Abstract
There is growing evidence that the complement activation product C5a positively or negatively regulates inflammatory functions. The studies presented here report that C5a exerts anti-inflammatory effects by altering production of the cytokines IL-17A and IL-23 during endotoxic shock in young adult male C57BL/6J mice and has similar effects on macrophages from the same mice. IL-17A and IL-23 both appeared in plasma during endotoxemia, and their neutralization improved survival. The relevant sources of IL-17A during endotoxemia were not CD4+ cells, γδ T cells, or NK cells but CD11b+F4/80+ macrophages. The addition in vitro of C5a to lipopolysaccharide-activated peritoneal macrophages dose dependently antagonized the production of IL-17A (IC50, 50–100 nM C5a) and IL-23 (IC50, 10 nM C5a). This suppression required the receptor C5aR, but was independent of the second C5a receptor, C5L2. Genetic absence of C5aR was associated with much higher levels of IL-17A and IL-23 during endotoxic shock. Mechanistically, C5a mediated its effects on the IL-17A/IL-23 axis in a 2-step process. C5a caused activation of the PI3K-Akt and MEK1/2-ERK1/2 pathways, resulting in induction of IL-10, which powerfully inhibited production of IL-17A and IL-23. These data identify previously unknown mechanisms by which the anaphylatoxin C5a limits acute inflammation and antagonizes the IL-17A/IL-23 axis.—Bosmann, M., Sarma, J. V., Atefi, G., Zetoune, F. S., Ward, P. A. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis.
Keywords: macrophages, interleukin-10, C5L2, endotoxic shock
The complement system is a first line of innate immune defenses, guarding the extracellular compartments, eliminating microbes, and contributing substantially to homeostasis (1, 2). The complement activation product C3b has an essential role as an opsonic factor that promotes phagocytosis, while complement anaphylatoxin C5a has been shown to display powerful biological activities, including recruitment and activation of phagocytes (3). C5a and its conversion product C5adesArg bind with high affinity to C5a receptors C5aR and C5L2, which are expressed abundantly on both myeloid and nonmyeloid cells (4, 5). The C5aR receptor belongs to the family of 7-transmembrane-spanning and G-protein-coupled receptors (6, 7). C5L2 represents a second C5a receptor that was first described as a “decoy receptor” due to the lack of Ca2+ signaling activity after receptor ligation with C5a or C5adesArg, and perhaps C3adesArg. However, engagement of the C5L2 receptor has recently been shown to activate some signaling pathways, although such conclusions are a matter of contention (8–10). Overall, C5a, via C5aR and possibly C5L2, promotes inflammation, including directing the influx and activation of polymorphonuclear neutrophils. Blockade of C5a or genetic absence of its receptors affected neutrophil functions and reduced acute systemic inflammation and mediator production (11, 12). On the other hand, high levels of C5a can also compromise innate immune functions (13).
IL-17A is essential for host defense against extracellular pathogens, such as Escherichia coli, Staphylococcus aureus, and Candida spp., but also for the intracellular pathogen, Mycobacterium tuberculosis (14). IL-17A predominantly interacts with nonleukocytic cells, such as epithelial cells, fibroblasts, and endothelial cells, but also with macrophages (15). From these cells, IL-17A initiates production of other proinflammatory mediators, such as IL-1, TNF-α, IL-6, and IL-8, as well as G-CSF, collectively resulting in an influx of neutrophils (15–17).
It is widely accepted that IL-17 and Th17 cells contribute to the pathogenesis of autoimmune diseases, based on findings in experimental models, such as autoimmune encephalomyelitis and collagen-induced arthritis (18, 19). Commitment of naive T cells to the Th17 lineage has been demonstrated to be induced by a combination of the cytokines TGFβ and IL-6 (20, 21), activating the transcription factor retinoid-related orphan receptor γt (RORγt; ref. 22). Later stages of Th17 cell differentiation (including clonal expansion, phenotype stabilization, and IL-17 production) also depend on IL-23 (p40/p19) expression (18, 19). However, under certain circumstances, IL-17A can also be produced independently of IL-23 (23). Despite the fact that much attention has been given to CD4+ T-helper cells (Th17) as the origin of IL-17A, it is now clear that during acute inflammatory responses, significant amounts of IL-17A may be derived from cells of the innate immune system (16). Release of IL-17A has been demonstrated from neutrophils, lymphocyte-tissue inducer cells, iNKT cells, γδ T cells, and paneth cells (16, 24). We have previously reported that depletion of γδ T cells reduces IL-17A and improves survival in the setting of polymicrobial sepsis, accompanied by substantial suppression of the cytokine storm (25). Production of IL-17A and IL-17F by cells of the macrophage lineage has also been described (26, 27), but so far, the evidence that macrophages contribute to IL-17A is limited.
In this report, we describe the ability of C5a to negatively regulate the IL-17A/IL-23 axis after endotoxic shock and in macrophages after lipopolysaccharide (LPS)-mediated activation of TLR4. Interestingly, we find the effects of C5a to be related to phosphatidylinositol 3-kinase (PI3K)-Akt and MAPK/extracellular signal-regulated kinase (ERK) kinase 1/2 (MEK1/2)–ERK1/2-mediated induction of IL-10 from macrophages, with IL-10 subsequently suppressing the IL-17A/IL-23 axis. Thus, C5a can exert predominantly anti-inflammatory properties under some circumstances.
MATERIALS AND METHODS
Animals
All procedures were performed in accordance with the U.S. National Institutes of Health guidelines and the University of Michigan Committee on Use and Care of Animals. Male mice of the strains C57BL/6J, IL-10−/−, γδ T cell−/−, αβ T cell−/−, CD4 T cell−/−, myeloid differentiation primary response gene 88 (MyD88)−/−, and Rorc(gt)gfp were purchased from the Jackson Laboratories (Bar Harbor, ME, USA). Male mice of the strains C5aR−/− and C5L2−/− were bred at the University of Michigan. All animals were housed under specific pathogen-free conditions.
Endotoxemia
The mice were injected with LPS i.p. (E. coli 0111:B4; Sigma-Aldrich, St. Louis, MO, USA). Body weight of each individual animal was measured directly before injection. Plasma was collected using EDTA (5 mM) as an anticoagulant. For survival studies, animals were monitored at least every 12 h for 8 d after challenge with LPS.
Isolation and incubation of macrophages
For elicitation of peritoneal-elicited macrophages (PEMs), mice received 1.5 ml thioglycollate 2.4% i.p. (Becton Dickinson, Franklin Lakes, NJ, USA) or casein sodium salt 9% (Sigma-Aldrich). PEMs were harvested 4 d later by peritoneal lavage. After centrifugation, cells were resuspended in RPMI 1640 [25 mM HEPES, 100 U/ml penicillin-streptomycin (Life Technologies), and 0.1% BSA (Sigma-Aldrich)], plated at 2 × 106 cells/ml and incubated at 37°C, 5% CO2.
For bone marrow-derived macrophages (BMDMs), the long bones were flushed with HBSS. Cells were cultured in RPMI 1640 (25 mM HEPES, 100 U/ml penicillin-streptomycin, 20% FCS, and 30% L-cell conditioned medium), supplemented with fresh medium after 3 d and incubated for a total of 7 d.
For isolation of alveolar macrophages, the lungs were removed and lavaged 20× with 1 ml PBS (0.5 mM EDTA). The MH-S cell line was a gift from Dr. J. Weinberg (University of Michigan).
At the end of all experiments, supernatants were cleared of nonadherent cells by centrifugation and stored at −80°C until further analysis. For light microscopic phenotyping of macrophage preparations, cytospins were stained with a modified Wright-Giemsa method (Hema 3 Stain Set; Fisher Scientific, Pittsburgh, PA, USA).
Measurement of mediator concentrations by ELISA and Luminex
The following mouse ELISA kits were used: IL-17A, TNF-α, IL-23(p19), IL-10 (all from R&D Systems, Minneapolis, MN, USA). ELISAs were performed according to the instructions of the manufacturer. The mouse C5a ELISA was performed with reagents from BD Biosciences (San Jose, CA, USA), as described earlier (28). For simultaneous measuring of 23 mouse cytokines, a bead-based assay was used (Luminex and BioPlex 200; Bio-Rad, Hercules, CA, USA). Processing of samples and data analysis was performed according to manufacturer's instructions as described before (29).
Isolation of mRNA and real-time PCR
Total RNA was obtained from macrophages by the TRIzol method. The cDNA was generated with TaqMan Reverse Transcription Reagents (Applied Biosystems, Carlsbad, CA, USA). Amplification was performed with SYBR Green Mastermix in the 7500 real-time PCR system (Applied Biosystems). Results were analyzed by the 2−ΔΔCt relative quantification method and normalized to GAPDH. Primer sequences were as follows: mouse IL-17A, forward 5′-CTCCAGAAGGCCCTCAGACTAC-3′ and reverse 5′-AGCTTTCCCTCCGCATTGACACAG-3′; mouse RORγt, forward 5′-CCGCTGAGAGGGCTTCAC-3′ and reverse 5′-TGCAGGAGTAGGCCACATTACA-3′; mouse GAPDH, forward 5′-TACCCCCAATGTGTCCGTCGTG-3′ and reverse 5′-CCTTCAGTGGGCCCTCAGATGC-3′ (all from Invitrogen, Carlsbad, CA, USA).
Flow cytometry
After incubation with Brefeldin A, cells were processed using the Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences) and BD Fc Block. For detection of phosphoproteins, cells were fixed and permeabilized with Perm Buffer III (BD Biosciences). A minimum of 50,000 events were acquired on a BD LSR II flow cytometer (BD Biosciences). All antibodies used were anti-mouse together with matched fluorochrome-labeled isotype controls. From BD Pharmingen: AF488 IL-17A (clone TC11-18H10), PE γδ T-cell receptor (clone GL3), PE Akt(pT308) (clone J1-223.371), and PE ERK1/2(pT202/pY204) (clone 20A). From eBioscience (San Diego, CA, USA): APC F4/80 (clone BM8), AF 700 CD11b (clone M1/70), PE CD11c (clone N418), PE CD4 (clone RM4–5), and PerCP-Cy5.5 CD3e (clone 145-2C11). From R&D Systems: PE CCR6 (clone 140706).
Microscopy
Spleens were snap-frozen, cryosectioned, and fixed with 4% formaldehyde solution (Thermo Scientific, Pittsburgh, PA, USA). After blocking with 7% BSA, 10% normal mouse serum, and endogenous biotin (blocking kit; Invitrogen), the sections were incubated overnight with primary antibodies, 1 h with secondary antibodies, and mounted with prolong gold/DAPI (Invitrogen). Antibodies used were goat anti-mouse IL-17A (R&D Systems), rat anti-mouse F4/80 (eBioscience), rabbit anti-goat biotinylated IgG, streptavidin AF488, and rabbit anti-rat AF594 (all from Invitrogen). Confocal images were acquired with a Zeiss LSM 510-META laser scanning confocal microscope (× 40/1.2 W, ×63/1.2 W; Carl Zeiss, Oberkochen, Germany) with LSM 510 software. Light microscopic images of cytospins were acquired using an Olympus BX-51 microscope (×40/0.9, ×60/1.4 oil, ×100/1.4 oil) with an Olympus DP-70 high-resolution digital camera and DP controller software (Olympus, Tokyo, Japan).
Antibodies and reagents
TLR agonists were obtained from Invivogen (San Diego, CA, USA): TLR2 (zymosan, Saccharomyces cerevisiae), TLR3 (poly I:C), TLR5 (flagellin, Bacillus subtilis), and TLR9 (type A CpG oligonucleotide ODN1518 and ODN1585 control). LPS (E. coli, 0111:B4) and N-formyl-Met-Leu-Phe (fMLP) were obtained from Sigma-Aldrich; LY294002, PD98059, and U0126 were obtained from Invivogen.
Neutralizing goat anti-mouse IL-23(p19) IgG, total goat IgG, rmC5a, rmIL-10, and rmIL-23 were obtained from R&D Systems; neutralizing anti-mouse IL-17A and control antibody from eBioscience; anti-asialo GM1 rabbit NK cell-depleting antiserum and normal rabbit serum from Wako Pure Chemicals (Richmond, VA, USA); rmIL-6 and rhTGFβ1 from PeproTech (Rocky Hill, NJ, USA); rat IgG2B isotype control antibody from BioLegend (San Diego, CA, USA). The neutralizing anti-F4/80 antibody was generated from clone HB-198 (American Type Culture Collection, Manassas, VA, USA) and used as described before by others (30, 31).
Statistical analysis
GraphPad Prism 5.01 software (GraphPad, San Diego, CA, USA) was used for figure preparation and statistical analysis. All values are expressed as means, and error bars represent means ± se. Data sets were analyzed by 1-way ANOVA and Student's t test and survival curves by log-rank (Mantel-Cox) test. In vivo experiments were done with numbers of animals per group as indicated in the figure legends (n≥3). In vitro experiments were independently performed ≥3 times, and representative experiments are shown. We considered differences significant at values of P < 0.05.
RESULTS
Blocking of IL-17A or IL-23(p19) improves survival after endotoxic shock
To investigate the role of IL-17A and IL-23(p19) during acute systemic inflammation, we challenged C57BL/6J mice with LPS [10 mg/kg body weight (BW) i.p.], a dose causing 60–80% lethality. Plasma collected from these mice at several time points showed an early, transient surge of IL-23(p19), peaking in 3 h, with levels of ∼800 pg/ml (Fig. 1A). The same samples revealed a 12 h peak for IL-17A (Fig. 1B), although IL-17A was already elevated over baseline levels by 3 h. Consequently, in all further experiments for detection of plasma IL-17A, a time point of 12 h was used unless otherwise indicated. Next, we pretreated C57BL/6J mice with a neutralizing polyclonal anti-IL-23 antiserum, specific for the p19 subunit of IL-23(p19/p40). Blockade of IL-23(p19) resulted in a significantly improved survival after challenge with LPS (Fig. 1C). Similarly, neutralization of IL-17A was also potently protective during endotoxemia and increased survival from 40 to 100% when anti-IL-17A was given immediately before LPS (Fig. 1D). Together, this indicated that the IL-17A/IL-23 axis drives lethal systemic inflammation in the endotoxemia model. To elucidate the cellular source of IL-17A release during endotoxemia, we compared C57BL/6J mice to mice genetically deficient of CD4+ or γδ T cells. Unexpectedly, the absence of CD4 or γδ T cells did not significantly affect levels of plasma IL-17A during endotoxic shock (Fig. 1E, F). IL-17A levels were also not reduced when NK cells were depleted before endotoxemia (Fig. 1G). However, pretreatment with a monoclonal antibody directed against the surface marker F4/80 for depletion of macrophages resulted in markedly diminished circulating IL-17A levels after endotoxic shock compared to mice treated with isotype control antibody (Fig. 1H). This anti-F4/80 antibody has been demonstrated before by others to effectively deplete F4/80+ macrophages (30, 31). Additional evidence that F4/80+ macrophages are a source of IL-17A in vivo was acquired by confocal microscopy. Sections of spleens from endotoxemic wild-type (Wt) mice were stained for F4/80, as well as for intracellular IL-17A and showed colocalization of IL-17A in F4/80+ cells in the spleen (Fig. 1I, bottom panel). Only a few IL-17A+F4/80+ cells were seen in spleens of endotoxemic mice, which may be due to rapid secretion of IL-17A. Sections from untreated healthy mice as controls displayed little detectable fluorescent intensity for IL-17A, whereas F4/80+ macrophages were also present in the spleens of these sham mice (Fig. 1I, top panel). No other cell type except F4/80+ macrophages stained consistently positive for IL-17A in the spleen of endotoxemic mice, when compared to staining with isotype controls (data not shown). Overall, the levels of IL-17A after endotoxemia appear to be generated at least to some extent by cells of the monocyte/macrophage lineage.
Figure 1.
Neutralizing of IL-17A or IL-23(p19) improves survival after endotoxemia. A) Time course of IL-23(p19) in plasma after LPS challenge of C57BL/6J mice in vivo (n=5/group), ELISA. B) Time course of IL-17A in plasma after LPS challenge in vivo (n=5/group). C) Survival of C57BL/6J mice in endotoxemia with pretreatment of neutralizing IL-23(p19) (n=9) antibody or control antibody (n=12), 20 μg antibody/animal i.p., LPS 12.5 mg/kg BW i.p. D) Survival of C57BL/6J mice in endotoxemia pretreated with neutralizing IL-17A antibody (n=9) or control antibody (n=10), 70 μg antibody/animal i.p. E) Plasma levels of IL-17A of endotoxemic CD4−/− mice (n=4) compared to C57BL/6J mice (n=5) as control, 12 h. F) Plasma levels of IL-17A in endotoxemia in γδTCR−/− mice (n=5) and C57BL/6J mice (n=8) as control, 12 h. G) Plasma levels of IL-17A 12 h after endotoxemia with depletion of NK cells by anti-asialo GM antiserum (50 μl/mouse i.p. on d 1) or control serum (n=9/group). H) Reduction of IL-17A plasma levels 12 h after endotoxemia after pretreatment with an anti-F4/80 macrophage-depleting antibody or IgG2B isotype control antibody (n=5; 500 μg i.p. on d −3 and −1). I) Confocal microscopy of spleen sections from sham-treated mice or mice 3 h after endotoxemia. Stainings for IL-17A (AF488, green), F4/80 (AF594, red), and merged images are shown. Scale bars = 15 μm). Dose of LPS was 10 mg/kg BW i.p. for all experiments except C. Error bars represent means ± se. n.s., not significant. *P < 0.05, ***P < 0.001; Student's t test.
Release of IL-17A by macrophages after TLR4 activation
To further investigate the possibility of macrophages being a source of IL-17A, mouse peritoneal and alveolar macrophages were assessed in vitro. Peritoneal cells isolated from C57BL/6J mice 4 d after intraperitoneal injection of thioglycollate were found to be ∼85–90% macrophages with extensive vacuole presence, based on light microscopic differential cell count (Fig. 2A) and expression of F4/80 and CD11b surface markers (Supplemental Table S1A, C). Macrophages were incubated for 10 h using a dose range of LPS (10 μg/ml to 10 ng/ml), and levels of IL-17A released into cell supernatant fluids were measured by ELISA (Fig. 2B). LPS concentrations >50 ng/ml induced a highly robust production of IL-17A. For further experiments, LPS at a concentration of 1 μg/ml was employed. Figure 2C shows the time course for release of IL-17A from LPS-stimulated macrophages, with a peak in IL-17A levels after 12 h, which is superimposable with the IL-17A kinetics described after endotoxemia (Fig. 1B). Comparable levels of LPS-induced IL-17A were detectable when casein, an alternative to thioglycollate, was used to elicit peritoneal macrophages (Fig. 2D). To determine the capability of mouse alveolar macrophages to produce IL-17A, these cells were harvested by lavage of the airways of normal C57BL/6J mice followed by in vitro cultivation. Cells from such BAL fluids were >98% macrophages, as determined by light microscopy (Fig. 2E). These alveolar macrophages released IL-17A after stimulation with LPS (Fig. 2F). The alveolar macrophage cell line, MH-S, also released IL-17A after LPS (Fig. 2G).
Figure 2.
IL-17A release by macrophages from different origins. A) PEMs from C57BL/6J (Wt) mice were isolated from the peritoneum after elicitation with thioglycollate and stained after Wright-Giemsa. B) Production of IL-17A by PEMs incubated for 10 h at 37°C with a dose range of LPS (0.01–10 μg/ml), ELISA. C) Time course for IL-17A production from PEMs incubated with LPS (1 μg/ml) for 3–24 h. D) IL-17A release by thioglycollate- or casein-elicited PEMs after incubation with LPS (1 μg/ml) for 10 h. E) Alveolar macrophages harvested from BAL fluids of untreated C57BL/6J mice and stained after Wright-Giemsa. F) IL-17A release by alveolar macrophages after incubation with LPS (1 μg/ml) for 10 h. G) Production of IL-17A by MH-S cells (SV40 transformed alveolar macrophage cell line), 10 h. H) Absence of detectable IL-17A release in LPS-stimulated BMDMs from C57BL/6J mice despite abundant production of TNF-α after 10 h. Control (Ctrl) denotes untreated macrophages cultured in medium alone. Error bars represent means ± se. *P < 0.05, **P < 0.01, ***P < 0.001; Student's t test.
BMDMs were also studied for IL-17A production. In supernatant fluids from LPS-stimulated BMDMs, IL-17A was not detectable (<15 pg/ml), despite abundant production of TNF-α (Fig. 2H). The polarization of BMDMs to the classic M1 phenotype after 24 h incubation with interferon-γ alone or in combination with low-dose LPS did not result in IL-17A production (data not shown).
Characterization for surface markers and mediator production by PEMs and BMDMs after TLR4 activation are summarized in Supplemental Table S1. PEMs and BMDMs showed abundant F4/80+ (86 vs. 98%, respectively) and CD11b+ (87 vs. 98%, respectively) expression and were typically F4/80+CD11b+ double-positive. These cells displayed low expression profiles for CD11c (<15%). There was a trend for PEMs to release higher quantities of IL-23(p19) compared to BMDMs. PEMs and BMDMs both displayed a TNF-αhigh, IL-1high, IL-12high, IL-10low phenotype, consistent with an M1 classic polarization (Supplemental Table S1B). This suggests that macrophages that express mediators of the M1 phenotype (as in PEMs and alveolar macrophages) may or may not (as in BMDMs) express IL-17A.
By RT-PCR, there was no measurable RORγt mRNA expression detectable after activation of macrophages with LPS (data not shown). In addition, there were no signs of the presence of RORγt in LPS-stimulated macrophages from RORγteGFP transgenic mice (Supplemental Fig. S1). Collectively, these data suggest that, under the conditions employed, there was no evidence of activation of RORγt, which is consistent with a recent report using macrophages (26).
TLR4 activation induces IL-17A expression in F4/80+CD11b+ macrophages
The PEM preparations contained small numbers of nonmacrophage cells, namely CD3+ cells (Supplemental Table S1C). To further investigate the nature of the IL-17A-producing cells after TLR4 activation, we used flow cytometry analysis. Costaining for intracellular IL-17A in combination with the presence of surface markers employing anti-CD11b and anti-F4/80 showed a distinct population of double-positive PEMs, consistent with IL-17A+ cells being macrophages (Fig. 3A). Macrophages displayed a CD11b+F4/80+ phenotype. Despite small contamination of PEM preparations with CD3+ cells, there was virtually no staining (<0.4%) for IL-17A in CD3+ CD4+ cells (Supplemental Table S1C).
Figure 3.
Characterization of IL-17A release by macrophages. A) Flow cytometry analysis for intracellular cytokine staining for IL-17A as a function of the macrophage surface markers, CD11b and F4/80. B) IL-17A release from LPS-activated PEMs from C57BL/6J mice compared to cells from γδ T-cell-deficient mice. C) Release of IL-17A from PEMs from αβ T-cell-deficient mice after LPS activation. D) Release of IL-17A from Wt macrophages after stimulation with agonists for TLR2, TLR3, TLR4, TLR5, and TLR9 (all at 1 μg/ml). E) Release of IL-17A from LPS-activated PEMs from C57BL/6J mice and MyD88−/− mice. F) RT-PCR analysis of mRNA levels for IL-17A in LPS-stimulated PEMs from C57BL/6J mice and MyD88−/− mice. Control (Ctrl) denotes untreated PEMs in cell culture medium alone. LPS (1 μg/ml) and 12 h incubation for all experiments. Error bars represent means ± se. **P < 0.01, ***P < 0.001; Student's t test.
We previously reported that γδ T cells are required for the appearance in plasma of IL-17A after cecal ligation and puncture (25). In PEM preparations, γδ T cells were virtually undetectable (Supplemental Table S1C). To further evaluate a role of γδ, T cells for maturation of macrophages into an IL-17A-producing phenotype, we studied PEMs from γδ TCR−/− mice. After elicitation of macrophages with thioglycollate, PEMs from γδ TCR−/− mice displayed, if anything, slightly higher levels of IL-17A compared to Wt cells (Fig. 3B). Similar results were obtained with PEMs from αβ T-cell−/− mice that contain no CD4−, CD8+ or CD4+CD8− cells (Fig. 3C), which should be associated with the absence of Th17 differentiated cells. These results suggest that the release of IL-17A by PEMs or alveolar macrophages is independent of γδ T cells and αβ T cells. Unlike for Th17 cells, in macrophages, IL-6 and TGFβ did not promote IL-17A production (Supplemental Fig. S2).
Release of IL-17A after TLR4 activation is MyD88 dependent
We sought to examine whether expression of IL-17A by macrophages could be induced by TLRs other than TLR4. Therefore, we stimulated PEMs with zymosan (TLR2), poly I:C (TLR3), LPS (TLR4), flagellin (TLR5), and CpG (TLR9) for 12 h. Only TLR4 activation by LPS resulted in robust accumulation of IL-17A in the cell culture supernatants (Fig. 3D).
The recognition of LPS by the TLR4 receptor on macrophages is known to initiate recruitment of the adaptor protein MyD88 to the ligand-receptor complex, although some responses after TLR4 activation are independent of MyD88 but dependent on TRIF. To investigate the role of MyD88 for IL-17A production from LPS-activated macrophages, we compared cells from C57BL/6J mice (Wt) with macrophage preparations from MyD88−/− mice. As shown in Fig. 3E, macrophages devoid of MyD88 produced very little IL-17A in response to LPS (1 μg/ml). In unstimulated Wt macrophages, mRNA levels for IL-17A were barely detectable. After activation of the TLR4 pathway by LPS, there was a steep increase of mRNA content for IL-17A in Wt macrophages but not in MyD88−/− macrophages (Fig. 3F). Similar results for IL-23(p19) were obtained, and they indicated that the release of IL-23(p19) from PEMs is also dependent on MyD88 (data not shown).
C5a suppresses IL-17A and IL-23(p19) release from macrophages
Complement activation results in the enzymatic cleavage of C5 and appearance of the 74–78 aa peptide (depending on species), potent anaphylatoxin, C5a. In macrophages incubated with LPS in the copresence of recombinant C5a, there was a dose-dependent reduction of IL-17A release with an IC50 of 50–100 nM C5a (Fig. 4A). This suppressive activity was specific for C5a, since addition of fMLP, which is a chemotactic peptide derived from bacteria and reactive with its G-protein-coupled receptor, did not affect LPS-induced release of IL-17A from PEMs (Fig. 4B). The appearance of IL-17A in culture supernatant fluids (Fig. 4C) and the suppressive effects of C5a were also reflected by changes in mRNA levels for IL-17A. After incubation with LPS, the relative mRNA expression levels for IL-17A were increased as early as 3 h and preceded the appearance of IL-17A in cell culture medium, and C5a was inhibitory at all the time points studied (Fig. 4D).
Figure 4.
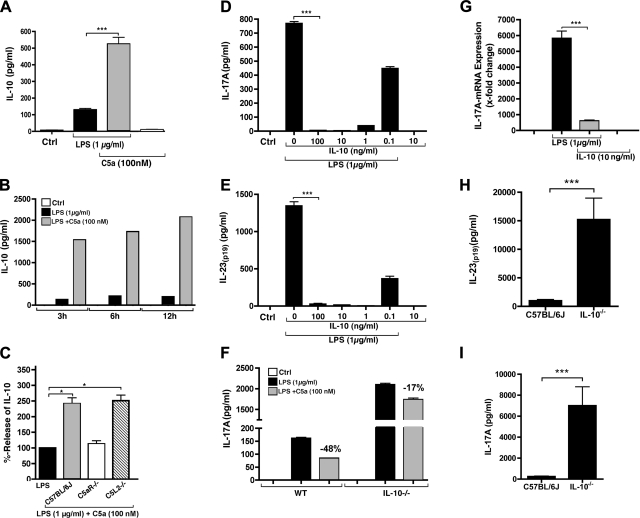
Ability of C5a to suppress IL-17A production in macrophages. A) PEMs (Wt) were incubated with LPS (1 μg/ml) in the copresence of the indicated concentrations of recombinant mouse C5a. Concentrations of IL-17A (ELISA) were detected in cell culture supernatants after 10 h. B) IL-17A release of PEMs after 10 h incubation with LPS alone or in combination with the bacterial chemotactic peptide, N-formyl-Met-Leu-Phe (fMLP). C) Time course for IL-17A release after stimulation with LPS or LPS plus C5a. D) Quantitative RT-PCR analysis of IL-17A mRNA expression at different time points after stimulation with LPS or LPS plus C5a (100 nM). E) Relative inhibition of LPS-induced IL-17A release by C5a (100 nM) in macrophages obtained from C57BL/6J, C5aR−/−, and C5L2−/− mice. Macrophages from the 3 mouse strains were isolated and stimulated in parallel with LPS or LPS plus C5a for 10 h. Data are expressed in percentage values, in which the IL-17A concentration with LPS alone for each animal strain and experiment was used as 100%. F) Comparison of IL-17A concentrations released from C57BL/6J and C5aR−/− macrophages. G) Release of IL-23(p19) after stimulation with LPS alone or together with different concentrations of C5a. H) Relative inhibition of IL-23(p19) release mediated by C5a from LPS-stimulated macrophages. Cells from C57BL/6J, C5aR−/−, and C5L2−/− mice were stimulated in parallel with LPS in the presence or absence of C5a. IL-23(p19) release after LPS alone was used as the 100% value for each individual mouse strain. Error bars represent means ± se. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001; Student's t test.
We assessed the relative contributions of the C5aR and C5L2 receptors to C5a-mediated negative regulation of IL-17A production. PEMs were obtained from Wt, C5aR−/−, and C5L2−/− mice, and cells were stimulated with either LPS or the combination of LPS (1 μg/ml) and C5a (100 nM). Relative changes due to the copresence of C5a were expressed as percentage values of IL-17A levels (Fig. 4E). The copresence of 100 nM C5a with LPS, when added to Wt macrophages, resulted in ∼55% inhibition in IL-17A production. C5a was unable to suppress IL-17A release in PEMs lacking C5aR. However, inhibition by C5a was fully expressed in PEMs deficient in the C5L2 receptor, suggesting that C5L2 is dispensable for mediating C5a-related inhibition of IL-17A, but not C5aR. Furthermore, macrophages from C5aR−/− mice displayed enhanced concentrations of IL-17A after LPS incubation when compared to cells from Wt mice (Fig. 4F). Taken together, these data indicate that C5a-induced suppression of IL-17A in LPS-stimulated PEMs requires engagement of C5aR.
Regarding IL-23 production in macrophages, we found that PEMs abundantly released IL-23(p19) within 10 h after TLR4 activation but not with TLR2, TLR3, TLR5, or TLR9 agonists (Supplemental Fig. S3). Interestingly, C5a, in a dose-dependent manner, inhibited IL-23(p19) release from PEMs, with an IC50 of ∼10 nM (Fig. 4G). This suppressive effect also required the presence of C5aR (Fig. 4H). Absence of the C5L2 receptor did not affect the ability of C5a to inhibit IL-23 expression.
C5a via C5aR regulates the IL-17A/IL-23 axis during endotoxic shock
Complement activation occurs during murine endotoxemia, as indicated by an increase of levels for C5a in plasma of endotoxemic C57BL/6J (Wt) mice (Fig. 5A). We have recently described that the peak of such C5a levels is observed after ∼6 h (28). Given the data presented above, that suppression of the IL-17A/IL-23 axis by C5a requires the C5aR receptor, we decided to study mediator production in C5aR−/− mice during endotoxic shock. Analysis of IL-23(p19) revealed that in C5aR−/− mice circulating plasma levels were 3-fold higher as compared to Wt mice after 12 h (Fig. 5B). In the same samples, the levels of IL-17A in the group of mice with genetic deficiency of C5aR averaged 2000 pg/ml, which was 6-fold higher than the levels in the Wt group (Fig. 5C). Interestingly, at the same time, plasma from C5aR−/− mice after endotoxemia had reduced levels of the anti-inflammatory cytokine IL-10 (Fig. 5D).
Figure 5.
Role of C5a and the C5aR receptor for mediator production in endotoxic shock. A) Levels of C5a in plasma from Wt mice 6 h after endotoxemia compared to untreated healthy Wt mice (sham, n=4/group), ELISA. B) Plasma levels of circulating IL-23(p19) in C5aR−/− and Wt mice (n>5/group) during endotoxemia, 12 h, ELISA. C) Hyperproduction of IL-17A as detected in plasma of C5aR−/− mice (n=7) and compared to Wt mice (n=9) during endotoxic shock, 12 h. D) Plasma levels of circulating IL-10 during endotoxic shock in C5aR−/− and Wt mice (12 h, n=5/group). LPS 7.5 mg/kg BW i.p. for all experiments. Error bars represent means ± se.*P < 0.05, **P < 0.01, ***P < 0.001; Student's t test.
C5a inhibits the IL-17A/IL-23 axis via IL-10
Because C5aR−/− mice displayed lower levels of circulating plasma IL-10 after in vivo challenge with LPS (Fig. 5D), we expanded our in vitro studies to the effects of C5a on IL-10, including a suggested relevance to the IL-17A/IL-23 axis. We assessed release of IL-10 from PEMs stimulated with LPS in the absence or copresence of recombinant mouse C5a (Fig. 6A). There was a 3–5-fold enhancement of IL-10 levels by C5a (100 nM) in the copresence of LPS. Notably, C5a in the absence of LPS did not induce IL-10 release. IL-10 was present in macrophage supernatant fluids already at early time points after addition of C5a and LPS, preceding the release of IL-17A (Fig. 6B). C5a was ineffective in altering IL-10 levels in C5aR−/− PEMs, but IL-10 was robustly enhanced in C5L2−/− PEMs (Fig. 6C).
Figure 6.
C5a suppresses the IL-17A/IL-23 axis via enhancement of IL-10. A) ELISA of IL-10 release after incubation with LPS (1 μg/ml, 10 h) in PEMs from C57BL/6J mice in the absence or copresence of recombinant C5a or C5a alone. B) Time course of IL-10 production from PEMs stimulated with LPS ± C5a. C) Enhancement of IL-10 production by C5a is signaled via C5aR but not C5L2. Relative stimulatory effect of C5a plus LPS compared to LPS alone (=100%) in C57BL/6J, C5aR−/− and C5L2−/− macrophages. D) Effect of different concentrations of recombinant IL-10 on release of IL-17A after LPS, 10 h. E) Effect of recombinant IL-10 on release of IL-23(p19) from LPS stimulated PEMs, 10 h. F) Reduced inhibition by C5a on IL-17A release in LPS-stimulated macrophages from IL-10−/− mice compared to Wt. Percentage numbers indicate the relative inhibitory effect of C5a. G) Quantitative RT-PCR analysis of the effects of recombinant IL-10 on mRNA levels for IL-17A, 8 h. H) Plasma levels of IL-23(p19) during endotoxic shock in C57BL/6J Wt mice (n=9) and IL-10−/− mice (n=6), 10 h. I) Plasma levels of IL-17A during endotoxic shock in Wt mice (n=9) and IL-10−/− mice (n=6), 10 h. Error bars represent means ± se. *P < 0.05, ***P < 0.001; Student's t test.
To investigate whether the enhancement of IL-10 by C5a relates to its ability to suppress IL-17A and IL-23(p19) release, we coincubated macrophages with recombinant IL-10 during TLR4 activation (Fig. 6D, E). The addition of IL-10-mediated potent suppression of the release of IL-17A and IL-23(p19), with an IC50 of ∼100 pg/ml. With IL-10 concentrations ≥1 ng/ml, production of IL-17A and IL-23(p19) was completely suppressed. Macrophages from IL-10−/− mice were stimulated with LPS, in parallel with Wt macrophages. Using IL-10−/− macrophages, we found that copresence of C5a with LPS reduced inhibition when compared to Wt macrophages (17 vs. 48%) of IL-17A release (Fig. 6F). In addition, the IL-10−/− macrophages displayed >10-fold elevated levels of IL-17A after LPS (Fig. 6F). Using RT-PCR, we assessed the suppression of IL-17A by IL-10 on the mRNA level. The addition of IL-10 to LPS-activated PEMs markedly reduced the message levels for IL-17A (Fig. 6G). The in vivo relevance of IL-10 for controlling the release of IL-17A and IL-23(p19) was demonstrated by the fact that IL-10−/− mice showed dramatically elevated levels of both mediators during endotoxemia (Fig. 6H, I). In the genetic absence of IL-10, the rise of plasma levels of IL-17A was 28-fold higher, and the plasma levels of IL-23(p19) were 15-fold higher, as compared to Wt mice. It is well known that IL-10−/− mice are hypersusceptible to endotoxic shock, and this is consistent with our own findings (data not shown). In summary, we found a robust and profound suppression of the IL-17A/IL-23 axis by IL-10, whose presence was markedly enhanced by the complement activation product C5a in macrophages, while C5aR−/− mice had diminished IL-10 levels in endotoxemia (Fig. 5D).
C5a enhances IL-10 release by activation of PI3K-Akt and MEK1/2-ERK1/2 pathways
To investigate intracellular signaling mechanisms by which C5a enhances the release of IL-10 from LPS-activated macrophages, we focused on the PI3K-Akt and MEK1/2-ERK1/2 signaling pathways. We and others have previously shown that C5a activates Akt and ERK1/2 pathways in neutrophils and macrophages (13, 28, 32). Indeed, incubation of PEMs with recombinant C5a (100 nM) for 10 min showed a significant increase of phosphorylated Akt as detected by flow cytometry (Fig. 7A). The antibody used was specific for phosphorylation of a tyrosine amino acid residue at position 308 of Akt. The stimulation with C5a also activated ERK1/2, as indicated by increased phosphorylation at the amino acid residues threonine 202 and tyrosine 204 (Fig. 7B). Next, the potential of C5a to modulate activation of Akt in macrophages from Wt vs. C5aR−/− mice was compared (Fig. 7C). A 20-min stimulation with LPS resulted in comparable proportions of F4/80+ macrophages being positive for phosphorylated Akt (30.7% in Wt vs. 32.0% in C5aR−/−). However, Akt activation in Wt macrophages was further amplified with costimulation of LPS plus C5a (44.3% in Wt vs. 32.5% in C5aR−/−). After incubation of Wt macrophages and C5aR−/− macrophages with LPS, 22.6% and 20.0% of the cells were double positive for F4/80 and phosphorylated ERK1/2, respectively (Fig. 7D). The addition of C5a together with LPS resulted in a 2-fold increased population of F4/80+p-ERK1/2+ macrophages (45.6%). This effect of C5a was not observed in C5aR−/− macrophages (22.7%). In macrophages from Wt mice, the C5aR receptor is abundantly expressed on F4/80+ macrophages (data not shown), but in C5aR−/− macrophages, C5a in the copresence of LPS failed to amplify Akt and ERK1/2 phosphorylation. Taken together, the data suggest that Akt and ERK1/2 activation by C5a both required the presence of the C5aR receptor, similar to the findings above, namely that C5aR is essential for C5a-mediated induction of IL-10. When the activation of the PI3K/Akt pathway was blocked with LY294002, a specific inhibitor of PI3K, thus preventing phosphorylation of downstream Akt, the production of IL-10 from PEMs was substantially suppressed (Fig. 7E). Furthermore, pretreatment with PD98059 or U0126, both selective inhibitors of the MEK1/2 and ERK1/2 pathways, blocked IL-10 release from macrophages (Fig. 7E, F). In conclusion, the PI3K-Akt and MEK1/2-ERK1/2 are activated by C5a in F4/80+ macrophages, and these pathways appear to be crucial for the release of IL-10.
Figure 7.
C5a induces IL-10 by activation of PI3K-Akt and MEK1/2-ERK1/2 signaling pathways. A) Flow cytometry detection of phosphorylation of Akt at amino acid position threonine 308 10 min after stimulation of Wt macrophages with recombinant mouse C5a. B) Phosphorylation of ERK1/2 at threonine 202 and tyrosine 204 in macrophages, detected by flow cytometry after 10 min incubation with C5a. C) Flow cytometry detection of phosphorylated Akt in F4/80+ macrophages from C57BL/6J or C5aR−/− mice. Macrophages were incubated with LPS or LPS plus C5a for 20 min or left untreated (Ctrl). D) Detection of phosphorylated ERK1/2 in F4/80+ macrophages from Wt or C5aR−/− mice. Macrophages were incubated with LPS or LPS plus C5a for 20 min. E) Inhibition of IL-10 release from macrophages by specific blockade of the PI3K-Akt (LY294002, 100 μM) and MEK1/2 (PD98059, 50 μM) pathways, 10 h. F) Suppression of IL-10 production by the MEK1/2 inhibitor U0126, 10 h. Concentrations for LPS were 1 μg/ml and for C5a 100 nM in all experiments. Error bars represent means ± se. **P < 0.01; Student's t test.
DISCUSSION
LPS is a highly reactive molecule able to induce acute inflammation in murine models of disease, as well as in humans, as established by the infusion of LPS into human volunteers at the National Institutes of Health several decades ago. The acute febrile response is accompanied by a robust activation of the coagulation cascades, accompanied by neutrophilia and the appearance of proinflammatory cytokines and chemokines in plasma (33, 34). In humans with sepsis, LPS has been detected in patients with gram-negative sepsis and occurs in patients with Klebsiella or Pseudomonas pneumonias, after penetrating abdominal injuries, and during urosepsis. Here, we report evidence that IL-17A and IL-23 play crucial roles after LPS-induced shock. IL-17A is an essential mediator in immune responses, as well as in innate immunity, and is produced not only by CD4+ Th17 cells but by innate immune cells, such as γδ T cells, neutrophils, paneth cells, etc. (16). In addition to existing reports (26, 27), we provide further evidence for the ability of PEMs to function as a source of IL-17A after activation by LPS in a MyD88-dependent manner. Such events may relate to the outcome of endotoxic shock. Initially, we were concerned about the possibility that PEM preparations might have been contaminated with other IL-17A-producing cell populations, such as Th17 or γδ T cells. However, flow cytometric studies and depletion of macrophages with an anti-F4/80 antibody confirmed macrophages as a relevant source of IL-17A. Alveolar macrophages also released IL-17A after in vitro stimulation with LPS.
We found robust suppressive activities of the complement product C5a on the IL-17A/IL-23 axis in the context of endotoxic shock and in LPS-activated PEMs. The C5a-mediated inhibition of mediators required relatively high concentrations of C5a (10–100 nM) in vitro, but comparable levels are found in endotoxemia (28). At higher concentrations, C5a appears to act in an anti-inflammatory manner, which is underscored by its ability to potently augment IL-10 release. We found a clear functional divergence of the C5aR and C5L2 receptors, with only the C5aR receptor being required for C5a to inhibit IL-17A and IL-23(p19). C5L2 was completely dispensable for the suppressive effects of C5a. Since the C5aR receptor is expressed on numerous cell types, it cannot be excluded that cells other than macrophages contribute directly or indirectly in the alterations of IL-17A, IL-23, and IL-10 levels after endotoxemia in C5aR−/− mice. Noteworthy, C5aR−/− mice have been reported to be protected in lethal endotoxemia, E. coli bacteremia and CLP (12, 35). Thus, it can be concluded that the suppressive effects of C5a on the IL-17A/IL-23 axis and IL-10 in these models are somewhat outbalanced by other biological effects of C5a resulting in death.
Mechanistically, C5a regulated the IL-17A/IL-23 axis in a 2-step process. C5a induced the release of IL-10 via the PI3K-Akt and MEK1/2-ERK1/2 pathways. Such pathways have been demonstrated earlier to be engaged in C5a effects on signaling (36, 37). Subsequently, IL-10 potently acted in an autocrine or paracrine fashion to antagonize IL-17A and IL-23. These data expand previous reports that C5a can regulate mediator production, such as IL-12 in human and mouse macrophages (36, 38). Furthermore, we have recently reported that C5a enhances release of the isoform IL-17F (28), which has a 10- to 30-fold lower bioactivity as compared to IL-17A, in terms of downstream gene activation, e.g., of CXCL1 (39). Therefore, C5a appears to down-modulate the more potent IL-17A while up-regulating the weaker IL-17F, perhaps shifting the balance of IL-17A and IL-17F toward less biological activity of the IL-17A/IL-17F system.
One major function of the complement system is protection and clearance of pathogens in extracellular compartments. By analogy, the immune functions of IL-17A are also associated mainly with defense against extracellular bacteria and fungi. Humans with a defect that nullifies the ability to mount Th17 responses and have reduced levels of IL-17A have recurrent C. albicans and S. aureus infections in the skin and lungs (40). Accordingly, this implies a somewhat redundant role for the complement system and IL-17A. On the other hand, there is abundant evidence demonstrating the detrimental role of IL-17A in the pathogenesis of autoimmune diseases, implying that the release of IL-17A must be tightly controlled in order to provide clearance of infectious pathogens without causing self-inflicted persistent tissue inflammation. Conversely, the absence of the C5aR and C3aR has been associated with a trend toward greater disease activity in experimental autoimmune encephalomyelitis (41). However, other reports have favored either an enhancing or negligible influence of the complement system for the development of autoimmune disease (42–44). It has been reported that C5a can skew release of Th1 cytokine release, such as IL-12; cause accentuation of Th2 responses; and promote T-cell expansion (36, 38, 45–47). In addition to our findings that IL-17A-producing macrophages are regulated via engagement of C5aR with C5a, other reports have recently described a role of the complement system and C5a in Th17 cell responses, e.g., in a disease model of asthma (42, 48, 49). For example, dendritic cells from C5aR−/− mice promoted the induction of Treg and Th17 cells after stimulation with ovalbumin or a TLR2 agonist (50). However, the influence of complement activation as an inhibitory or stimulatory factor on Th17 cell development might depend on the experimental model studied, as well as the time course and the magnitude of C5a generation. IL-17, like C5a, is a powerful enhancer of innate immunity, provided IL-17 and C5a are present locally, but not systemically. Once these powerful agents appear in the plasma, this sets the stage for an exuberant and destructive systemic inflammatory response, as has been described in the setting of polymicrobial sepsis in rodents (11, 12, 25). Therefore, tight regulation of both IL-17A and C5a is essential if effective regulation of innate immune systems is to be achieved.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health, grants GM-29507 and GM-61656 (to P.A.W.), along with the Deutsche Forschungsgemeinschaft, project 571701, BO 3482/1-1 (to M. B.). The authors cordially thank Beverly Schumann, Sue Scott, and Robin Kunkel for assistance in the preparation of the manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMDM
- bone marrow-derived macrophage
- C5a
- complement anaphylatoxin C5a
- C5aR
- G-protein-dependent C5a receptor
- C5L2
- G-protein-independent C5a receptor
- ERK1/2
- extracellular signal-regulated kinase 1/2
- fMLP
- N-formyl-Met-Leu-Phe
- MEK1/2
- MAPK/ERK kinase 1/2
- MyD88
- myeloid differentiation primary response gene 88
- PEM
- peritoneal elicited macrophage
- PI3K
- phosphatidylinositol 3-kinase
- RORγt
- retinoid-related orphan receptor γt.
REFERENCES
- 1. Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010) Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peerschke E. I., Yin W., Ghebrehiwet B. (2010) Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol. Immunol. 47, 2170–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo R. F., Ward P. A. (2005) Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23, 821–852 [DOI] [PubMed] [Google Scholar]
- 4. Ohno M., Hirata T., Enomoto M., Araki T., Ishimaru H., Takahashi T. A. (2000) A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol. Immunol. 37, 407–412 [DOI] [PubMed] [Google Scholar]
- 5. Atefi G., Zetoune F. S., Herron T. J., Jalife J., Bosmann M., Al-Aref R., Sarma J. V., Ward P. A. (2011) Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 25, 2500–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerard C., Gerard N. P. (1994) C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu. Rev. Immunol. 12, 775–808 [DOI] [PubMed] [Google Scholar]
- 7. Gerard N. P., Gerard C. (1991) The chemotactic receptor for human C5a anaphylatoxin. Nature 349, 614–617 [DOI] [PubMed] [Google Scholar]
- 8. Chen N. J., Mirtsos C., Suh D., Lu Y. C., Lin W. J., McKerlie C., Lee T., Baribault H., Tian H., Yeh W. C. (2007) C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446, 203–207 [DOI] [PubMed] [Google Scholar]
- 9. Gerard N. P., Lu B., Liu P., Craig S., Fujiwara Y., Okinaga S., Gerard C. (2005) An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J. Biol. Chem. 280, 39677–39680 [DOI] [PubMed] [Google Scholar]
- 10. Gao H., Neff T. A., Guo R.-F., Speyer C. L., Sarma J. V., Tomlins S., Man Y., Riedemann N. C., Hoesel L. M., Younkin E., Zetoune F. S., Ward P. A. (2005) Evidence for a functional role of the second C5a receptor C5L2. FASEB J. 19, 1003–1005 [DOI] [PubMed] [Google Scholar]
- 11. Czermak B. J., Sarma V., Pierson C. L., Warner R. L., Huber-Lang M., Bless N. M., Schmal H., Friedl H. P., Ward P. A. (1999) Protective effects of C5a blockade in sepsis. Nat. Med. 5, 788–792 [DOI] [PubMed] [Google Scholar]
- 12. Rittirsch D., Flierl M. A., Nadeau B. A., Day D. E., Huber-Lang M., Mackay C. R., Zetoune F. S., Gerard N. P., Cianflone K., Kohl J., Gerard C., Sarma J. V., Ward P. A. (2008) Functional roles for C5a receptors in sepsis. Nat. Med. 14, 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riedemann N. C., Guo R. F., Bernacki K. D., Reuben J. S., Laudes I. J., Neff T. A., Gao H., Speyer C., Sarma V. J., Zetoune F. S., Ward P. A. (2003) Regulation by C5a of neutrophil activation during sepsis. Immunity 19, 193–202 [DOI] [PubMed] [Google Scholar]
- 14. Miossec P., Korn T., Kuchroo V. K. (2009) Interleukin-17 and type 17 helper T cells. New Engl. J. Med. 361, 888–898 [DOI] [PubMed] [Google Scholar]
- 15. Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J. J., Garrone P., Garcia E., Saeland S., Blanchard D., Gaillard C., Das Mahapatra B., Rouvier E., Golstein P., Banchereau J., Lebecque S. (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. [see comment] J. Exp. Med. 183, 2593–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cua D. J., Tato C. M. (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 [DOI] [PubMed] [Google Scholar]
- 17. Wilson R. H., Whitehead G. S., Nakano H., Free M. E., Kolls J. K., Cook D. N. (2009) Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180, 720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy C. A., Langrish C. L., Chen Y., Blumenschein W., McClanahan T., Kastelein R. A., Sedgwick J. D., Cua D. J. (2003) Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langrish C. L., Chen Y., Blumenschein W. M., Mattson J., Basham B., Sedgwick J. D., McClanahan T., Kastelein R. A., Cua D. J. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mangan P. R., Harrington L. E., O'Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 21. McGeachy M. J., Bak-Jensen K. S., Chen Y., Tato C. M., Blumenschein W., McClanahan T., Cua D. J. (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 22. Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 23. Liu X. K., Clements J. L., Gaffen S. L. (2005) Signaling through the murine T cell receptor induces IL-17 production in the absence of costimulation, IL-23 or dendritic cells. Mol. Cells 20, 339–347 [PubMed] [Google Scholar]
- 24. Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L. A., Lubberts E., van den Berg W. B., Libert C. (2008) IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205, 1755–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flierl M. A., Rittirsch D., Gao H., Hoesel L. M., Nadeau B. A., Day D. E., Zetoune F. S., Sarma J. V., Huber-Lang M. S., Ferrara J. L., Ward P. A. (2008) Adverse functions of IL-17A in experimental sepsis. FASEB J. 22, 2198–2205 [DOI] [PubMed] [Google Scholar]
- 26. Gu Y., Yang J., Ouyang X., Liu W., Li H., Bromberg J., Chen S. H., Mayer L., Unkeless J. C., Xiong H. (2008) Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38, 1807–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Da Silva C. A., Hartl D., Liu W., Lee C. G., Elias J. A. (2008) TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 181, 4279–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bosmann M., Patel V. R., Russkamp N. F., Pache F., Zetoune F. S., Sarma J. V., Ward P. A. (2011) MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 25, 4222–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosmann M., Russkamp N. F., Patel V. R., Zetoune F. S., Sarma J. V., Ward P. A. (2011) The outcome of polymicrobial sepsis is independent of T and B cells. Shock 36, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bedoret D., Wallemacq H., Marichal T., Desmet C., Quesada Calvo F., Henry E., Closset R., Dewals B., Thielen C., Gustin P., de Leval L., Van Rooijen N., Le Moine A., Vanderplasschen A., Cataldo D., Drion P. V., Moser M., Lekeux P., Bureau F. (2009) Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 119, 3723–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tidball J. G., Wehling-Henricks M. (2007) Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 578, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo R. F., Sun L., Gao H., Shi K. X., Rittirsch D., Sarma V. J., Zetoune F. S., Ward P. A. (2006) In vivo regulation of neutrophil apoptosis by C5a during sepsis. J. Leukoc. Biol. 80, 1575–1583 [DOI] [PubMed] [Google Scholar]
- 33. Taylor F. B., Jr. (2001) Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit. Care Med. 29, S78–89 [DOI] [PubMed] [Google Scholar]
- 34. Taylor F. B., Jr., Hack E., Lupu F. (2006) Observations on complement activity in the two-stage inflammatory/hemostatic response in the baboon and human models of E. coli sepsis and endotoxemia. Adv. Exp. Med. Biol. 586, 203–216 [DOI] [PubMed] [Google Scholar]
- 35. Hollmann T. J., Mueller-Ortiz S. L., Braun M. C., Wetsel R. A. (2008) Disruption of the C5a receptor gene increases resistance to acute Gram-negative bacteremia and endotoxic shock: opposing roles of C3a and C5a. Mol. Immunol. 45, 1907–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hawlisch H., Belkaid Y., Baelder R., Hildeman D., Gerard C., Kohl J. (2005) C5a negatively regulates Toll-like receptor 4-induced immune responses. Immunity 22, 415–426 [DOI] [PubMed] [Google Scholar]
- 37. Riedemann N. C., Guo R. F., Hollmann T. J., Gao H., Neff T. A., Reuben J. S., Speyer C. L., Sarma J. V., Wetsel R. A., Zetoune F. S., Ward P. A. (2004) Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 18, 370–372 [DOI] [PubMed] [Google Scholar]
- 38. Wittmann M., Zwirner J., Larsson V. A., Kirchhoff K., Begemann G., Kapp A., Gotze O., Werfel T. (1999) C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J. Immunol. 162, 6763–6769 [PubMed] [Google Scholar]
- 39. Gaffen S. L. (2009) Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milner J. D., Brenchley J. M., Laurence A., Freeman A. F., Hill B. J., Elias K. M., Kanno Y., Spalding C., Elloumi H. Z., Paulson M. L., Davis J., Hsu A., Asher A. I., O'Shea J., Holland S. M., Paul W. E., Douek D. C. (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramos T. N., Wohler J. E., Barnum S. R. (2009) Deletion of both the C3a and C5a receptors fails to protect against experimental autoimmune encephalomyelitis. Neurosci. Lett. 467, 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hashimoto M., Hirota K., Yoshitomi H., Maeda S., Teradaira S., Akizuki S., Prieto-Martin P., Nomura T., Sakaguchi N., Kohl J., Heyman B., Takahashi M., Fujita T., Mimori T., Sakaguchi S. (2010) Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J. Exp. Med. 207, 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reiman R., Gerard C., Campbell I. L., Barnum S. R. (2002) Disruption of the C5a receptor gene fails to protect against experimental allergic encephalomyelitis. Eur. J. Immunol. 32, 1157–1163 [DOI] [PubMed] [Google Scholar]
- 44. Liu J., Lin F., Strainic M. G., An F., Miller R. H., Altuntas C. Z., Heeger P. S., Tuohy V. K., Medof M. E. (2008) IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J. Immunol. 180, 5882–5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kohl J., Baelder R., Lewkowich I. P., Pandey M. K., Hawlisch H., Wang L., Best J., Herman N. S., Sproles A. A., Zwirner J., Whitsett J. A., Gerard C., Sfyroera G., Lambris J. D., Wills-Karp M. (2006) A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J. Clin. Invest. 116, 783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X., Kimura Y., Fang C., Zhou L., Sfyroera G., Lambris J. D., Wetsel R. A., Miwa T., Song W.-C. (2007) Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lalli P. N., Strainic M. G., Yang M., Lin F., Medof M. E., Heeger P. S. (2008) Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112, 1759–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fang C., Zhang X., Miwa T., Song W.-C. (2009) Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood 114, 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lajoie S., Lewkowich I. P., Suzuki Y., Clark J. R., Sproles A. A., Dienger K., Budelsky A. L., Wills-Karp M. (2010) Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 11, 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weaver D. J., Reis E. S., Pandey M. K., Kohl G., Harris N., Gerard C., Kohl J. (2010) C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur. J. Immunol. 40, 710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.