Abstract
Self-resolving inflammatory exudates and lipid mediator metabolomics recently uncovered a new family of potent anti-inflammatory and proresolving mediators biosynthesized by macrophages (MΦs), denoted maresins. Here we determined that maresin 1 (MaR1) produced by human MΦs from endogenous docosahexaenoic acid (DHA) matched synthetic 7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid. The MaR1 alcohol groups and Z/E geometry of conjugated double bonds were matched using isomers prepared by total organic synthesis. MaR1's potent defining actions were confirmed with synthetic MaR1, i.e., limiting polymorphonuclear neutrophil (PMN) infiltration in murine peritonitis (ng/mouse range) as well as enhancing human macrophage uptake of apoptotic PMNs. At 1 nM, MaR1 was slightly more potent than resolvin D1 in stimulating human MΦ efferocytosis, an action not shared by leukotriene B4. MaR1 also accelerated surgical regeneration in planaria, increasing the rate of head reappearance. On injury of planaria, MaR1 was biosynthesized from deuterium-labeled (d5)-DHA that was blocked with lipoxygenase (LOX) inhibitor. MaR1 dose-dependently inhibited TRPV1 currents in neurons, blocked capsaicin (100 nM)-induced inward currents (IC50 0.49±0.02 nM), and reduced both inflammation- and chemotherapy-induced neuropathic pain in mice. These results demonstrate the potent actions of MaR1 in regulating inflammation resolution, tissue regeneration, and pain resolution. These findings suggest that chemical signals are shared in resolution cellular trafficking, a key process in tissue regeneration. Moreover, immunoresolvents of the innate immune response, such as MaR1, offer new opportunities for assessing MΦs and their local DHA metabolome in the return to tissue homeostasis.—Serhan, C. N., Dalli, J., Karamnov, S., Choi, A., Park, C.-K., Xu, Z.-Z., Ji, R.-R., Zhu, M., Petasis, N. A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain.
Keywords: DHA metabolome, eicosanoids, inflammation resolution, neutrophils, resolvins
Uncontrolled inflammation is now considered to be a link between many widely occurring diseases, including classic inflammatory diseases, cardiovascular disease, and asthma, as well as certain neurodegenerative diseases, and so much attention has turned to controlling the innate inflammatory response and its local chemical mediators (1). In this regard, phagocytes have essential roles: polymophonuclear neutrophils (PMNs) as the first white cell responders to invaders and tissue injury, and macrophages (MΦs), with pivotal roles in the resolution of acute inflammation by virtue of their ability to take up apoptotic PMNs and debris, as well as orchestrating tissue remodeling of the site (2). During resolution, specialized proresolving mediators (SPMs) are produced from essential polyunsaturated fatty acids, for example, resolvins and protectins, that limit PMN infiltration and enhance MΦ homeostatic functions (3, 4). Proresolution MΦs are apparently specially equipped for these homeostatic functions (5–9). Hence, MΦs play key roles in innate host responses and local inflammation (1, 10, 11), as well as in neovascularization, resolution of inflammation, and wound healing (12, 13). Thus, the chemical signals and mediators produced by MΦs are of wide interest.
In this regard, SPMs are unique in that they are temporally biosynthesized locally by resolving exudates of acute inflammation, where they act in both nonphlogistic and antiphlogistic fashions to, for example, limit further PMN recruitment to the site as well as stimulate MΦ efferocytosis of apoptotic PMNs. The SPM families of autacoids include the lipoxins, resolvins, protectins, and the most recent addition to these potent mediators, the macrophage mediators in resolving inflammation (maresins; see below; for a recent review, see ref. 14). Given the well-appreciated and critical roles of MΦs in resolution as well as wound-healing events, novel lipid-derived local mediators derived from MΦs that enhance resolution are of considerable interest. For example, the newly uncovered docosahexaenoic acid (DHA) metabolome, resolvin D1 (RvD1; 7S,8R,17S trihydroxydocosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid; ref. 15) regulates tissue MΦ accumulation and reduces both obesity-induced diabetes in mice (16) and arthritic pain (17, 18). Notably, failures or uncoupling of active tissue resolution programs of the acute inflammatory response can lead to persistent inflammation-associated diseases. In support of this notion, MΦs from localized aggressive periodontitis display impaired phagocytosis and persistent local inflammation that is rescued with the eicosapentaenoic acid-derived resolvin E1 (RvE1; 5S, 12R,18R-trihydroxyeicosa-6Z,8E,10E,14Z,16E-pentaenoic acid; ref. 19). Hence, MΦs and the local mediators they produce are vital in tissue homeostasis and host defense as well as the resolution of inflammation.
Along these lines, we identified a novel family of potent DHA-derived molecules produced by MΦs, denoted the maresins, that act directly on phagocytes (20). The new DHA-metabolome is initiated in MΦs by 14-lipoxygenation of DHA, producing the hydroperoxy-containing intermediate 14S-hydroperoxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S-HpDHA) that undergoes further conversion via 13(14)-epoxidation, which is a key process in the biosynthesis of potent mediators such as 7,14-dihydroxydocosa-4Z,8Z,10,12,16Z,19Z-hexaenoic acid, denoted maresin 1 (MaR1). MaR1, from this new DHA-metabolome, is defined as possessing potent anti-inflammatory and proresolving actions both in vivo and in vitro (20). In addition to MaR1 in the resolving exudates, a double dioxygenation product, 7S,14S-dihydroxydocosa-4Z,8E,10Z,12E,16Z,19Z-hexaenoic acid (7S,14S-diHDHA), formed by consecutive lipoxygenation, was also identified using molecular oxygen incorporation, and proved less active than MaR1. Given the different biosynthetic origin, namely, 2 sequential lipoxygenation steps, it was anticipated that its stereochemistry at carbon position 7 would be predominantly in the 7S configuration, and the double-bond geometry in its conjugated triene moiety was expected to have the 8E,10Z,12E configuration (20). It is well established that stereochemistry of small molecule mediators (for example, individual prostaglandins and leukotrienes) is dictated by their biosynthesis and is critical to their potent bioaction (21). Subtle changes in stereochemistry of mediators such as resolvins and protectins can result in dramatic changes in potency (14). Since the enzymes [human 12-lipoxygenase (LOX) and murine 12/15-LOX] that initiate DHA 14-lipoxygenation for maresin biosynthesis are widely occurring in the animal and plant kingdoms (22), it is likely that maresins and related compounds originally identified with MΦs (20) are also biosynthesized by a wide range of cell types across phyla. In the present report, we matched MaR1 stereochemistry with human leukocyte MaR1 and confirm with synthetic MaR1 its potent anti-inflammatory and proresolving actions in vivo as well as document MaR1 production by Platyhelminthes (Dugesia tigrina) and novel potent actions of MaR1 in both tissue regeneration and pain control.
MATERIALS AND METHODS
Materials
Materials used included hr-IL4 and hr-M-CSF (R&D Systems, Minneapolis, MN, USA); 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA), RPMI 1640, trypan blue, H2O2, Triton-X 100, bovine serum albumin (BSA), cold water fish gelatin, and zymosan (Sigma, St. Louis, MO); IgG-free BSA (Jackson Laboratories, Bar Harbor, ME, USA); Ly6G-PE conjugated antibody (clone 1A8; BioLegend, San Diego, CA, USA); brown planaria (Dugesia tigrina; Wards Scientific, Rochester, NY, USA); 5S,12R-dihydroxy-5-cis-9,10-trans,14-cis-eicosatetraenoic acid (LTB4), d5-DHA, and baicalein (Cayman Chemical, Ann Arbor, MI, USA); HPLC-grade methanol (MeOH; Fisher Scientific, Pittsburgh, PA, USA); DAPI containing Vectashield, and FITC-conjugated Erythrina cristagalli lectin (ECL; Vector Laboratories, Burlingame, CA, USA); and human serum (Lonza, Portsmouth, NH). A second source of synthetic MaR1 was obtained from Greg Keyes (Cayman Chemical). Male FvB mice 6–8 wk of age were purchased from Charles River (Wilmington, MA, USA).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based metabolomics
All LC-MS/MS solvents and solid-phase extraction materials were as previously described (20, 23), and LC-MS/MS was carried out using an ABI Qtrap 5500 (Applied Biosystems, Foster City, CA, USA; see below and figures). The total organic synthesis and preparation of each 7,14-diHDHA isomer I–IV will be reported separately (unpublished results). All isomers and MaR1 were validated just prior to experiments using LC-MS/MS, and amounts were determined with UV extinction coefficient of ε ≈ 40,000.
Human primary MΦs and endogenous MaR1
Human peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient Ficoll-Histopaque isolation. Isolated PBMCs were washed 3 times to remove platelets, and monocytes were purified using a monocyte isolation kit (StemCell Technologies, Vancouver, BC, Canada) yielding a 96–99% CD14+ monocyte population. The cells (4×106 cells/incubation) were then cultured for 7 d in phenol red-free RPMI 1640 medium supplemented with 10% human serum and 20 ng/ml M-CSF for 7 d. Next, the cells were incubated for 48 h (37°C) with 20 ng/ml IL-4 (10). On d 9, 2 vol of MeOH was added, and products were extracted (23). Extracted samples were subject to LC-MS/MS-based lipidomics [Applied Biosystems QTrap 5500 equipped with an Agilent HP1100 binary pump and diode-array detector (DAD); Agilent Technologies, Santa Clara, CA, USA]. An Agilent Eclipse Plus C18 column (50 × 4.6 mm × 1.8 μm) was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (v/v/v) to 100:0:0.01 at 0.4-ml/min flow rate. Instrument control and data acquisition were performed using AnalystQ 1.4.2 software (Applied Biosystems). Ion pair transition (m/z 359.2/221.1) multireaction monitoring (MRM) was used for profiling and quantitation of MaR1 and related isomers.
Efferocytosis with apoptotic PMNs
Briefly, monocytes and PMNs were isolated separately from fresh peripheral blood of healthy volunteers (protocol 1999-P-001297, approved by the Partners Human Research Committee, Boston, MA, USA) and differentiated in the presence of 10 ng/ml of hr-GM-CSF (R&D Systems). Phagocytosis was conducted as described previously (24). Fluorescently labeled apoptotic human PMNs were obtained following overnight incubation in RPMI 1640 of freshly isolated PMNs prelabeled with CFDA (10 μM; Sigma). On d 7, MΦs were incubated with either vehicle, MaR1, 7S isomer I, or 12E isomer II (10 pM to 100 nM) for 15 min prior to addition of apoptotic PMNs at a ∼3:1 ratio (PMN:MΦ) and incubated for 1 h at 37°C. Subsequently, nonphagocytosed PMNs were washed away, and extracellular fluorescence was quenched using trypan blue (1:50 dilution). The number of phagocytosed cells (i.e., intracellular) was assessed using a SpectraMax M3 plate reader (Molecular Devices, Sunnyvale, CA, USA) by monitoring fluorescence emission at 525 nM.
Zymosan peritonitis
Male FvB mice 6–8 wk of age (Charles River) were treated with either vehicle or MaR1 (0.1 to 10 ng/mouse i.v.) 10 min prior to intraperitoneal administration of zymosan (0.1 mg/mouse). After 4 h, the peritoneum was lavaged, total number of infiltrated cells was assessed using trypan blue, and infiltrated PMNs were enumerated by light microscopy and immunofluorescent staining of the lavage cells with an anti-mouse Ly6G-PE-conjugated antibody (clone 1A8; Biolegend) as previously reported (24). Murine peritonitis procedures were approved by the Standing Committee on Animals of Harvard Medical School (protocol no. 02570) and performed in accordance with institutional and U.S. National Institutes of Health (NIH) guidelines.
Tissue regeneration and MaR1 biosynthesis in D. tigrina
Brown planaria (D. tigrina; Ward's Scientific) were maintained in spring water (21°C). On d 0, planaria ∼1.0–1.5 cm in length were subjected to resection of the head postpharyngeally. The posterior portion of the planaria was then placed in spring water (21°C) containing either vehicle, RvE1 (100 nM) or MaR1 (1–100 nM). To assess the extent of tissue regeneration for a 7-d period, images of the portion of the planaria were taken at 24-h intervals and analyzed using ImageJ software (NIH, Bethesda, MD, USA). A tissue regeneration index (TRI) was employed that took into consideration the size of the regenerated tissue total area (A) and the size of the planaria (here we employed the postpharyngeal width W of the animal as a surrogate), where TRI = A/W.
Brown planaria were subjected to either head resection and placed in fresh water containing 1 μg d5-DHA or placed in d5-DHA without surgery (21°C). At the indicated time intervals, 2 vol of MeOH was added, and the tissues were homogenized using a Bullet Blender Plus (NextAdvance, Cambridge, MA, USA) and subjected to solid-phase extraction (23). The extracted samples were next analyzed using an LC-UV-MS/MS system (QTrap 5500; Applied Biosystems) as above. Ion pair transitions for profiling and quantification were d5-MaR1 (364.2/221.1) and d5-14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (d5-14-HDHA; 348.2/219.1). Criteria for identification included LC retention time (tR) and a minimum of 6 fragment diagnostic ions within the MS/MS spectrum matching those of synthetic and authentic standards. Deuterium-labeled internal standard d8-5S-HETE and d4-LTB4 were added prior to extraction as internal standard for recovery calculations (23). Calibration curves were obtained for each product; MaR1 gave a value of r = 0.97; d4-LTB4, r = 0.99; d4-PGE2, r = 0.91; and d8-5-HETE, r = 0.99. To assess the contribution of LOX enzymes to the production of 14-HDHA and MaR1, planaria were subject to injury/surgery (see above) and then incubated in the presence of either d5-DHA alone or d5-DHA plus baicalein (10 μM) for 30 min at room temperature. Subsequently, 2 vol of cold methanol was added and solid-phase extracted as above. In a separate set of experiments, after surgery, planaria following surgery were kept in spring water containing either vehicle, baicalein (0.1 μM) or baicalein (0.1 μM) plus MaR1 (100 nM). Assessment of head regeneration was conducted as above for a 7-d time course.
Planaria fluorescent lectin-conjugated staining
Staining was carried out as described previously (25). Briefly, planaria were treated with 2% HCl for 5 min on ice, fixed with Carnoy's fixative for 2 h at 4°C, and washed for 1 h at 4°C in methanol. They were then bleached overnight in 6% H2O2 in methanol at room temperature. After bleaching, animals were rehydrated in 75, 50, and 25 methanol/PBTX (PBS with 0.3% Triton-X 100) and twice with PBTX for 5 min. Samples were then incubated in blocking buffer (0.6% IgG-free BSA, Jackson Laboratories; and 0.45% fish gelatin, Sigma) in PBTX for 2–4 h with shaking. After blocking, samples were incubated in FITC-conjugated ECL at 1 mg/ml (Vector Laboratories) in blocking buffer overnight at 4°C to visualize secretory cells (25). Subsequently, the planaria were washed with PBTX 6 times for 1 h at room temperature, mounted in DAPI containing Vectashield (Vector Laboratories), and imaged with a Zeiss YFL (Carl Zeiss, Thornwood, NY, USA) and ImagePro Plus 7 software (Media Cybernetics, Bethesda, MD, USA).
MaR1 and pain
Adult CD1 mice (male, 8–10 wk) were used for behavioral and pharmacological studies. Young CD1 mice (4–6 wk) were used for electrophysiological studies in dorsal root ganglion (DRG) neurons. To produce acute inflammatory pain, capsaicin (1 mg, 20 ml; Sigma) was injected into the plantar surface of a hind paw under a brief anesthesia of isoflurane. To produce chemotherapy-induced neuropathic pain, vincristine sulfate (Sigma) was injected (0.2 mg/kg i.p.; ref. 26). MaR1 and Trolox, a vitamin E analog, were prepared in PBS. All animal procedures were approved by the Animal Care Committee of Harvard Medical School (protocol no. 03649) and performed in accordance with the guidelines of the International Association for the Study of Pain.
Whole-cell patch-clamp recordings in cultured DRG neurons
DRGs were removed aseptically and incubated with collagenase (1.25 mg/ml)/dispase-II (2.4 U/ml) at 37°C for 90 min, then digested with 0.25% trypsin for 8 min at 37°C. Cells were mechanically dissociated with a flame-polished Pasteur pipette in the presence of 0.05% DNase I. DRG cells were plated on glass coverslips and grown in a neurobasal defined medium (with 2% B27 supplement; Invitrogen) with 5 μM AraC and 5% carbon dioxide at 36.5°C. DRG neurons were cultured for 24 h before use. Whole-cell current-clamp recordings were performed at room temperature to measure currents and action potentials, respectively, with an Axopatch-200B amplifier (Axon Instruments, Foster City, CA, USA). The recording chamber (300 μl) was continuously superfused (2–3 ml/min). Series resistance was compensated for (>80%), and leak subtraction was performed. Data were low-pass-filtered at 2 kHz, sampled at 10 kHz. The pClamp8 (Axon Instruments) software was used during experiments and analysis. Voltage-clamp experiments were performed at a holding potential of −60 mV.
Pain behavioral analysis
Animals were habituated to the testing environment daily for ≥2 d before baseline testing. Animals were put in plastic boxes for 30 min habituation before examination. For testing capsaicin-induced spontaneous pain (nocifensive behavior), capsaicin was intraplantarly injected, and the time spent on nocifensive behavior (flinching and licking) was recorded for 5 min. For testing mechanical sensitivity, the plantar surface of the hind paw was stimulated with a series of von Frey hairs with logarithmically incrementing stiffness (0.02–2.56 g; Stoelting, Wood Dale, IL, USA), presented perpendicular to the plantar surface. The 50% paw withdrawal threshold was determined using Dixon's up-down method. For all behavioral tests, the experimenters were blinded to the treatments.
Statistical analysis
All data were expressed as means ± se. Differences between groups were compared using Student's t test (2 groups) or 1-way ANOVA (multiple groups) followed by post hoc Bonferroni test. The criterion for statistical significance was P < 0.05.
RESULTS
Human MΦ MaR1
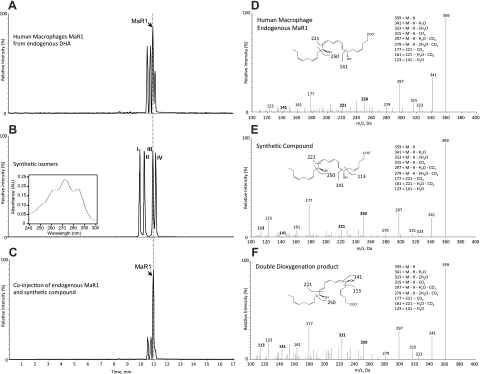
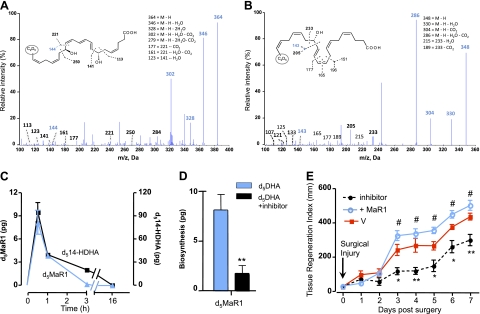
Since both human and murine MΦs produce MaR1 and several related isomers from DHA (20), it was important to establish MaR1 stereochemistry as well as confirm its assigned structure and actions with synthetic materials prepared by total organic synthesis. Figure 1 reports the matching of MaR1 produced by human MΦs with 4 synthetic 7,14-diHDHA isomers, labeled compounds I–IV (Fig. 2). The assignment of the stereochemistry of the hydroxyl group at position 7 in MaR1 and the double-bond geometry within its conjugated triene double-bond system remained to be determined with MaR1 from endogenous DHA. To address this, it was essential to design and synthesize R/S and Z/E stereoisomers for direct matching (Figs. 1B and 2). Their total synthesis will be reported separately. To this end, biological MaR1 was obtained and profiled from human MΦs according to the published criteria. Authentic MaR1 produced from endogenous DHA was chromatographed using LC-MS/MS-based lipidomics (Fig. 1A) for direct comparisons to those of synthetic I–IV isomers (Fig. 1B).
Figure 1.
Endogenous MaR1 from human MΦs and synthetic isomers. A) Selected ion chromatograms (m/z 359>221) depicting human MΦ-derived endogenous MaR1 and related natural isomers. B) Synthetic isomers I–IV. Inset: characteristic UV-absorption spectrum. C) Coinjection of human MΦ-derived endogenous MaR1 with synthetic isomer III. D) MS/MS spectrum of human MΦ-derived endogenous MaR1 (tR=11.05 min). E) Synthetic MaR1 (tR=11.05 min). F) Double dioxygenation product 7S,14S-diHDHA (isomer IV; tR=11.25 min). Vertical dashed and dotted line indicates coelution tR. Representative MRM chromatograms and MS/MS spectra of n = 6. See text for details.
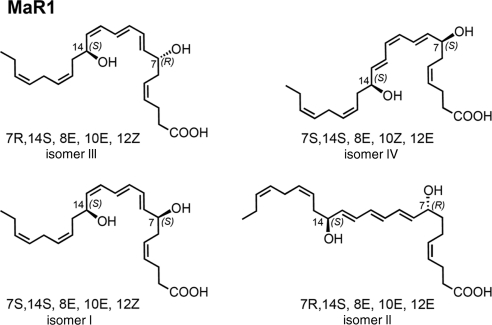
Figure 2.
Synthetic stereoisomers. Stereoisomers I–IV synthesized for comparison with authentic MaR1 and related isomers.
Figure 2 shows the 4 stereoisomers used for LC-MS/MS matching. These included the 7R,14S-(8E,10E,12E)-trans isomer (compound II) as well as the double dioxygenation product (7S,14S-diHDHA) with the double geometry of 8E,10Z,12E in the conjugated triene portion of this isomer (compound IV, Fig. 2). The authentic MaR1 from both human (Fig. 1A) and murine MΦs (not shown) produced a sharp peak in liquid chromatography with tR = 11.05 min. Also illustrated in the Fig. 1B inset are the characteristic conjugated triene chromophore absorbance bands with λmaxMeOH ∼ 270 nm and shoulders on either side that are characteristic of each of the 4 isomers (I–IV). MaR1 clearly separated from the 12E isomer (compound II), which eluted earlier, as well as the 7S,14S-(8E,10E,12Z)-containing isomer (compound I), which also showed a chromatographic behavior, eluting before authentic MΦ-derived MaR1. Coinjection with the material prepared by total organic synthesis and authentic MaR1 demonstrated coelution with synthetic compound III (Fig. 1C). Thus, the complete stereochemistry of MaR1 was determined as 7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid.
Of note, although the chromatographic behaviors of MaR1 and its I–IV isomers were each distinct in tR (Fig. 1B), the fragmentation of MaR1 and its stereoisomers was essentially identical (Fig. 1D–F). It is noteworthy that each gave m/z 359 = M-H, m/z 341 = M-H-H2O, m/z 323 = M-H-2H2O, m/z 315 = M-H-CO2, m/z 297 = M-H-H2O-CO2, m/z 279 = M-H-2H2O-CO2, m/z 205 = 250-CO2+H, m/z 177 = 221-CO2, m/z 161 = 221-H2O-CO2, and m/z 123 = 141-H2O. The endogenous MaR1 MS/MS spectrum (Fig. 1D) displayed the same prominent diagnostic ions in its mass spectrum. Hence, these results also confirm the original structural assignments for MaR1 (20) and provide the stereochemistry of MaR1 produced from endogenous DHA by MΦ, since the tR and prominent ions were matched with synthetic compound III (Figs. 1 and 2).
MaR1, a potent anti-inflammatory and proresolving molecule
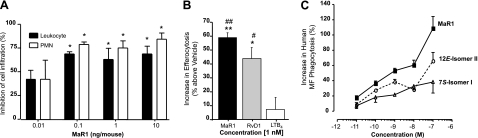
Having identified the synthetic MaR1, we also required confirmation of its potent anti-inflammatory and proresolving actions. At doses as low as 0.1 ng/mouse, synthetic MaR1 reduced PMN infiltration in response to zymosan-initiated acute peritonitis (Fig. 3A). It is noteworthy that, at doses of 10 ng/mouse, MaR1 reduced PMN infiltration by as much as 50–80%. A recently appreciated key action for SPM is stimulating MΦs to uptake of apoptotic PMN, which is also the case for MaR1 (20). In Fig. 3B, synthetic MaR1 proved to be a potent efferocytosis agonist, enhancing human MΦ uptake of apoptotic human PMN. In this system, MaR1 at 1 nM was more potent than RvD1. This RvD1 proresolving property was used for direct comparison as a known positive control (27), an activity that was not shared by LTB4 (Fig. 3B). We next assessed the related MaR1 natural isomers; these results indicate that MaR1 was more potent than either the 12E-triene-containing isomer (II) or its 7S-containing isomer (I; see Figs. 2 and 3C). Together, these results also demonstrate and confirm the potent anti-inflammatory and proresolving actions defining MaR1 with synthetic MaR1.
Figure 3.
MaR1 is a potent anti-inflammatory and proresolving mediator. A) Murine peritonitis. Zymosan (0.1 mg) was administered to mice (see Materials and Methods) 10 min after i.v. MaR1. After 4 h, peritonea were lavaged, and the total numbers of leukocytes, PMNs, and mononuclear cells along with the number of Ly6G-positive cells were determined (n=4 mice/group). *P < 0.05 vs. vehicle group. B) MΦ efferocytosis of apoptotic PMNs. MΦs (24-well plate, 105 cells/well) were exposed to either MaR1, RvD1, or LTB4 mediators at 1 nM (37°C, 15 min) followed by CFDA-labeled apoptotic human PMNs (90 min, 37°C). Results are expressed as percentage increase above vehicle (n=3). *P ≤ 0.05, **P ≤ 0.01 vs. vehicle; #P ≤ 0.05, ##P ≤ 0.01 vs. LTB4. C) MaR1 enhances efferocytosis: Apoptotic neutrophils prelabeled with CFDA were coincubated in the presence of vehicle or the indicated amounts of MaR1, 7S isomer I, or 12E isomer II (1 h at 37°C) in 5% CO2. Nonphagocytosed cells were washed, and the extent of phagocytosis was determined (see Materials and Methods; n=3–4 separate donors). Results are means ± se.
MaR1 stimulates tissue regeneration
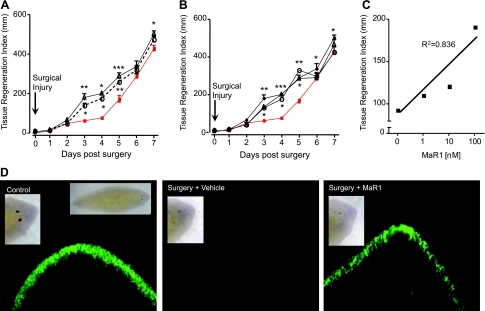
Planaria are simple organisms capable of rapid regeneration, the process wherein mammalian tissue MΦs play distinct roles. Hence, we questioned whether MaR1 demonstrated properties in tissue regeneration. To this end, planaria were grown, and their anterior portions were surgically removed and exposed to proresolving mediators (Fig. 4A). Planaria are increasingly recognized as a useful model system for tissue regeneration (28). Factors are known to be involved in planaria tissue regeneration (28); however, they remained to be identified. MaR1 and RvE1 at 100 nM each enhanced the rate of tissue regeneration, with the appearance of the anterior portion (i.e., head regeneration) evident as early as 3 d postsurgery (Fig. 4A). We next examined MaR1 dose dependency (Fig. 4B). MaR1 at doses as low as 1, 10, and 100 nM enhanced tissue regeneration in the anterior head that was statistically significant by d 3 and 4. By d 6 and 7, the actions were less prominent. MaR1-enhanced tissue regeneration was concentration dependent (Fig. 4C). Figure 4D shows the untreated planaria and whole animals (left panel, inset) on d 4 after surgery and exposure to either MaR1 (Fig. 4D, right panel) or vehicle alone (Fig. 4D, center panel) following staining with fluorescent conjugated ECL (see Materials and Methods).
Figure 4.
Maresin 1 stimulates tissue regeneration. A) Anterior portions of brown planaria were surgically removed, and remaining planaria were exposed to vehicle (squares), RvE1 (100 nM; circles), or MaR1 (100 nM; triangles) for 7 d. B) Planaria were exposed to increasing concentrations of MaR1 (circles, 1 nM; triangles, 10 nM; diamonds, 100 nM), and regeneration was assessed for 7 d. C) Concentration dependence. D) Planaria 4 d after surgery with or without MaR1 were stained using fluorescently conjugated ECL to visualize secretory cells; see Materials and Methods. Inset (left panel): gross whole worm. Results are means ± se (n=5 planaria/group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle alone.
We next assessed whether planaria are able to biosynthesize MaR1 following tissue injury. To this end, planaria were incubated with deuterium-labeled DHA (d5-DHA). Figure 5A shows the LC-MS/MS spectrum of d5-MaR1 as well as d5-14-HDHA obtained from planaria (Fig. 5B). These results demonstrate that MaR1 biosynthesis was activated within surgically injured planaria. The time course of formation of both 14-HDHA (a MaR pathway biomarker) and MaR1 are shown in Fig. 5C. The LOX inhibitor baicalein blocked MaR1 biosynthesis (Fig. 5C). Moreover, baicalein delayed the regeneration time course, and MaR1, when added back, rescued this block and enhanced the rate of regeneration (Fig. 5E).
Figure 5.
MaR1 production by brown planaria in response to tissue injury. Anterior portions of brown planaria were surgically removed, and worms were incubated for indicated time points with deuterium-labeled DHA. A, B) LC-MS/MS spectrum of d5MaR1 (A) and d514-HDHA (B) obtained from planaria with diagnostic ions with deuterium incorporated. C) Time course and LC-MS/MS of d514-HDHA and d5MaR1 produced at the indicated time points postsurgery. D) LOX inhibitor. **P < 0.01. E) LOX inhibitor reduced regeneration and MaR1 (nM) rescue. *P < 0.05, **P < 0.01 vs. vehicle (V); #P < 0.05 vs. LOX inhibitor. Results are means ± se (n=5 planaria/group).
MaR1 blocks transient receptor potential V1 (TRPV1) currents in primary sensory neurons and inhibits pain in vivo
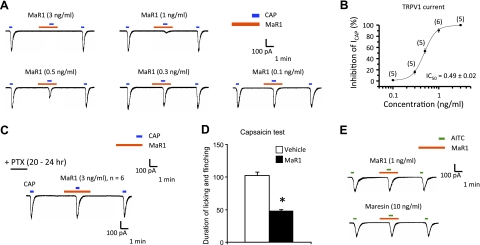
TRPV1 is expressed in primary sensory neurons and plays an important role in mediating heat pain and heat hyperalgesia after injury (29). Since we recently found that RvE1 reduces inflammatory pain via blocking TRPV1 activity (30), we used patch-clamp recordings in dissociated mouse DRG primary sensory neurons to test whether MaR1 also modulates the activity of TRPV1. Perfusion of DRG neurons with capsaicin (100 nM) elicited a marked TRPV1 current, and this current was dose-dependently inhibited by maresin (Fig. 6A). Notably, MaR1 inhibited TRPV1 currents with a very low IC50, 0.16 nM (i.e., 0.49 ng/ml); see Fig. 6B. For comparison, AMG9810, a commonly used TRPV1 antagonist, inhibited TRPV1 currents with an IC50 of 163 nM (data not shown), which is ≈1000 times higher than MaR1. Proresolving mediators such as LXA4, RvE1, and RvD1 each signal via specific G-protein-coupled receptors (GPCRs; ref. 31); hence, to assess the potential involvement of GPCRs in MaR1 actions, we treated DRG cultures with pertussis toxin (PTX; 0.5 mg/ml), which blocks Gαi-coupled GPCRs. After PTX treatment, MaR1 did not inhibit TRPV1 currents (P>0.05, n=6 neurons; Fig. 6C).
Figure 6.
MaR1 inhibits TRPV1 currents in DRG neurons and reduces inflammatory pain. A) Capsaicin (CAP; 100 nM)-induced inward currents. MaR1 dose-dependent inhibition of TRPV1 currents. B) MaR1 dose-response inhibition of TRPV1 currents. Inset: IC50 of TRPV1 current inhibition; n = 5–6 neurons/dose. C) Treatment of DRG cultures with pertussis toxin (PTX; 0.5 mg/ml, 18 h) blocked inhibitory actions of MaR1 (3 ng/ml) on TRPV1 current (n=6 neurons). D) Intraplantar administration of MaR1 (10 ng/mouse) reduces capsaicin (1 mg)-induced spontaneous pain (n=8 mice). *P < 0.05 vs. vehicle. E) Mustard oil allyl isothiocyanate (AITC; 300 mM)-induced inward currents. Notably, MaR1 had no direct action on TRPA1 currents.
We further examined whether MaR1 could control inflammatory pain in mice. Intraplantar injection of capsaicin (1 mg) elicited marked spontaneous pain behaviors (flinching/licking) that was reduced by intraplantar MaR1 (10 ng; Fig. 6D). We also tested whether synthetic MaR1 modulates TRPA1, which is also key in inflammatory pain (32, 33). MaR1, even at high concentrations (10 ng/ml), did not inhibit TRPA1 current induced by mustard oil allyl isothiocyanate (AITC; 300 mM; Fig. 6D).
Intraperitoneal injection of vincristine, a chemotherapy agent, elicited profound mechanical allodynia, a cardinal feature of neuropathic pain, as indicated by a reduction in paw withdrawal threshold following stimulation of von Frey hairs. Vincristine-induced mechanical allodynia was evident after 1 d and maintained after 14 d. Strikingly, MaR1 sharply reduced vincristine-induced mechanical allodynia (Supplemental Fig. S1). Together, these results show that MaR1 selectively inhibits TRPV1 in DRG neurons, as well as TRPV1-induced inflammatory pain and neuropathic pain.
DISCUSSION
Local lipid mediators produced by MΦs that control and enhance the resolution of acute inflammation and organ regeneration are of general interest. Here we report the stereochemical assignment of endogenous MaR1 produced by human MΦs and its potent novel actions. The maresins are a newly identified family of products biosynthesized via a pathway in the DHA metabolome initiated by 14-lipoxygenation of DHA (20). Several novel 7,14S-diHDHA products were identified that possess potent anti-inflammatory and innate immunoresolvent actions. Specifically, the first maresin, 7,14-dihydroxydocosa-7Z,8,14,12,16Z,19Z-hexaenoic acid, is the founding member of this family, and given its potent actions in limiting PMN infiltration and enhancing MΦ phagocytosis, was denoted MaR1. The basic structural elucidation of MaR1 was recently confirmed by others (34). Yet, the absolute stereochemistry of MaR1 remained to be established, a key step in unraveling its actions and biosynthesis. Using a total organic synthesis and matching approach, we established the complete stereochemistry of MaR1, which proved to be 7R,14S-dihydroxydocosa-4Z,8E,10E, 12Z,16Z,19Z-hexaenoic acid. The matching of authentic MΦ-derived with synthetic material also confirmed the defining potent anti-inflammatory (i.e., reducing PMN infiltration) and proresolving (i.e., enhancing MΦ phagocytosis of apoptotic PMNs) actions of MaR1.
MaR1 reduced further PMN infiltration in vivo in murine peritonitis (Fig. 3) and reduced the chemotaxis of isolated peripheral blood neutrophils (not shown), translating the potent bioactions of MaR1 to human leukocytes. MaR1 enhanced efferocytosis of apoptotic human PMNs at 1 nM, which showed it to be more potent than RvD1, a known positive control (27) of efferocytosis. When directly compared to the arachidonate-derived proinflammatory mediator LTB4 (21) at equimolar concentrations, LTB4 did not enhance efferocytosis. Hence, LTB4 proved to be a useful negative control that is also a LOX product for this vital process in resolution. In these actions, MaR1 demonstrated greater potency than either its 7S-isomer I or the double-bond (8E,10E,12E) 12E isomer II, emphasizing the importance of stereochemistry in the actions of endogenously biosynthesized lipid mediators such as MaR1 and their related natural isomers (Fig. 3C). Hence, the maresin family possess this high degree of stereoselectivity in evoking biological responses.
Along with MaR1, we also identified and confirmed the stereochemistry of the double dioxygenation product 7S,14S-diHDHA (isomer IV). Only the 7R,14S-dihydroxy-8E,10E,12Z-containing isomer III coeluted with both human MΦ (Fig. 1) and murine MΦ MaR1 (not shown). This finding, together with earlier results from acidic alcohol trapping, 18O incorporation studies, and LOX-deficient mice (20), suggests that, following insertion of molecular oxygen at carbon 14, a 13,14-epoxide is enzymatically biosynthesized that opens during enzymatic conversion to MaR1. This conversion sets the geometry and configuration of the alcohol as 7R via addition from H2O and the geometry of the conjugated double-bond system to that of the 8E,10E,12Z geometry. In theory, enzymatic conversion of a 13S,14S-epoxide intermediate can then set the triene double-bond configuration to 8E,10E,12Z, which, together with the orientation of the alcohol groups (e.g., 7R,14S), evokes potent action. The matching with synthetic isomers and materials obtained from human MΦs was carried out with endogenous DHA (Fig. 1A) and separately with 14S-HpDHA as precursor (not shown) as previously reported (20). This matching approach with LC-MS/MS was needed because the bioactive MaR1 was formed in only nanogram amounts; the amounts that could be isolated in pure form precluded direct nuclear magnetic resonance determinations for double-bond geometry and chirality of the alcohol groups because this approach requires >100 μg of pure material.
The roles of the MΦs in tissue homeostasis, host response to injury, and resolution of acute inflammation are now widely appreciated (10). MaR1 provides a new link between MΦs and these local processes within tissues. We turned to planaria to address the action of MaR1 in tissue regeneration. These organisms have emerged as a useful system to assess organ and tissue regeneration (28), yet the small-molecule factors that mediate this process remain to be identified. In this regard, MaR1 enhanced tissue regeneration, shortening the time interval required for regeneration. MaR1 was more potent than EPA-derived RvE1, which also stimulated regeneration, demonstrating a degree of SPM selectivity in this process. Of interest, after surgery or injury, planaria also biosynthesized, from deuterium-labeled d5-DHA, MaR1 that carried the d5-deuterium label from the precursor. Both the formation of MaR1 and 14S-HDHA biosynthesis were blocked in planaria with a LOX inhibitor and regeneration that was rescued by add-back of MaR1 (Fig. 5E). These findings point to the likely possibility that local mediators in inflammation resolution and tissue regeneration, such as human MΦ-derived mediator MaR1, may be shared signals in these complex homeostatic processes. Also, the present results with planaria and their ability to biosynthesize MaR1 emphasize the primordial nature of these bioactive structures in that they are phylogenetically highly conserved. Along these lines, E-series and D-series resolvins and related products were recently identified in marine organisms, such as Engraulis, the Peruvian anchovy, a major source of ω-3 essential fatty acids (35), and earlier in neural and hematopoietic organs of the rainbow trout (36). Of interest, these highly conserved structures, including resolvins, prostaglandins, and enzymatically biosynthesized hydroxy-fatty acids, are also present in salmon and are lost with cooking (37).
Resolvins and protectins are also potent regulators of inflammatory pain (17, 18, 38–40). Given the role of MΦs in resolution and in inflammatory pain, our findings that MaR1 regulates TRPV1 currents in a PTX-sensitive fashion is intriguing. In this regard, the action of MaR1 proved to be potent, with an IC50 of 0.49 ± 0.2 nM. Intraplantar injections of MaR1 at 10 ng/injection significantly reduced pain responses induced by capsaicin as well as in the widely used formalin-induced inflammatory pain model (Fig. 6). It is well known that cancer treatments, such as chemotherapy, induce neuropathic pain, the control of which remains to be fully addressed. In the present experiments, MaR1 dramatically reduced vincristine-initiated neuropathic pain (Supplemental Fig. S1). These findings, taken together, suggest that MaR1 serves as a potent analgesic for regulating and controlling local inflammation resolution and associated inflammatory pain, as well as neuropathic pain.
Neural tissues and retina that accumulate DHA store it within membrane phospholipids, where it is released by specific phospholipases for conversion to local mediators (41, 42). In resolving inflammatory exudates, DHA is initially supplied to exudate cells via edema directly from circulating n-3 levels for local utilization at the site of inflammation and production of both D-series resolvins and protectins (43). Results from many clinical and basic studies have implicated DHA in cardiovascular diseases (recently reviewed in ref. 44), and decreased DHA levels correlate with disease in patients with nonresolving inflammation (45). Also, untargeted MS-based metabolomics of human stem cells recently identified another member of the SPM DHA metabolome, neuroprotectin D1/protectin D1 (46), a mediator we elucidated earlier in resolving inflammatory exudates (15, 41). In stem cells, neuroprotectin D1 selectively enhances neuronal differentiation, an action not shared by eicosanoids, such as LTB4 or leukotriene C4 (46). Related to the MaR pathway, a novel 14S,21R-diHDHA was recently identified from DHA, which accelerates wound healing with reepithelialization when administered together with mesenchymal stem cells in diabetic mice (47). Also, 17S-hydroxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid (17-HDoHE; or also known as 17S-HDHA), RvD1, and RvE1 were each identified in human plasma of healthy individuals using synthetic standards (48). Hence, establishing the complete stereochemistry of MaR1 and related isomers will now permit their evaluation in many experimental and in vivo settings. In summation, our present findings that MaR1 demonstrates potent actions (in vivo and in vitro) as well as production by MΦs and planaria provide new pathways in the DHA metabolome that can potentially link organ regenerative responses and wound healing with the resolution of local acute inflammation and pain.
Supplementary Material
Acknowledgments
The authors thank Mary Halm Small for expert assistance in manuscript preparation, Thad W. Vickery for technical assistance, and Dr. Rong Yang for initial LC-MS/MS methods to isolate isomers of MaR1 and related structures. The authors also thank Iris Kao for assistance in the initial planaria experiments.
The work was supported in part by U.S. National Institutes of Health grants 1P01GM095467 (C.N.S., N.A.P.), 1R01DE019938 (C.N.S.), and 1R01NS067686 (R.R.J., C.N.S.). The authors report no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 7S,14S-diHDHA
- 7S,14S-dihydroxydocosa-4Z, 8E,10Z,12E,16Z,19Z-hexaenoic acid
- 14S-HDHA
- 14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid
- 14S-HpDHA
- 14S-hydroperoxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid
- 17S-HDHA
- 17S-hydroxydocosa-4Z, 7Z,10Z,13Z,15E,19Z-hexaenoic acid
- BSA
- bovine serum albumin
- CFDA
- 5-carboxyfluorescein diacetate acetoxymethyl ester
- d5-14-HDHA
- d5-14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid
- DHA
- docosahexaenoic acid
- DRG
- dorsal root ganglion
- ECL
- Erythrina cristagalli lectin
- GPCR
- G-protein-coupled receptor
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LOX
- lipoxygenase
- LTB4
- 5S,12R-dihydroxy-5-cis-9,10-trans,14-cis-eicosatetraenoic acid
- MaR
- maresin
- maresin
- macrophage mediator in resolving inflammation
- MaR1
- maresin 1, 7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid
- MΦ
- macrophage
- MRM
- multireaction monitoring
- PMN
- polymorphonuclear neutrophil
- PTX
- pertussis toxin
- RvD1
- resolvin D1, 7S,8R,17S trihydroxydocosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid
- RvE1
- resolvin E1, 5S,12R,18R-trihydroxyeicosa-6Z,8E,10E,14Z,16E-pentaenoic acid
- tR
- retention time
- SPM
- specialized proresolving mediator
- TRPV1
- transient receptor potential V1.
REFERENCES
- 1. Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 [DOI] [PubMed] [Google Scholar]
- 2. Majno G., Joris I. (2004) Cells, Tissues, and Disease: Principles of General Pathology, 2nd ed Oxford University Press, New York [Google Scholar]
- 3. Serhan C. N. (2007) Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 [DOI] [PubMed] [Google Scholar]
- 4. Serhan C. N. (2010) Novel resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Satiated-efferocytosis generates pro-resolving CD11blow macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bystrom J., Evans I., Newson J., Stables M., Toor I., van Rooijen N., Crawford M., Colville-Nash P., Farrow S., Gilroy D. W. (2008) Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 112, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. (2004) Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 [DOI] [PubMed] [Google Scholar]
- 8. Freire-de-Lima C. G., Xiao Y. Q., Gardai S. J., Bratton D. L., Schiemann W. P., Henson P. M. (2006) Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 281, 38376–38384 [DOI] [PubMed] [Google Scholar]
- 9. Rossi A. G., Sawatzky D. A., eds. (2008) The Resolution of Inflammation, Birkhäuser Verlag, Basel, Switzerland [Google Scholar]
- 10. Russell D. G., Gordon S., eds. (2009) Phagocyte-Pathogen Interactions: Macrophages and the Host Response to Infection, ASM Press, Washington, DC [Google Scholar]
- 11. Zak D. E., Aderem A. (2009) Systems biology of innate immunity. Immunol. Rev. 227, 264–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majno G., Cotran R. S., Kaufman N., eds. (1982) Current Topics in Inflammation and Infection, Williams & Wilkins, Baltimore [Google Scholar]
- 13. Cotran R. S. (1982) The endothelium and inflammation: new insights. In Current Topics in Inflammation and Infection (Majno G., Cotran R. S., Kaufman N., eds) pp 18–37, Williams & Wilkins, Baltimore [Google Scholar]
- 14. Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation-resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R.-L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellmann J., Tang Y., Kosuri M., Bhatnagar A., Spite M. (2011) Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 25, 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lima-Garcia J., Dutra R., da Silva K., Motta E., Campos M., Calixto J. (2011) The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 164, 278–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu Z. Z., Ji R. R. (2011) Resolvins are potent analgesics for arthritic pain. Br. J. Pharmacol. 164, 274–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fredman G., Oh S. F., Ayilavarapu S., Hasturk H., Serhan C. N., Van Dyke T. E. (2011) Impaired phagocytosis in localized aggressive periodontitis: rescue by resolvin E1. PLoS ONE 6, e24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. (2009) Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J. Exp. Med. 206, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samuelsson B., Dahlen S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. (1987) Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 [DOI] [PubMed] [Google Scholar]
- 22. Rowley A. F., Kühn H., Schewe T., eds. (1998) Eicosanoids and Related Compounds in Plants and Animals, Portland Press, London [Google Scholar]
- 23. Yang R., Chiang N., Oh S. F., Serhan C. N. (2011) Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr. Protoc. Immunol. 95, 14.26.11–14.26.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. (2011) Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zayas R. M., Cebrià F., Guo T., Feng J., Newmark P. A. (2010) The use of lectins as markers for differentiated secretory cells in planarians. Dev. Dyn. 239, 2888–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joseph E. K., Levine J. D. (2004) Caspase signalling in neuropathic and inflammatory pain in the rat. Eur. J. Neurosci. 20, 2896–2902 [DOI] [PubMed] [Google Scholar]
- 27. Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.-H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc. Natl. Acad. Sci. U. S. A. 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pellettieri J., Fitzgerald P., Watanabe S., Mancuso J., Green D. R., Alvarado A. S. (2010) Cell death and tissue remodeling in planarian regeneration. Dev. Biol. 338, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 [DOI] [PubMed] [Google Scholar]
- 30. Xu Z.-Z., Zhang L., Liu T., Park J.-Y., Berta T., Yang R., Serhan C. N., Ji R.-R. (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serhan C. N., Krishnamoorthy S., Recchiuti A., Chiang N. (2011) Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 11, 629–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bautista D. M., Jordt S. E., Nikai T., Tsuruda P. R., Read A. J., Poblete J., Yamoah E. N., Basbaum A. I., Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 [DOI] [PubMed] [Google Scholar]
- 33. Dai Y., Wang S., Tominaga M., Yamamoto S., Fukuoka T., Higashi T., Kobayashi K., Obata K., Yamanaka H., Noguchi K. (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Invest. 117, 1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sasaki K., Urabe D., Arai H., Arita M., Inoue M. (2011) Total synthesis and bioactivities of two proposed structures of maresin. Chem. Asian J. 6, 534–543 [DOI] [PubMed] [Google Scholar]
- 35. Oh S. F., Vickery T. W., Serhan C. N. (2011) Chiral lipidomics of E-series resolvins: aspirin and the biosynthesis of novel mediators. Biochim. Biophys. Acta 1811, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong S., Tjonahen E., Morgan E. L., Yu L., Serhan C. N., Rowley A. F. (2005) Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins—mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 78, 107–116 [DOI] [PubMed] [Google Scholar]
- 37. Raatz S. K., Golovko M. Y., Brose S. A., Rosenberger T. A., Burr G. S., Wolters W. R., Picklo M. J., Sr. (2011) Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar). J. Agric. Food Chem. 59, 11278–11286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang L., Wang C.-F., Serhan C. N., Strichartz G. (2011) Enduring prevention and transient reduction of post-operative pain by intrathecal Resolvin D1. Pain 152, 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price S. (2010) Pain: resolvins show promise for treating inflammatory pain. Nat. Rev. Rheumatol. 6, 379. [DOI] [PubMed] [Google Scholar]
- 40. Bang S., Yoo S., Yang T. J., Cho H., Kim Y. G., Hwang S. W. (2010) Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 161, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bazan N. G. (2006) Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 29, 263–271 [DOI] [PubMed] [Google Scholar]
- 42. Salem N., Jr., Litman B., Kim H.-Y., Gawrisch K. (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36, 945–959 [DOI] [PubMed] [Google Scholar]
- 43. Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Caterina R. (2011) n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 364, 2439–2450 [DOI] [PubMed] [Google Scholar]
- 45. Freedman S. D., Blanco P. G., Zaman M. M., Shea J. C., Ollero M., Hopper I. K., Weed D. A., Gelrud A., Regan M. M., Laposata M., Alvarez J. G., O'Sullivan B. P. (2004) Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 350, 560–569 [DOI] [PubMed] [Google Scholar]
- 46. Yanes O., Clark J., Wong D. M., Patti G. G., Sánchez-Ruiz A., Benton H. P., Trauger S. A., Desponts C., Ding S., Siuzdak G. (2010) Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 6, 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian H., Lu Y., Shah S. P., Hong S. (2011) 14S,21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J. Biol. Chem. 286, 4443–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Psychogios N., Hau D. D., Peng J., Guo A. C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., Young N., Xia J., Knox C., Dong E., Huang P., Hollander Z., Pedersen T. L., Smith S. R., Bamforth F., Greiner R., McManus B., Newman J. W., Goodfriend T., Wishart D. S. (2011) The human serum metabolome. PLoS ONE 6, e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.