B).
Figure 2.
RanBP9 disrupts focal adhesion signaling. A) NIH3T3 cells were transfected with either RanBP9 or empty vector for 24 h, and cells were replated on fibronectin-coated 6-well plates. Equal amounts of cell lysates were subjected to immunoblotting for paxillin, phospho-paxillin, pyk2, and phospho-pyk2. Note that RanBP9 induces a marked reduction in phospho-pyk2 and phospho-paxillin despite no changes in total pyk2 or paxillin. B) NIH3T3 cells were transfected with control or RanBP9 siRNA twice over a 48-h period, and cells were replated on fibronectin-coated 6-well plates. Equal amounts of cell lysates were subjected to immunoblotting for paxillin, phospho-paxillin, pyk2, phospho-pyk2, and RanBP9. Note that RanBP9 siRNA dramatically increases phospho-pyk2 and phospho-paxillin without substantial changes in total pyk2 or paxillin. Asterisks at right of immunoblots indicate protein band positions.
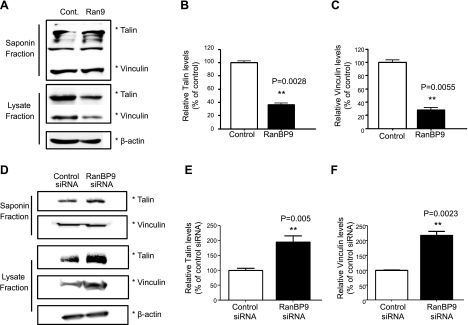
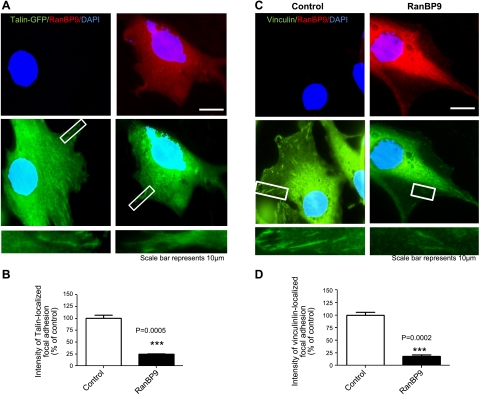
To determine whether the structural assembly of focal adhesions is also altered by RanBP9, we next examined two structural focal adhesion components that link β-integrins to the actin cytoskeleton, vinculin and talin. To biochemically assay for the amount of talin and vinculin within focal adhesions vs. cytosol, we used saponin, a surfactant that permeabilizes the plasma membrane without solubilization. The saponin fraction therefore contains the cytosolic nonfocal adhesion pool, whereas the remaining lysate fraction is enriched in the focal adhesion pool. RanBP9 markedly reduced both talin and vinculin in the focal adhesion pool (lysate fraction), whereas there were no detectable changes in the cytosolic saponin fraction (nonfocal adhesion pool; Fig. 3A–C). In contrast, knockdown of endogenous RanBP9 by siRNA transfection significantly increased the amount of talin and vinculin in the focal adhesion pool without appreciably changing the cytosolic pool (Fig. 3D–F), indicating that endogenous RanBP9 normally functions to inhibit focal adhesion assembly. To demonstrate the status of focal adhesions in a different way, we cotransfected talin-GFP with vector control or RanBP9. After replating cells on fibronectin, GFP-talin could be visualized as diffuse cytosolic signals, as well as in focal adhesion complexes. The intensity of talin-GFP localized focal adhesions relative to diffuse cytosolic talin-GFP was significantly reduced in RanBP9-transfected cells (Fig. 4A, B). Furthermore, the intensity of endogenous vinculin in focal adhesion complexes was also dramatically reduced in RanBP9-transfected cells (Fig. 4C, D). Therefore, these results clearly demonstrate that RanBP9 disrupts focal adhesion assembly and inhibits the localization of talin and vinculin to focal adhesion sites.
Figure 3.
RanBP9 inhibits focal adhesion assembly. A) NIH3T3 cells were transfected with either RanBP9 or empty vector for 48 h; cells were sequentially treated with saponin buffer on ice, saponin extractable material was collected, and remaining cells were extracted by SDS sample buffer and sonicated. Both saponin (nonfocal adhesion pool) and lysate fractions (focal adhesion enriched pool) were resolved on SDS-PAGE gel and subjected to immunoblotting for talin and vinculin. A representative experiment is shown. B, C) Quantitations from 3 experiments show that RanBP9 significantly reduces talin (B) and vinculin (C) in the lysate fraction without changes in the saponin fraction. Asterisks at right of immunoblots indicate protein band positions. D) NIH3T3 cells were transfected with control or RanBP9 siRNA twice over a 48-h period, and cells were subjected to cytosolic and focal adhesion fractionation, as above, followed by immunoblotting for talin and vinculin. Asterisks at right of immunoblots indicate protein band positions. On surface biotinylation and removal of surface biotin without internalization (SR0), no LRP or β1-integrin signals were detected. A representative experiment is shown. E, F) Quantitations from 3 experiments show that RanBP9 siRNA significantly increases talin (E) and vinculin (F) in the lysate fraction without changes in the saponin fraction. Asterisks at right of immunoblots indicate protein band positions. *P < 0.05; ***P < 0.001.
Figure 4.
RanBP9 strongly reduces talin and vinculin localization in focal adhesions. A) NIH3T3 cells were transfected with talin-GFP and either empty vector or RanBP9, and cells were replated 24 h after transfection for 2 h. Cells were then fixed and viewed under a fluorescence microscope (Nikon, Japan). Bottom panels present magnified views of boxed areas. B) Intensity of talin-GFP localized focal adhesions relative to diffuse cytoplasmic talin-GFP demonstrates a ∼75% reduction in the presence of RanBP9 cotransfection (red, P=0.0005). C) NIH3T3 cells were transfected with empty vector or RanBP9, replated 24 h after transfection for 2 h, and subjected to immunofluorescence for endogenous vinculin. Bottom panels present magnified views of boxed areas. D) Intensity of vinculin-localized focal adhesions relative to diffuse cytoplasmic vinculin demonstrates a ∼80% reduction in the presence of RanBP9 transfection (red, P=0.0002). Red signal was adjusted to show little to no endogenous RanBP9. ***P < 0.001.
Accelerated endocytosis of β1-integrin and LRP by RanBP9 underlies inhibition of cell adhesion
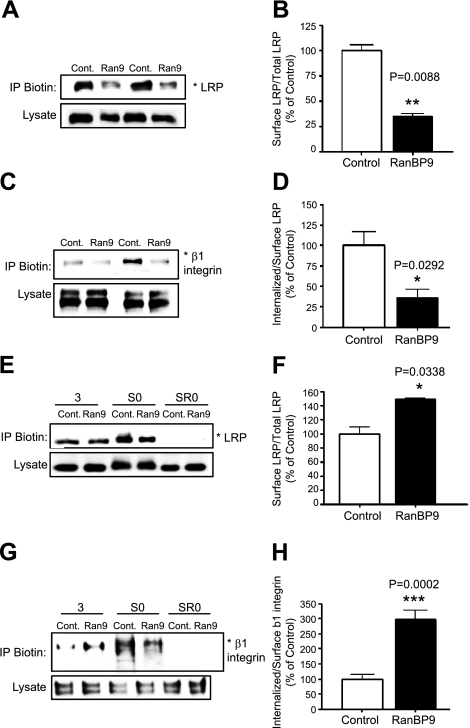
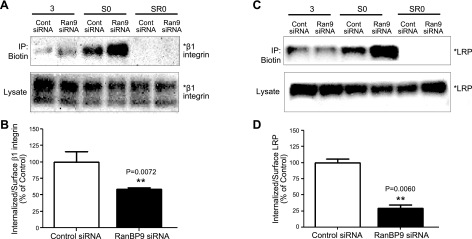
Given that RanBP9 impaired cell adhesion and focal adhesion assembly and signaling, we next assessed whether RanBP9 affects the level of LRP and β1-integrin on the cell surface. Thus, vector control or RanBP9-transfected NIH3T3 cells were biotinylated on ice, and lysates were subjected to immunoprecipitation with antibiotin antibody to pull down biotinylated surface proteins and immunoblotting for LRP and β1-integrin. Indeed, RanBP9 markedly reduced cell surface levels of both LRP (Fig. 5A, B) and β1-integrin (Fig. 5C, D) without altering their total cellular content. As RanBP9 reduced cell surface APP and accelerated its endocytosis (11), we next assessed whether the reduced cell surface levels of LRP and β1-integrin might be due to faster internalization. Therefore, vector control and RanBP9-transfected cells were surface biotinylated with a cleavable biotin derivative on ice and returned to 37°C for 3 min for internalization to proceed. Then noninternalized surface biotin was completely removed, and lysates were subjected to immunoprecipitation for biotin and detection of biotinylated and internalized LRP and β1-integrin. As shown in Fig. 5E, G, although RanBP9 reduced the levels of LRP and β1-integrin on the cell surface without internalization (S0), similar levels of LRP and increased amounts of β1-integrin were detected after 3 min of internalization. Therefore, quantitation from multiple internalization experiments (n=4) demonstrated that RanBP9 accelerated LRP endocytosis by ∼50% and β1-integrin endocytosis by ∼3-fold (Fig. 5F, H). On surface biotinylation and removal of surface biotin without internalization (Fig. 5E, G; SR0), no LRP or β1-integrin signal could be detected, indicating that the removal of surface biotin was complete and that the surface pool did not contribute to the internalized pool of LRP and β1-integrin in this assay (Fig. 5E, G). In contrast, knockdown of endogenous RanBP9 by siRNA dramatically increased the amount of cell surface LRP and β1-integrin (Fig. 6A, C). Similar biotin internalization experiments demonstrated that the increased amounts of surface LRP and β1-integrin secondary to RanBP9 siRNA are largely due to reduced internalization (Fig. 6). These data indicate that both endogenous and exogenous RanBP9 reduces cell surface LRP and β1-integrin largely via accelerating their internalization, essentially identical to that seen with APP (11). Therefore, accelerated endocytosis of β1-integrin and LRP underlies the disruption of cell adhesion and focal adhesion assembly/signaling induced by RanBP9.
Figure 5.
RanBP9 overexpression reduces cell surface LRP and β1-integrin by promoting endocytosis. A, C) Vector control or RanBP9-transfected NIH3T3 cells were biotinylated on ice, and lysates were subjected to immunoprecipitation with antibiotin antibody and immunoblotting for endogenous LRP (A) and β1-integrin (C). Note that RanBP9 transfection markedly reduces cell surface levels of both LRP and β1-integrin without altering their total cellular content. Asterisks at right of immunoblots indicate protein band positions. A representative experiment is shown. B, D) Quantitation from multiple experiments (n=4) demonstrates a significant reduction in surface LRP (B, P=0.0088) and β1-integrin (D, P=0.0292) relative to total LRP and β1-integrin levels. E, G) Control or RanBP9-transfected cells were subjected to surface biotin internalization experiments. Note that although RanBP9 reduces the levels of LRP (E) and β1-integrin (G) on the cell surface without internalization (S0), similar levels of LRP and increased amounts of β1-integrin were detected after 3 min of internalization. On surface biotinylation and removal of surface biotin without internalization (SR0), no LRP or β1-integrin signals were detected. A representative experiment is shown. F, H) Quantitation from multiple internalization experiments (n=4) demonstrates that RanBP9 accelerates LRP endocytosis by ∼50% (F, P=0.0338) and β1-integrin endocytosis by ∼3-fold (H, P=0.002). Error bars = se. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 6.
RanBP9 knockdown enhances cell surface LRP and β1-integrin by reducing endocytosis. A, C) Control or RanBP9 siRNA-transfected NIH3T3 cells were subjected to surface biotin internalization experiments. Note that RanBP9 siRNA dramatically increases the levels of β1-integrin (A) and LRP (C) on the cell surface without internalization (S0). However, reduced proportions of LRP and β1-integrin are detected after 3 min of internalization. On surface biotinylation and removal of surface biotin without internalization (SR0), no LRP or β1-integrin signals were detected. Asterisks at right of immunoblots indicate protein band positions. A representative experiment is shown. B, D) Quantitation from multiple internalization experiments (n=4) demonstrates that RanBP9 siRNA inhibits LRP endocytosis by ∼70% (B, P=0.0060; B) and β1-integrin endocytosis by ∼40% (D, P=0.0072). Error bars = se. **P < 0.01.
RanBP9 reduces cell surface β1-integrin, LRP, and APP and interferes with neurite arborization in primary hippocampal neurons
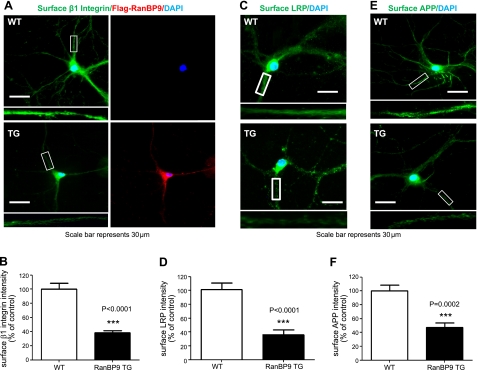
We next determined whether RanBP9 alters the surface levels of β1-integrin, LRP, and APP in primary hippocampal neurons. To this end, we generated RanBP9-transgenic mice using the neuron-specific Thy-1 promoter (Supplemental Fig. S2). In line 629, the human Flag-RanBP9 expression was enriched in the cortex and hippocampus (Supplemental Fig. S2). Therefore, we cultured primary hippocampal neurons from line 629 and wild-type littermates at P0. At DIV14, neurons were stained for the extracellular portions of β1-integrin, LRP, and APP without membrane permeabilization to detect the specific surface proteins, followed by permeabilization by Triton X-100 and detection of intracellular Flag-RanBP9. Indeed, surface levels of β1-integrin, LRP, and APP were all dramatically reduced in RanBP9-transgenic neurons compared to control littermate neurons (Fig. 7). As expected, the RanBP9-transgenic neurons expressed the transgene, as evidenced by M2 staining of Flag-RanBP9 (Fig. 7A). Therefore, RanBP9 consistently reduces surface levels of β1-integrin, LRP, and APP in both nonneuronal and neuronal cells.
Figure 7.
RanBP9 reduces cell surface β1-integrin, LRP, and APP in primary hippocampal neurons. A, C, E) Primary hippocampal neurons isolated from P0 RanBP9 transgenic (TG) and wild-type (WT) littermate controls were grown on coverslips until DIV14. Neurons were then subjected to immunofluorescence staining for β1-integrin (A, green), LRP (C, green), or APP (E, green) without membrane permeabilization to detect cell surface proteins. After permeabilization with Triton X-100, intracellular Flag-RanBP9 was then detected using the M2 antibody (red). Note the reduction in surface β1-integrin, LRP, and APP levels in RanBP9 transgenic primary neurites. Insets: magnified views of boxed areas. A representative experiment is shown. B, D, F) Quantitations from multiple experiments (n=3) demonstrate that RanBP9 reduces surfaces levels of β1-integrin by ∼60% (B, P<0.0001), LRP by ∼65% (D, P<0.0001), and APP by ∼55% (F, P=0.0002; F) in primary hippocampal neurons. Error bars = se. ***P < 0.001.
Because β1-integrin, LRP, and APP are all critical to cell-adhesive events and neurite development (4, 14), primary neurons were transfected with EYFP at DIV10 and subjected to Sholl analysis on DIV12 to examine neurite complexity and arborization. This is achieved by drawing concentric circles every 10 μm from the cell body and counting the number of intersections of EYFP-transfected neurons. As seen in Supplemental Fig. S3, RanBP9-transgenic mouse primary neurons appeared to develop noticeably fewer neuritic branches compared to their wild-type counterparts. Quantitation of Sholl analysis indeed demonstrated that RanBP9-transgenic neurons contain significantly reduced neurite complexity and arborization compared to nontransgenic neurons, particularly from 30 to 180 μm from the cell body (Supplemental Fig. S3). Therefore, RanBP9 inhibits neurite arborization in primary neurons, consistent with reduced levels of surface β1-integrin, LRP, and APP.
DISCUSSION
In this study, we made a number of novel observations that implicate a pivotal role of RanBP9 in focal adhesion signaling and assembly, which couples the mechanism of Aβ generation with focal adhesion inhibition via a single molecular pathway. First, overexpression of RanBP9 dramatically impaired cell attachment and spreading, events that were linked to inhibition of focal adhesion signaling and assembly. Second, knockdown of RanBP9 strongly promoted cell attachment, spreading, and focal adhesion signaling and assembly, indicating that endogenous RanBP9 normally functions to interfere with cell-adhesive events. Third, inhibition of cell adhesion was due to the ability of RanBP9 to reduce cell surface β1-integrin and LRP by accelerating their endocytosis in a manner analogous to APP (11). Finally, RanBP9 dramatically reduced the cell surface levels of β1-integrin, LRP, and APP and potently reduced neurite arborization in primary hippocampal neurons.
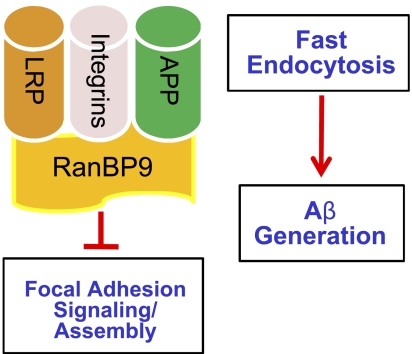
RanBP9 physically interacts with β-integrins, LRP, and APP, all of which also form complexes with each other and are involved in integrin-mediated cell-adhesive processes (4, 7, 9–11, 13). APP forms a complex with β1-integrin and reelin, which together promote neurite outgrowth and arborization in primary neurons and in vivo. Activation of integrin signaling via reelin depends on the presence of the APP-integrin complex, which, in turn, promotes α-secretase processing of APP and inhibition of Aβ generation (4). LRP also forms a complex with β1-integrin and APP and mediates their trafficking to the cell surface, as well as endocytosis (6, 9, 10, 15, 16). We previously demonstrated that RanBP9 promotes Aβ generation by scaffolding APP/BACE1/LRP complexes and promoting APP endocytosis, both of which are necessary for Aβ generation (11). Our observations that RanBP9 not only accelerates the endocytosis of APP but also LRP and β1-integrin raises the intriguing possibility that inhibition of integrin-mediated cell adhesion and Aβ generation by RanBP9 are coordinated and mechanistically coupled events (Fig. 8). However, it remains to be determined whether RanBP9 promotes the endocytosis of β1-integrin, LRP, and APP as protein complexes, individual proteins, or a combination thereof. Although RanBP9 overexpression accelerated β1-integrin endocytosis more than LRP, RanBP9 siRNA decreased the β1-integrin endocytosis more than LRP, suggesting that overexpressed and endogenous RanBP9 may differentially affect LRP and β1-integrin endocytosis to variable degrees. However, the extent of cell surface reductions in β1-integrin, LRP, and APP were within a similar range of (∼55–65%) in RanBP9 transgenic neurons, as well as in RanBP9-transfected NIH3T3 cells (∼70%). Because it is known that LRP alters β1-integrin and APP trafficking to the cell surface (10, 17), we cannot rule out the possibility that RanBP9 may also influence the rate of β1-integrin, LRP, and APP trafficking to the surface in addition to endocytosis.
Figure 8.
Schematic of RanBP9 in Aβ generation and focal adhesions. Binding of RanBP9 to β1-integin, LRP, and/or APP accelerates their endocytosis, thereby promoting Aβ generation and simultaneously disrupting focal adhesions. Although APP, LRP, and β1-integrin all form complexes with each other, it remains to be determined whether RanBP9 acts on the protein complex, individual proteins, or a combination thereof.
The ability of integrins to bind the extracellular matrix and provide linkage to the actin cytoskeleton via talin and vinculin is critical for fundamental biological processes, including cell adhesion, cell survival and death, cell migration, and neurite outgrowth (18). Our observation that RanBP9 dramatically promoted β1-integrin and LRP internalization places RanBP9 as a pivotal molecular regulator of these processes. Accelerated β1-integrin and LRP endocytosis not only disrupted the binding of integrins to the extracellular matrix (cell attachment) but also the linkage to the cytoskeleton by talin and vinculin (cell spreading), since RanBP9 disrupted focal adhesion assembly, as seen by the reduction of talin and vinculin in focal adhesion complexes. Whether such sites are the more dynamic focal complexes, such as those seen with APP and FE65 (19), or the more stable and mature focal adhesions remains to be determined. Nonetheless, such disruptions in cell-adhesive events are well known to be linked to perturbations in cell migration and promotion of cell death via ANOIKIS (i.e., loss of cell adhesion), as well as integrin-mediated death (IMD; refs. 20–23). IMD is characterized by unligation or misligation of integrins to inappropriate substrates, leading to integrin clustering and recruitment of caspase 8. Indeed, it has been shown that the binding of Aβ to integrins is required for Aβ-induced neurotoxicity (24–26). Integrin signaling is also associated with changes in synaptic plasticity and neuronal excitability, and interfering with integrin-dependent adhesion by antagonists or conditional deletion of α3, α5, and/or β1 integrins leads to defective long-term potentiation (27). As RanBP9 levels are highly elevated in brains of patients with AD (12), such an increase in RanBP9 is predicted not only to positively affect Aβ levels but also to negatively regulate vital integrin-dependent processes. Indeed, neurite arborization was dramatically reduced in RanBP9 transgenic primary neurons, and RanBP9 strongly promoted Aβ/amyloid plaque accumulation in vivo and diminished synaptic integrity (unpublished results). Taken together, our findings here demonstrate that RanBP9 simultaneously accelerates the endocytosis of β1-integrin, LRP, and APP, events that play critical roles in focal adhesion signaling/assembly, as well as Aβ generation.
Supplementary Material
Acknowledgments
The authors thank Dr. Elisabetta Bianchi (Pasteur Institute, Paris, France) for the RanBP9 monoclonal antibody and Dr. Mark Ginsberg (University of California, San Diego, CA, USA) for talin-GFP construct, as well as helpful discussions.
This work was supported in part by the American Health Assistance Foundation (A2007-05, D.E.K.), the Alzheimer's Association (IIRG-08-91662, D.E.K.), the U.S. National Institutes of Health/National Institute on Aging (1R01AG033055- 01A1 and 1K02AG031920-10A1, D.E.K.; 1R03AG032064-01 and 1R01AG036859-01, M.K.L.), and by a World Class University–Neurocytomics Project grant from the National Research Foundation of Korea (D.E.K.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Aβ
- amyloid β
- AD
- Alzheimer's disease
- APP
- amyloid precursor protein
- BACE1
- β-site APP-cleaving enzyme 1
- LRP
- low-density lipoprotein receptor-related protein
- RanBP9
- Ran-binding protein 9.
REFERENCES
- 1. De Strooper B., Annaert W. (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 113, 1857–1870 [DOI] [PubMed] [Google Scholar]
- 2. Perez R. G., Soriano S., Hayes J. D., Ostaszewski B., Xia W., Selkoe D. J., Chen X., Stokin G. B., Koo E. H. (1999) Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J. Biol. Chem. 274, 18851–18856 [DOI] [PubMed] [Google Scholar]
- 3. Wahrle S., Das P., Nyborg A. C., McLendon C., Shoji M., Kawarabayashi T., Younkin L. H., Younkin S. G., Golde T. E. (2002) Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23 [DOI] [PubMed] [Google Scholar]
- 4. Hoe H. S., Lee K. J., Carney R. S., Lee J., Markova A., Lee J. Y., Howell B. W., Hyman B. T., Pak D. T., Bu G., Rebeck G. W. (2009) Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J. Neurosci. 29, 7459–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young-Pearse T. L., Bai J., Chang R., Zheng J. B., LoTurco J. J., Selkoe D. J. (2007) A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 27, 14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabodi S., Di S. P., Leal M. P., Tinnirello A., Bisaro B., Morello V., Damiano L., Aramu S., Repetto D., Tornillo G., Defilippi P. (2010) Integrins and signal transduction. Adv. Exp. Med. Biol. 674, 43–54 [DOI] [PubMed] [Google Scholar]
- 7. Hu K., Wu C., Mars W. M., Liu Y. (2007) Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J. Clin. Invest. 117, 3821–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akkawi S., Nassar T., Tarshis M., Cines D. B., Higazi A. A. (2006) LRP and alphavbeta3 mediate tPA activation of smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 291, H1351–H1359 [DOI] [PubMed] [Google Scholar]
- 9. Cao C., Lawrence D. A., Li Y., Von Arnim C. A., Herz J., Su E. J., Makarova A., Hyman B. T., Strickland D. K., Zhang L. (2006) Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 25, 1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salicioni A. M., Gaultier A., Brownlee C., Cheezum M. K., Gonias S. L. (2004) Low-density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J. Biol. Chem. 279, 10005–10012 [DOI] [PubMed] [Google Scholar]
- 11. Lakshmana M. K., Yoon I. S., Chen E., Bianchi E., Koo E. H., Kang D. E. (2009) Novel role of RanBP9 in BACE1 processing of amyloid precursor protein and amyloid beta peptide generation. J. Biol. Chem. 284, 11863–11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lakshmana M. K., Chung J. Y., Wickramarachchi S., Tak E., Bianchi E., Koo E. H., Kang D. E. (2010) A fragment of the scaffolding protein RanBP9 is increased in Alzheimer's disease brains and strongly potentiates amyloid-beta peptide generation. FASEB J. 24, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denti S., Sirri A., Cheli A., Rogge L., Innamorati G., Putignano S., Fabbri M., Pardi R., Bianchi E. (2004) RanBPM is a phosphoprotein that associates with the plasma membrane and interacts with the integrin LFA-1. J. Biol. Chem. 279, 13027–13034 [DOI] [PubMed] [Google Scholar]
- 14. Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., Schwartz A. L. (1995) Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. U. S. A. 92, 9480–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietrzik C. U., Busse T., Merriam D. E., Weggen S., Koo E. H. (2002) The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 21, 5691–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon I. S., Chen E., Busse T., Repetto E., Lakshmana M. K., Koo E. H., Kang D. E. (2007) Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 21, 2742–2752 [DOI] [PubMed] [Google Scholar]
- 17. Waldron E., Heilig C., Schweitzer A., Nadella N., Jaeger S., Martin A. M., Weggen S., Brix K., Pietrzik C. U. (2008) LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol. Dis. 31, 188–197 [DOI] [PubMed] [Google Scholar]
- 18. Reddig P. J., Juliano R. L. (2005) Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24, 425–439 [DOI] [PubMed] [Google Scholar]
- 19. Sabo S. L., Ikin A. F., Buxbaum J. D., Greengard P. (2001) The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J. Cell Biol. 153, 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taddei M., Giannoni E., Fiaschi T., Chiarugi P. (2012) Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226, 380–393 [DOI] [PubMed] [Google Scholar]
- 21. Vachon P. H. (2011) Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J. Signal Transduct. 2011, 738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stupack D. G., Cheresh D. A. (2002) Get a ligand, get a life: integrins, signaling and cell survival. J. Cell Sci. 115, 3729–3738 [DOI] [PubMed] [Google Scholar]
- 23. Stupack D. G., Puente X. S., Boutsaboualoy S., Storgard C. M., Cheresh D. A. (2001) Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155, 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson K. L., Ferreira A. (2004) Alpha1 integrin activation: a link between beta-amyloid deposition and neuronal death in aging hippocampal neurons. J. Neurosci. Res. 75, 688–697 [DOI] [PubMed] [Google Scholar]
- 25. Wright S., Malinin N. L., Powell K. A., Yednock T., Rydel R. E., Griswold-Prenner I. (2007) Alpha2beta1 and alphaVbeta1 integrin signaling pathways mediate amyloid-beta-induced neurotoxicity. Neurobiol. Aging 28, 226–237 [DOI] [PubMed] [Google Scholar]
- 26. Wang Q., Klyubin I., Wright S., Griswold-Prenner I., Rowan M. J., Anwyl R. (2008) Alpha v integrins mediate beta-amyloid induced inhibition of long-term potentiation. Neurobiol. Aging 29, 1485–1493 [DOI] [PubMed] [Google Scholar]
- 27. Becchetti A., Pillozzi S., Morini R., Nesti E., Arcangeli A. (2010) New insights into the regulation of ion channels by integrins. Int. Rev. Cell. Mol. Biol. 279, 135–190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.