Abstract
Estrogen receptors (ERs) are expressed in leukocytes and in every ocular tissue. However, sex-specific differences and the role of estradiol in ocular inflammatory-reparative responses are not well understood. We found that female mice exhibited delayed corneal epithelial wound closure and attenuated polymorphonuclear (PMN) leukocyte responses, a phenotype recapitulated by estradiol treatment both in vivo (topically in male mice) and in vitro (corneal epithelial cell wound healing). The cornea expresses 15-lipoxygenase (15-LOX) and receptors for lipoxin A4 (LXA4), which have been implicated in an intrinsic lipid circuit that regulates corneal inflammation and wound healing. Delayed epithelial wound healing correlated with lower expression of 15-LOX in the regenerated epithelium of female mice. Estradiol in vitro and in vivo down-regulated epithelial 15-LOX expression and LXA4 formation, while estradiol abrogation of epithelial wound healing was completely reversed by treatment with LXA4. More important, ERβ and ERα selectively regulated epithelial wound healing, PMN cell recruitment, and activity of the intrinsic 15-LOX/LXA4 circuit. Our results demonstrate for the first time a sex-specific difference in the corneal reparative response, which is mediated by ERβ and ERα selective regulation of the epithelial and PMN 15-LOX/LXA4 circuit. These findings may provide novel insights into the etiology of sex-specific ocular inflammatory diseases.—Wang, S. B., Hu, K. M., Seamon, K. J., Mani, V., Chen, Y., Gronert, K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea.
Keywords: 17β-estradiol, lipoxin A4, ERβ, ERα, 15-LOX, sex-specific
The role of sex steroids, especially estrogen, in inflammation is complex and highly tissue-specific for a given inflammatory or immune response. Estrogen has well documented pro- and anti-inflammatory actions yet lacks general unifying bioactions in the pathogenesis of inflammatory diseases (1–4). Several animal models have shown clearly that estrogen inhibits both acute and chronic inflammation and accelerates dermal wound healing (1, 3, 5). In sharp contrast to these reparative anti-inflammatory functions, estrogen promotes B-lymphocyte-driven immune responses, is linked to the pathogenesis of autoimmune diseases, and exhibits proinflammatory actions in prostatitis (2–4). Receptors for estrogen are expressed in leukocytes and every major tissue in the eye (6), which is one of the few organs where estrogen has reported proinflammatory activities. An epidemiological analysis of 39,878 female health professionals demonstrated that estrogen replacement therapy is associated with a significant increase in dry eye symptoms (7, 8), a finding that has important implications, as dry eye syndrome primarily affects women. The etiology for this highly sex-specific inflammatory and immune disease is unknown, but key features are chronic inflammation, irritation, and epithelial injury (9–11).
Several lines of evidence indicate that a relative deficiency in androgens in menopausal women correlates with initiation of autoimmune responses (10–12). However, the role of androgen in ocular surface diseases and inflammatory-reparative responses is far from clear, despite an impressive body of work. The role of estrogen in ocular surface diseases and inflammatory-reparative responses has been largely ignored, despite the facts that its receptors are expressed in every ocular tissue and most leukocytes and that estrogen induces proinflammatory gene expression in corneal epithelial cells and meibomian glands (13–16). Pronounced changes in estrogen levels during pregnancy, menopause, and hormone replacement therapy have been linked to striking effects on inflammatory and immune response in nonocular tissues. Furthermore, animal and in vitro studies place estrogen and its nuclear receptors [estrogen receptor α and β (ERα and ERβ)] as key and selective regulators of wound-healing and immune responses in these tissues (1–4).These studies provide a compelling argument and rationale for an important role of estrogen in ocular surface inflammation and wound healing. Dry eye animal studies are performed exclusively in females due to disease incidence in humans; however, a striking gap of knowledge remains regarding even basic sex-specific differences in inflammatory and wound-healing responses in the eye.
A key feature of essential frequent inflammatory and wound-healing responses is that they are acute and self-resolving by design. It is now recognized that dysregulation of inflammatory resolution is an early and critical event that leads to chronic inflammation and diseases (17–20). Among the earliest inflammatory and immune regulators that are released in response to injury, infection, or stress are lipid mediators, such as eicosanoids. Specific eicosanoid circuits, such as the lipoxin A4 (LXA4) and 15-lipoxygenase (15-LOX) circuit, have emerged as key mediators of inflammatory resolution and anti-inflammation (18, 21). The protective actions of LXA4 are mediated by G-protein coupled receptors in both humans (ALX) and mice (ALX1, ALX2). Elegant studies (22–28) have demonstrated that LXA4 is formed endogenously, regulates adaptive and innate immune responses and pain, and drives inflammatory resolution. We recently reported that the cornea expresses a unique 15-LOX/LXA4 circuit that has essential roles in inflammatory responses of the eye and is critical for endowing the cornea with an amplified anti-inflammatory tone to ensure privileged injury responses (21, 29–32). LXA4 is formed via the interaction of 5-LOX and 15-LOX, which are both expressed in corneal epithelial cells. This pathway is significantly amplified by the recruitment of specific polymorphonuclear (PMN) leukocytes and macrophage populations that carry 5-LOX and/or 15-LOX, which sets in motion a temporally defined counterregulatory program that drives inflammatory resolution. A striking feature in both mouse and human corneas is the high epithelial expression of 15-LOX (21, 33) and the expression of the ALX receptors (29, 31, 33, 34). Acute and chronic inflammation selectively regulates expression of 15-LOX and ALX receptors. Genetic deletion of the LXA4 biosynthetic pathway (15-LOX and 5-LOX) leads to a phenotype of delayed epithelial wound healing, impaired induction of cytoprotective genes, and amplified chronic inflammation, which can be rescued by adding back topical LXA4. The relevance of intrinsic protective lipid circuits in ocular health is underscored by recent reports that demonstrate that 5-LOX, 15-LOX, and/or LXA4 have key roles in pathological angiogenesis (29), uveitis (35), retinopathy (36, 37), and protection of retinal pigmented epithelial cell against oxidative stress (37, 38).
We set out to assess whether acute inflammatory-reparative responses are regulated by estrogen in the cornea and whether sex-specific differences in this fundamental response involve regulation of the intrinsic 15-LOX/LXA4 circuit. Our findings demonstrate for the first time a female-specific phenotype of delayed corneal epithelial wound healing that can be induced by topical treatment with estradiol. More importantly, sex-specific differences and the estradiol-mediated phenotype of delayed wound healing and attenuated PMN cell response are paralleled by concomitant and ER-specific inhibition of 15-LOX expression and LXA4 formation, while LXA4 abrogates the epithelial actions of estradiol. In view of the intrinsic role of the 15-LOX/LXA4 circuit in self-resolving inflammation and wound healing, these results have potentially important implications for estrogen's role in regulating routine ocular inflammatory-reparative responses.
MATERIALS AND METHODS
Animal experiments
All animal studies have been approved by the University of California, Berkeley, in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Age-matched (6- to 10-wk-old) Balb/c female and male mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained on a 12-h light-dark cycle and fed a standard diet ad libitum (rat/mouse diet LM-485; Harlan Tekland, Madison, WI, USA).
Corneal epithelial abrasion
Mice were anesthetized with ketamine (50 mg/kg) and xylazine (20mg/kg) intraperitoneally. A drop of proparacaine hydrochloride ophthalmic solution (0.5%) was applied to the eye to deliver local corneal anesthesia before injury. Epithelial abrasion was achieved using an Algerbrush II with a 0.5-mm corneal rust ring remover (Alger Co., Inc., Lago Vista, TX, USA), as described previously (30, 31, 39). The corneal epithelium was mechanically removed up to the limbal border under a dissection microscope, and full removal was verified using fluorescein stain as a direct marker of epithelial defect. A 2- to 3-epithelial cell layer (100% wound healing) was regenerated within 5 d postinjury in both males and females (31). Reinjury was initiated by complete reabrasion of the regenerated epithelium at 7 d after initial abrasion. Epithelial defect was visualized with fluorescein. Wound area was quantified using imaging software (ImagePro Express 6.0; Media Cybernetics, Inc., Bethesda, MD, USA) as described previously (30, 31, 39).
Topical treatments
17β-Estradiol (Sigma Aldrich, St. Louis, MO, USA), ERα-specific agonist propylpyrazole triol (PPT; Cayman Chemical, Ann Arbor, MI, USA), and ERβ-specific agonist diarylpropiolnitrile (DPN; Cayman Chemical) were used in topical applications to the eye. Each compound was dissolved in ethanol and diluted to treatment concentrations of 1 μM in sterile HBSS (<0.1% ethanol). The topical doses were selected based on published human clinical trials with 17β-estradiol eye drops in menopausal women with dry eye syndrome (40). Solution (5 μl) was administered topically to each eye ter in die (t.i.d.) 24 h prior to abrasion and subsequent to injury up to 5 d after reinjury. The control group received 5 μl of sterile HBSS containing <0.1% ethanol.
Sample isolation
Mice were killed at 2 or 7 d after injury and 2 or 5 d after reinjury. Corneas were removed using a stainless steel surgical blade. All noncorneal tissue was cleaned from the cornea in sterile phosphate-buffered saline (on ice) under a dissecting microscope with sterile instruments. To isolate the epithelial sheet, cleaned corneas were incubated in 2% ethylenediaminetetraacetic acid (EDTA) for 30 min at 37°C. The epithelium was then lifted from the cornea using sterile instruments.
Histological sections
Whole eyes were removed and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). The samples were then allowed to set at −80°C for ≥2 h before being sectioned lengthwise into 5-μm-thick slices (29, 41). Hematoxylin and eosin (H&E) was used as a physical stain to distinguish cell types.
Assessment of inflammation
Myeloperoxidase (MPO) activity was used as an index of tissue leukocyte infiltration (29–31). Corneas (1 cornea/data point) were homogenized mechanically in a solution of 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, followed by 3 cycles of freeze-thaw and a 10-s sonication. The homogenates were then centrifuged, and supernatants were collected. MPO activity was measured by spectrophotometry using o-dianisidine dihydrochloride reduction as a colorimetric indicator. MPO activity was converted into total PMN cell number using calibration curves established from PMN cells collected from zymosan A-induced peritonitis exudates in Balb/c mice.
Cell culture
Immortalized human corneal epithelial (HCE) cells were a kind gift from Haydee Bazan (Louisiana State University Health Science Center, New Orleans, LA, USA) and maintained in serum-free keratinocyte growth medium (KGM; Lonza Walkersville Inc., Walkersville, MD, USA) supplemented with the following growth factors and antibiotics: bovine pituitary extract, human epidermal growth factor (hEGF), insulin (bovine), hydrocortisone, GA-1000 (gentamicin, amphotericin-B), epinephrine, and transferrin. For treatment, medium was removed from confluent HCE cells and replaced with fresh KGM without growth factors. After 24 h, HCE cells were treated with 1 pM of 17β-estradiol, PPT, or DPN in medium for a period of 24 h. A concentration range of 1–100 pM of estradiol induces physiological responses in primary and immortalized HCE cells in culture (14). Cells were harvested rapidly by mechanical scraping and collected by centrifugation at 4°C.
In vitro scratch-wound assay
HCE cells were cultured on 35- × 10-mm tissue culture dishes and allowed to grow to 80% confluence. After an incubation period of 24 h in growth factor-free KGM, cells were wounded by making one horizontal scratch with a sterile 200-μl pipette tip. To ensure that the same areas were compared between time points, another scratch directly perpendicular to and bisecting the first mark was made. The cells were then washed (to remove detached cells) and incubated in fresh growth factor-free KGM (with and without 1 pM estradiol). Images of HCE monolayers were taken using a Zeiss confocal microscope (Carl Zeiss, Oberkochen, Germany), and wound area was quantified using ImagePro Express 6.0.
Gene expression
mRNA from corneas, corneal epithelium, and cultured HCE cells was isolated using a RNA Easy Mini Kit (Qiagen Sciences, Germantown, MD, USA) and quantified via spectrophotometry. The mRNA was then reverse transcribed using a High-Capacity cDNA Kit (Applied Biosystems, Foster City, CA, USA), and real-time PCR was performed using SYBR Green Master Mix (Applied Biosystems) with a Step One Plus qPCR system (Applied Biosystems) as described previously (29, 41). Primer pairs used are listed in Supplemental Table S1. All were selected from the Harvard Primer Bank (Boston, MA, USA) and verified by the NIH GenBank database. β-Actin was used as a reference gene. mRNA quantities were expressed relative to universal mouse reference RNA that was generated from mRNA obtained from pooled Balb/c kidney and spleen. Universal human reference mRNA was purchased from Stratagene (La Jolla, CA, USA) and amplified according to specifications. The ΔΔCT method was used. Amplifications were run in duplicate, and efficiency curves for all primers were established.
Lipid mediator lipidomics
For lipid autacoid analysis (29, 36, 41), injured HCE monolayers or isolated mouse corneal stroma were incubated with 2 μM calcium ionophore (A23187; Fisher Bioreagents, Fair Lawn, NJ, USA) in KGM without growth factors for 30 min (37°C). The reaction was then stopped by the addition of 66% methanol containing deuterated internal standards, prostaglandin (PG)E2-d4, 15(S)-HETE-d8, and leukotriene B4 (LTB4)-d4 (400 pg/each), to calculate the recovery of prostanoids or monohydroxy- and dihydroxy-containing fatty acids. Lipid autacoids were extracted by solid phase using Accubond ODS-C18 cartridges (Agilent Technologies, Santa Clara, CA, USA). Eicosanoids were identified and quantified by liquid chromatography (LC)/mass spectrometry (MS)/MS-based lipidomics. In brief, extracted samples were analyzed by a triple-quadrupole linear ion trap LC/MS/MS system (MDS SCIEX 3200 QTRAP; Applied Biosystems) equipped with a Kinetex C18 minibore column (Phenomenex, Torrance, CA, USA) using a mobile phase gradient of water/acetonitrile/acetic acid (72:28:0.01, v/v/v) and isopropanol/acetonitrile (60:40, v/v) with a 450 μl/min flow rate. MS/MS analyses were performed in negative ion mode, and prominent fatty acid metabolites were quantified by multiple reaction monitoring (MRM mode) using established transitions for LXA4 (351→115 m/z; refs. 2, 4–6). Calibration curves (1–1000 pg) and specific LC retention times for LXA4 were established with synthetic standards (Cayman Chemical).
Statistics
One-tailed, unpaired Student's t test was used to evaluate the significance of differences between 2 groups. One-way ANOVA was performed on groups of 3 or greater using GraphPad InStat software (GraphPad, San Diego, CA, USA). One-tailed Fisher's exact test (a variation of a χ2 test) was used to evaluate the significance of differences between groups in wound closure. Values of P < 0.05 were considered significant. All data reported as means ± se unless otherwise indicated.
RESULTS
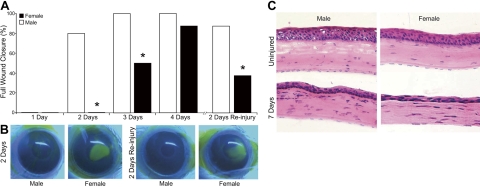
To determine whether sex-specific differences are inherent in the corneal reparative response during an acute and self-resolving epithelial injury, age-matched female and male Balb/c mice were wounded by complete mechanical removal of the corneal epithelium up to the limbus border. Corneas of male mice consistently healed faster than corneas of female mice (Fig. 1A, B). At 2 and 3 d after epithelial injury, 80 and 100% of the males (n=5) had achieved full epithelial wound healing, compared to 0% at d 2 and 50% at d 3 for females (n=5, P=0.001). By d 7, both male and female corneas were 100% reepithelialized. Histology (Fig. 1C) demonstrated that the normal corneal epithelial layer in the uninjured healthy cornea of males and females contains 5–7 cell layers, while this normal epithelium is only partially restored at 7 d postinjury (2–3 cell layers). More important, the stroma of healed corneas in both male and female mice contained significant numbers of remnant leukocytes from the acute inflammatory response. A pattern of increased H&E straining in the cornea at 7 d postinjury potentially indicates more infiltrating cells in males than in females. The most abundant infiltrating cells in the injured cornea are PMN cells. Quantification by MPO assay (Fig. 2) clearly indicates that there are no sex-specific differences in corneal PMN cell content at 7 d postinjury. The identity of all H&E-staining cells in the cornea cannot be determined by H&E staining and cell morphology alone. Cells in the tissue section may include dendritic cells, γδ T cells, and macrophages, and their potential role in the sex-specific difference in epithelial wound healing are of interest but remain to be defined.
Figure 1.
Corneal epithelial wound healing is delayed in female mice. Corneas of age-matched Balb/c male and female mice were injured by complete epithelial abrasion. In the reinjury group, the regenerated epithelium was removed at 7 d after initial epithelial injury. Wound healing was quantified via flourescein stain at 1–4 d after initial injury and 2 d after reinjury. A) Wound healing is expressed as percentage of corneas with complete epithelial wound closure. Significant differences were assessed by Fisher's exact test (n=5). *P < 0.05 vs. males. B) Representative images of male and female fluorescein-stained eyes at 2 d after initial injury and 2 d after reinjury. C) Representative H&E images of 5-μm cross sections of uninjured corneas and healed corneas at 7 d after injury.
Figure 2.
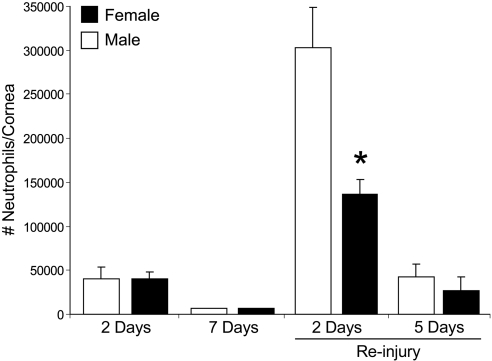
Attenuated PMN cell response to recurrent epithelial injury in females. Corneas from male and female mice were harvested at indicated time points postinjury, and tissue MPO activity was quantified (n=5–8). MPO activity is expressed as PMN cells/cornea based on calibration curves that were established with isolated peritoneal PMN cells. *P < 0.05 vs. males.
A key feature of corneal inflammatory diseases such as dry eye syndrome is recurrent epithelial injury. To assess whether sex-specific differences in wound healing persist during recurrent epithelial injury, the regenerated corneal epithelium was reabraded at 7 d postinjury. Despite reinjury of the healing epithelium and inflamed cornea, the second injury induced a rapid and accelerated wound-healing response, with 88% of male mice achieving full epithelial wound closure by d 2. In sharp contrast, only 48% of females achieved full epithelial wound closure at 2 d after reinjury (P=0.026). These findings provide the first evidence for a sex-specific difference in the rate of epithelial wound healing in corneas of female mice.
Inflammation is intimately linked to wound healing. In the corneal reparative response to acute epithelial abrasion injury, PMN cell infiltration is an essential and beneficial factor that drives corneal reepithelialization (31, 42–44). Hence, we next assessed whether sex-specific differences in wound healing correlate with a difference in levels of recruited PMN cells in male and female corneas. Despite a marked difference in epithelial wound closure at 2 d postinjury, corneal PMN cell content did not differ in injured corneas of males and females, nor was PMN cell resolution delayed in the reepithelialized cornea at d 7 (Fig. 2). However, epithelial reinjury induced markedly amplified recruitment of PMN cells to the cornea at 2 d after a second abrasion when directly compared to the initial abrasion injury. Specifically, a second epithelial abrasion induced >7.6-fold increase in the male PMN cell response (302,800±46,284 cells/cornea) than was observed with the initial 2-d epithelial injury (39,920±13,541 cells/cornea). Amplified inflammation in response to a recurrent epithelial injury also revealed a marked sex-specific difference, as females had an average of 166,443 fewer PMNs per cornea (55% lower response) when directly compared to age-matched males (Fig. 2). This finding correlates with a significant delay in full wound closure in females (36% females with full wound closure vs. 88% males with full wound closure, Fig. 1A). These results provide strong evidence for a specific intrinsic difference in the corneal inflammatory-reparative response of male and female mice.
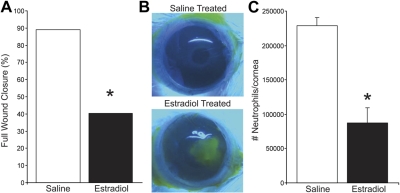
Estrogen has been shown to regulate and promote cutaneous wound healing in rodent models (5, 45), which may explain reported differences in healing abilities between sexes and between postmenopausal and premenopausal females (1, 2, 4). The role of estrogen in ocular reparative responses is unknown, even though estrogen is present in the tear film (46) and its biosynthetic enzyme (aromatase) and receptors (ERα and ERβ) are expressed in the cornea (refs. 47, 48; see Fig. 5A). To assess the in vivo action of estrogen on epithelial wound healing, we treated male mice topically with 17β-estradiol throughout the wound-healing response. The action of estradiol on the inflammatory-reparative response was assessed at 2 d after reinjury, as this time point exhibits marked sex-specific differences in both epithelial wound healing and the PMN cell response to injury. Treatment of males with estradiol recapitulated the female phenotype of delayed corneal reepithelialization and attenuated PMN cell recruitment (Fig. 3). Specifically, topical estradiol reduced the number of mice that achieved complete reepithelialization by 56% (Fig. 3A, B), which correlated with a 71% reduction (Fig. 3C) in the amplified PMN cell infiltration that is a consequence of recurrent epithelial injury.
Figure 5.
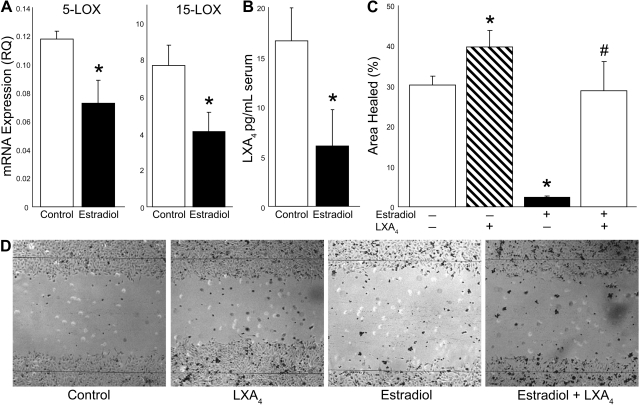
Estradiol regulates epithelial wound healing and LXA4 formation. A) HCE monolayers were treated with estradiol (1 pM) for 24 h. Cells were harvested, and 5-LOX and 15-LOX mRNA expression was quantified by qPCR (n=6). B) Epithelial LXA4 formation. HCE cells were treated with estradiol (1 pM) or pure medium for 24 h, cells were activated with calcium ionophore for 15 min, and medium was collected for LC/MS/MS quantification of endogenous LXA4 formation (n=6). C) In vitro epithelial wound healing. Confluent cells were incubated with or without LXA4 (1 nM) in the presence or absence of estradiol (1 pM) for 24 h prior to scratch wounding. Wound healing was assessed at 8 h postinjury and is expressed as percentage area healed (n=5). D) Representative image of epithelium at 8 h after scratch injury. Lines indicate initial wound area. *P < 0.05 vs. control; #P < 0.05 vs. estradiol treatment.
Figure 3.
Estradiol treatment induces a female-specific wound-healing and PMN cell response in male mice. Eyes of male mice were treated topically with 17β-estradiol (1 μM, 5 μl drop/eye, t.i.d.) or saline alone from d 0 to 9, and full wound closure was quantified at 2 d after epithelial reinjury. A) Percentage of eyes in each group with full epithelial wound closure (Fisher's exact test, n=5). B) Representative fluorescein-stained corneas at 2 d after reinjury. C) Estradiol regulation of the amplified PMN response to reinjury. Corneas were harvested at 2 d after reinjury, and MPO activity was quantified. MPO activity is expressed as number of PMN cells/cornea (n=5–8). *P < 0.05 vs. saline group.
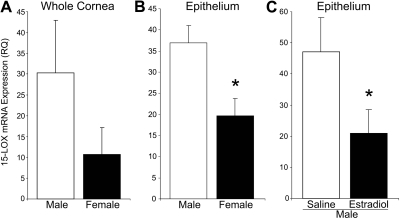
Corneal epithelial cells have a critical function in defending the ocular surface while also expressing high levels of 15-LOX, a key enzyme for the formation of LXA4 and regulator of epithelial wound healing in the cornea (31, 49, 50). Hence, we next assessed whether 15-LOX (Alox15) expression in male and female corneas exhibits sex-specific differences 2 d after epithelial reinjury. The average mRNA level of 15-LOX was lower in the cornea of females (Fig. 4A). However, this difference did not reach statistical significance, which is likely due to the presence of distinct 15-LOX-expressing leukocyte populations in the stroma of the healing cornea. To focus on the sex-specific differences in epithelial 15-LOX expression, we isolated corneal epithelial sheets 2 d after reinjury from both male and female mice. qPCR analysis demonstrated a significant 47% lower expression of 15-LOX in the regenerated epithelium of females when directly compared to males (Fig. 4B). Topical treatment of male eyes with estradiol throughout the wound-healing response reduced the expression of 15-LOX in the regenerated male epithelium to levels measured in the epithelium of females. These findings provide the first evidence for sex-specific differences in the expression of corneal epithelial 15-LOX and for the ability of estrogens to down-regulate intrinsic epithelial 15-LOX expression.
Figure 4.
Regenerated corneal epithelium has sex-specific 15-LOX expression that is induced by estradiol. A) Whole corneas were isolated from male and female mice at 2 d after reinjury, and 15-LOX mRNA expression was quantified by qPCR (P=0.14, n=4). B) Corneal epithelial sheets were isolated from corneas at 2 d after reinjury, and 15-LOX mRNA expression was quantified by qPCR in male and female mice (n=3–4). C) Male mice were treated topically with saline alone or 17β-estradiol (1 μM, t.i.d., 5 μl drop/eye) from d 0 to 9, epithelial sheets were isolated at 2 d after reinjury, and 15-LOX expression was quantified by qPCR (n=3–4). *P < 0.05.
To assess the direct action of estrogen on epithelial wound healing and functional expression of the LXA4 circuit, we used HCE cells in an established in vitro scratch-injury model. Uninjured confluent human epithelial monolayers, like mouse corneal epithelial cells (31), functionally express both 5-LOX and 15-LOX, as evidenced by mRNA expression (Fig. 5A) and generate LXA4 (16.7±3.3 pg/ml) following calcium ionophore activation (Fig. 5B). Activation of epithelial cells with a low concentration of estradiol (1 pM) significantly reduced expression of 15-LOX and 5-LOX by 47 and 38% (Fig. 5A), respectively, after 24 h. More importantly, estradiol inhibited endogenous LXA4 formation by ionophore-activated epithelial cells by 64% (Fig. 5B). A defined scratch injury to the epithelial monolayer results in rapid reepithelialization of the denuded area. At 8 h after injury, 30.4 ± 2.1% of the wound area was reepithelialized (Fig. 5C, D). Activation of epithelial cells with a low concentration of LXA4 (1 nM) at 24 h prior to the epithelial injury significantly increased epithelial wound healing by 31%, which is consistent with our previous findings that topical LXA4 in mice increases the rate of epithelial wound healing in injured corneas at 24 h and 48 h after injury (31). In sharp contrast, treatment with estradiol (1 pM) at 24 h prior to injury reduced the normal rate of epithelial wound healing by 93%, to 2.4 ± 0.3% (Fig. 5C, D). LXA4 could rescue epithelial cells from the estradiol effect, as the wound-healing rate of epithelial monolayers treated with a combined dose of estradiol and LXA4 at 24 h prior to injury was not significantly different from untreated cells (Fig. 5C, D). These results indicate that estradiol down-regulates biosynthetic pathways for LXA4 formation in epithelial cells and that this inhibition directly correlates with impaired epithelial wound healing.
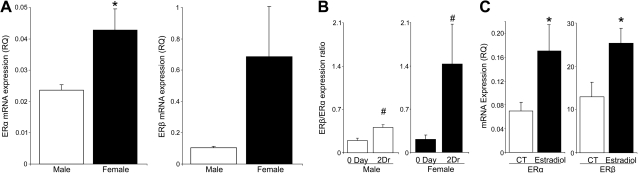
The bioactions of estradiol are primarily mediated by two nuclear hormone receptors, namely ERα and ERβ. Both of these receptors are expressed in immortalized HCE cell lines (Fig. 6C) and mouse and human corneas (15, 47, 51). qPCR analysis of gene expression in freshly isolated corneal epithelial sheets demonstrated that murine expression of both ERα and ERβ is markedly higher in females than in males (Fig. 6A). Based on relative RNA expression, ERα is the most abundant ER in the corneal epithelium; its RNA expression levels are ∼5 fold higher than ERβ in both sexes. The wound-healing response in female epithelium markedly upregulated the expression ratio of ERβ:ERα from 0.21 ± 0.07 to 1.43 ± 0.65 at 2 d after reinjury (Fig. 6B). Hence, corneal injury in females results in sex-specific up-regulation of epithelial ERβ, which is of interest as each ER regulates distinct but yet to be defined gene cassettes (3, 52). Estradiol (1 pM) significantly up-regulates both ERα and ERβ expression (Fig. 6C) in HCE cells within 24 h, suggesting a feed-forward loop for the action of estradiol in these cells.
Figure 6.
Corneal inflammatory-reparative response regulates epithelial ERα and ERβ expression. A) Corneas from uninjured male and female mice were removed, and epithelial sheets were isolated for qPCR analysis of ERα and ERβ mRNA expression (n=5). B) Expression ratio of ERβ to ERα. Epithelial sheets were collected from uninjured corneas and corneas at 2 d after reinjury, and relative expression of ERβ:ERα was assessed by qPCR (n=5). C) Estradiol regulation of ERα and ERβ. HCE cells were treated with estradiol (1 pM) or medium control (CT) for 24 h, and expression of ERα and ERβ was quantified by qPCR (n=5). *P < 0.05 vs. males or control; #P<0.05 vs. uninjured group.
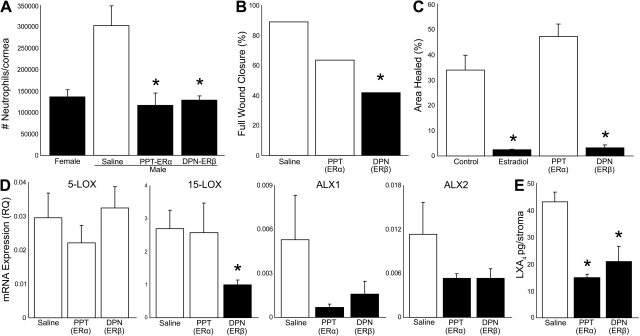
To assess the in vivo action of ERα and ERβ, we treated male mouse eyes topically with selective and established receptor agonists (1 μM, 5 μl drop, t.i.d.) throughout the duration of the inflammatory-reparative response. The therapeutic dose was selected based on a clinical trial in menopausal women that used an ophthalmic topical dose of 1 μM estradiol (40). DPN was used as an ERβ-specific agonist, which has a 70-fold higher binding affinity for ERβ, and PPT was used as an ERα-specific agonist, which has a 410-fold higher binding affinity for ERα. Topical treatment with both DPN and PPT reduced PMN content at 2 d after reinjury by 58–62% in male mice, effectively inducing a female PMN response to recurrent epithelial injury (Fig. 7A). Reduced PMN infiltration correlated with a >50% reduction in the rate of full wound healing in the ERβ treatment group (Fig. 7B). Even though a trend was found, the ERα agonist PPT did not significantly delay full reepthelialization of the corneal wound, which suggests that the epithelial and leukocyte-targeted actions of estrogen are receptor specific.
Figure 7.
ER-specific regulation of the PMN cell response, wound healing, and 15-LOX/LXA4 circuit. A) ER regulation of PMN cell recruitment. Male mice were treated topically with PPT, DPN, or saline alone (1 μM, 5 μl t.i.d., d 0–9) and were compared to females. Corneal PMN cell content at 2 d after reinjury was measured by quantifying MPO activity (n=4). B) ERβ regulation of epithelial wound healing. Wound closure was assessed at 2 d after reinjury in males treated topically with PPT, DPN, or saline alone (n=4). C) ERβ regulation of epithelial wound healing in vitro. HCE cells were incubated with 1 pM estradiol, PPT, or DPN 24 h prior to scratch wounding. Wound area was quantified by image analysis at 8 h postinjury. (n=4–5). D) Expression of the LXA4 circuit. Male mice were treated topically with saline, PPT, or DPN, and mRNA expression of 15-LOX, 5-LOX, and LXA4 receptors (ALX1, ALX2) was quantified by qPCR in the isolated epithelial sheets (n=5). E) ER regulation of LXA4 in the leukocyte-rich stroma. Corneas were isolated from saline-, PPT-, or DPN-treated male mice at 2 d after reinjury; the corneal epithelium was removed, and the leukocyte-rich stroma was activated with calcium ionophore. Released LXA4 in the medium was quantified by LC/MS/MS (n=5). *P < 0.05 vs. saline or control.
To assess direct epithelial action of the ER agonists, we employed the in vitro epithelial injury model. Consistent with experiments in Fig. 3, estradiol abrogated reepithelialization of denuded area (94% inhibition, Fig. 7C). The epithelial action of estradiol was selectively mediated by ERβ, as DPN reduced wound healing from 33 ± 6 to 3 ± 1%. In contrast, the ERα selective agonist PPT did not affect the epithelial wound-healing rate (Fig. 7C). Next, we assessed the selective in vivo action of ERα and ERβ on the intrinsic expression of the 15-LOX/ALX circuit in the regenerated corneal epithelium. Eyes were treated topically with DPN, PPT or saline throughout the initial wound-healing response (d 0–7) and the recurrent epithelial injury (d 7–9). Expression of 5-LOX in the regenerated epithelial sheets was not affected by treatment with either ER agonist. In contrast, ERβ activation during the in vivo reparative response reduced 15-LOX expression in the regenerated epithelium by 63%, while the ERα agonist PPT did not change epithelial 15-LOX expression. This finding indicates that estradiol's ability to markedly reduce epithelial 15-LOX expression (Figs. 4B and 5B) and endogenous LXA4 formation (Fig. 5C) is selectively mediated by the epithelial ERβ receptor. The expression levels of the two mouse LXA4 receptors, ALX1 and ALX2, showed trends for reduced expression following topical treatment with either ERα or ERβ agonist, but this difference did not reach significance (ANOVA, P>0.05, n=5) due to variability in the saline treatment group (Fig. 7D). Functional expression of the LXA4 circuit in the cornea depends on LXA4 formation, which is driven by both epithelial cells and leukocytes during inflammatory responses. Hence, we next assessed whether the leukocyte-rich stroma 2 d after epithelial reinjury could generate LXA4 in the absence of epithelial cells and whether ERα and/or ERβ receptors regulate leukocyte-dependent LXA4 formation. On activation with calcium ionophore, the isolated leukocyte-rich stroma generated significant levels of LXA4 (43±4 pg/cornea, n=4). More importantly, endogenous LXA4 formation was significantly impaired in mice treated with either the ERα (PPT, 65% inhibition, 15±1 pg/cornea) or ERβ (DPN, 52% inhibition, 21±6 pg/cornea) selective agonist.
DISCUSSION
To the best of our knowledge, this study provides the first findings demonstrating a sex-specific difference in ocular reparative-inflammatory responses. The clinical relevance of our findings in mice is underscored by results from a post hoc analysis of a trial of 120 patients that received treatment for fungal corneal ulcers (53). The analysis demonstrated sex-specific differences in the clinical outcome of corneal ulcers. Specifically, reepithelialization of corneal wounds was significantly delayed in female patients, suggesting worse prognoses for ocular surface wound healing in women. Even though sex-specific prevalence and incidence of ocular diseases are carefully tracked, no studies have reported sex-specific differences in ocular surface wound healing or the clinical outcome of ocular inflammatory or immune events. Ocular diseases are not triggered by single events but likely by the recurrent dysregulation of intrinsic circuits essential for executing routine tissue maintenance, wound-healing, and innate or adaptive immune responses. Sex-specific differences in the dynamic execution of routine epithelial wound healing in the cornea identifies a potential factor in the etiology of ocular surfaces diseases in females, as recurrent epithelial injury is a hallmark of dry eye syndrome. Wound healing and inflammation are fundamental and intimately linked responses. Hence, delays in wound healing may contribute to shifting healthy ocular inflammation during the reparative response to disease causing inflammation and adaptive immune responses if epithelial injury is recurrent or in the context of other stress factors, such as deficient tear film, infection, or hypoxia.
Our results demonstrate that recurrent epithelial injury, which resulted in greatly amplified PMN cell infiltration into the stroma, correlated with accelerated reepithelialization. Furthermore, the magnitude of the increased PMN cell response to recurrent epithelial injury was markedly reduced in female mice (>50%) compared to males, which correlated with a significant delay in wound closure in females. These findings seem counterintuitive based on epidermal wound-healing studies that indicate that PMN cells in general impair wound healing. However, it is important to recognize that a unique feature of corneal epithelial wound-healing responses to self-resolving and minor epithelial injuries is the requirement of inflammation, PMN cell infiltration into the stroma, and platelet activation at the vascular border (31, 42–44, 54). Reduced PMN cell infiltration directly correlates with delayed reepithelialization of corneal wounds that normally heal within 2–5 d. This unique privileged injury response dictates that redundant circuits are in place to tightly control the precarious activation and recruitment of beneficial PMN cells and is highly evolved in the delicate visual axis (32). Consistent with the beneficial role of PMN cells in mild and self-resolving corneal injury responses, we have demonstrated previously that genetic disruption of the protective LXA4 biosynthetic pathway decreases, while topical treatment with LXA4 significantly increases, PMN cell content in the cornea, which correlates with decreased or increased epithelial wound healing, respectively (31). In sharp contrast, LXA4 in chronic or severe corneal injury responses (29, 30) or in every other reported severe inflammatory model (27) inhibits PMN cell infiltration. This in vivo attenuation of PMN cell content in inflamed tissues by LXA4 is likely indirectly mediated by decreased expression of chemotactic gradients and increased phagocytosis by macrophages. Results from the rapidly self-resolving epithelial injury model point toward a novel injury-specific and PMN cell-targeted bioaction of LXA4 that is the subject of future studies.
Sex-specific differences in corneal PMN cell content were observed only after recurrent epithelial injury, while the rate of wound closure was delayed in females both in the initial and recurrent reparative response. This finding suggests that both epithelial and leukocyte responses to injury exhibit sex-specific differences. The notion of sex-specific regulation of epithelial and leukocyte function in the cornea is indirectly supported by the expression of distinct ERs in these cell types (1, 3, 51) and the presence of estrogen and estrogen biosynthetic enzymes in the cornea (46, 48).
17β-Estradiol, the most prominent human estrogen, induced a female phenotype of wound healing in corneas of male mice that was mirrored by concomitant decrease in the PMN cell response to recurrent epithelial injury and expression of epithelial 15-LOX. These data provide evidence that topical estradiol is sufficient to induce a female reparative-inflammatory response in male mice and provides further evidence for the critical role of PMN and epithelial cell 15-LOX in acute wound-healing responses. The fact that estradiol at concentrations as low as 1 pM delayed wound healing in monolayers of HCE cells demonstrates that estradiol has direct and physiologically relevant epithelial actions. More important, estradiol significantly reduced the constitutive expression of 5-LOX and 15-LOX in HCE cells. These two LOX enzymes are required for the formation of LXA4 in the cornea and are expressed in mouse and HCE cells (21, 31). LXA4 alone could increase the rate of epithelial wound healing in vitro, which is consistent with previous studies that have demonstrated that LXA4 and 15-LOX promote epithelial wound healing in vivo (30, 31) and that the EGF mediates its epithelial effects in part through LXA4 (49). Epithelial cells that were treated with LXA4 and estradiol retained normal wound-healing responses. This add-back experiment provides strong evidence that estradiol's actions on epithelial proliferation and migration are mediated in part by inhibition of the constitutive LXA4 biosynthetic pathway in corneal epithelial cells. A recent study (55) demonstrated that LXA4 can bind to ERα and can regulate estradiol-targeted gene expression in endometrial epithelial cells. Bioactions of estradiol and LXA4 are likely distinct for tissue-specific epithelial cell types. However, regulation of estradiol-targeted genes offers a potential mechanism for the ability of LXA4 to retain normal wound healing in estrogen-treated epithelial cells. Expression of 15-LOX is markedly lower in the regenerated epithelium of female mice, and topical estradiol reduces expression of 15-LOX in male to the levels measured in the corneal epithelium of females. The mechanism for estrogen regulation of 15-LOX or 5-LOX expression remains to be defined. Gene regulation by ERs is complicated and is often not via response elements in the promoter region of a target gene (3, 52). Taken together, these multiple lines of in vivo and in vitro evidence link the sex-specific differences in the corneal reparative-inflammatory response to regulation of the intrinsic epithelial LXA4 biosynthetic pathway.
Estrogens regulate expression of distinct gene targets by two nuclear hormone receptors, ERα and ERβ (1, 3, 52). The expression of both receptors in the cornea and corneal epithelium has been established in both males and females (6, 47, 51), though expression of both receptors is markedly higher in females (Fig. 5). Our results demonstrate for the first time that epithelial expression of ERs is regulated by the corneal inflammatory-reparative response, as the receptor expression ratio shifts from predominantly ERα in the resident epithelium to ERβ in the regenerated epithelium. ERβ is of particular interest, as this receptor has been linked to many of estrogen's immune regulatory functions (4). Activation of either ERα or ERβ in the cornea resulted in reduced PMN cell infiltration in response to epithelial reinjury. By contrast, only ERβ activation significantly delayed epithelial wound healing in vivo in mice and in vitro in HCE cells. These findings identify ERβ as the specific receptor that mediates estradiol's epithelial-targeted bioactions and indicate that, by either direct or indirect actions, both ERβ and ERα can regulate PMN cell recruitment to the injured cornea. Consistent with ERβ-specific regulation of epithelial wound healing, only ERβ markedly down-regulated expression of 15-LOX in the regenerated epithelium of reinjured corneas. Neither ERα nor ERβ altered the expression of 5-LOX in the regenerated epithelium. This finding is in contrast to estradiol's ability to markedly reduce constitutive 5-LOX expression in vitro in HCE cells. These differences are likely due to distinct differences between cultured human epithelial cells and in vivo regenerated mouse epithelium, as well as multiple tissue-specific factors that regulate 5-LOX expression in vivo in the inflamed cornea. Epithelial reinjury induces profound up-regulation of PMN cell migration into the stroma that correlates with rapid epithelial wound healing. Even though the corneal epithelium expresses low levels of functional 5-LOX, the predominant source of 5-LOX activity in the inflamed cornea are leukocytes, especially PMN cells and macrophages. Consistent with the ability of both ERα and ERβ to markedly attenuate PMN cell infiltration, activation of either receptor markedly reduced endogenous formation of LXA4 by the leukocyte-rich stroma. Transcellular biosynthesis is the predominant endogenous route for LXA4 formation (20, 21, 26, 28). During healthy inflammation, epithelial cells, which highly express 15-LOX, interact with 5-LOX-expressing leukocytes to set in place a temporal amplification loop. This intrinsic feed-forward loop is likely an important factor in the cornea and counterbalances proinflammatory circuits, stimulates noninflammatory activation of macrophages, and drives normal execution of inflammatory-reparative responses. Estradiol attenuates functional expression of this intrinsic corneal circuit at multiple levels by receptor specific regulation in both epithelial cells and leukocytes, which correlates with marked sex-specific differences in the inflammatory and wound-healing response.
In summary, our results provide the first evidence linking sex-specific differences and the action of estradiol to the 15-LOX/LXA4 circuit. Specifically, ERβ down-regulates epithelial expression and function of key enzymes in the formation of LXA4, while both ERα and ERβ markedly reduce PMN infiltration and leukocyte-dependent LXA4 formation. LXA4, its receptor ALX, and biosynthetic enzymes 15-LOX and 5-LOX have emerged as intrinsic lipid circuits across the visual axis that are critical to the routine execution of healthy inflammatory-reparative responses and the counterregulation of circuits that lead to dysregulated or chronic inflammation (20, 21, 29, 56). Our finding that estrogen negatively regulates the intrinsic LXA4 circuits, especially ERβ-specific regulation of epithelial 15-LOX, may provide novel insights into the etiology or pathogenesis of sex-specific ocular inflammatory diseases.
Supplementary Material
Acknowledgments
This work was supported in part by U.S. National Eye Institute grants EY016136 and P30EY003176 and a Hellman's Family Faculty Fund grant from the University of California, Berkeley.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ALX
- LXA4 receptor
- LOX
- lipoxygenase
- LXA4
- lipoxin A4
- ER
- estrogen receptor
- PMN
- polymorphonuclear
- PPT
- propylpyrazole triol
- DPN
- diarylpropiolnitrile
- OCT
- optimal cutting temperature
- MPO
- myeloperoxidase
- HCE
- human corneal epithelial
- KGM
- keratinocyte growth medium.
REFERENCES
- 1. Deroo B. J., Korach K. S. (2006) Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilliver S. C. (2010) Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 120, 105–115 [DOI] [PubMed] [Google Scholar]
- 3. Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Strom A., Treuter E., Warner M., Gustafsson J. A. (2007) Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 87, 905–931 [DOI] [PubMed] [Google Scholar]
- 4. Straub R. H. (2007) The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 [DOI] [PubMed] [Google Scholar]
- 5. Ashcroft G. S., Mills S. J., Lei K., Gibbons L., Jeong M.-J., Taniguchi M., Burow M., Horan M. A., Wahl S. M., Nakayama T. (2003) Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Invest. 111, 1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wickham L. A., Gao J., Toda I., Rocha E. M., Ono M., Sullivan D. A. (2000) Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 78, 146–153 [DOI] [PubMed] [Google Scholar]
- 7. Suzuki T., Schaumberg D. A., Sullivan B. D., Liu M., Richards S. M., Sullivan R. M., Dana M. R., Sullivan D. A. (2002) Do estrogen and progesterone play a role in the dry eye of Sjogren's syndrome? Ann. N. Y. Acad. Sci. 966, 223–225 [DOI] [PubMed] [Google Scholar]
- 8. Schaumberg D. A., Buring J. E., Sullivan D. A., Dana M. R. (2001) Hormone replacement therapy and dry eye syndrome. JAMA 286, 2114–2119 [DOI] [PubMed] [Google Scholar]
- 9. Schaumberg D. A., Sullivan D. A., Dana M. R. (2002) Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 506, 989–998 [DOI] [PubMed] [Google Scholar]
- 10. Sullivan D. A. (2004) Sex and sex steroid influence on dry eye syndromes. In Dry Eye and Ocular Surface Disease (Pflugfelder S., Beuerman R., Stern M. E., eds) pp. 165–190, Marcel Dekker, New York [Google Scholar]
- 11. Barabino S., Dana M. R. (2007) Dry eye syndromes. Chem. Immunol. Allergy 92, 176–184 [DOI] [PubMed] [Google Scholar]
- 12. Sullivan D. A. (2004) Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul. Surf. 2, 92–123 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki T., Schirra F., Richards S. M., Jensen R. V., Sullivan D. A. (2008) Estrogen and progesterone control of gene expression in the mouse meibomian gland. Invest. Ophthalmol. Vis. Sci. 49, 1797–1808 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki T., Sullivan D. A. (2006) Comparative effects of estrogen on matrix metalloproteinases and cytokines in immortalized and primary human corneal epithelial cell cultures. Cornea 25, 454–459 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki T., Sullivan D. A. (2005) Estrogen stimulation of proinflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea 24, 1004–1009 [DOI] [PubMed] [Google Scholar]
- 16. Sullivan D. A., Schaumberg D. A., Suzuki T., Schirra F., Liu M., Richards S., Sullivan R. M., Dana M. R., Sullivan B. D. (2002) Sex steroids, meibomian gland dysfunction and evaporative dry eye in Sjogren's syndrome. Lupus 11, 667. [DOI] [PubMed] [Google Scholar]
- 17. Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 [DOI] [PubMed] [Google Scholar]
- 18. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence T., Willoughby D. A., Gilroy D. W. (2002) Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunology 2, 787–795 [DOI] [PubMed] [Google Scholar]
- 20. Gronert K. (2010) Resolution, the grail for healthy ocular inflammation. Exp. Eye Res. 91, 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liclican E. L., Gronert K. (2010) Molecular circuits of resolution in the eye. Sci. World J. 10, 1029–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godson C., Brady H. R. (2000) Lipoxins: Novel anti-inflammatory therapeutics? Curr. Op. Invest. Drugs 1, 380–385 [PubMed] [Google Scholar]
- 23. McMahon B., Mitchell S., Brady H. R., Godson C. (2001) Lipoxins: revelations on resolution. Trends Pharmacol. Sci. 22, 391–395 [DOI] [PubMed] [Google Scholar]
- 24. McMahon B., Godson C. (2004) Lipoxins: endogenous regulators of inflammation. Am. J. Physiol. Renal Physiol. 286, F189–201 [DOI] [PubMed] [Google Scholar]
- 25. Maderna P., Godson C. (2009) Lipoxins: resolutionary road. Br. J. Pharmacol. 158, 947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W., Rovati G. E., Shimizu T., Yokomizo T., Brink C. (2006) The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 58, 463–487 [DOI] [PubMed] [Google Scholar]
- 28. Chiang N., Arita M., Serhan C. N. (2005) Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglan. Leukot. Essent. Fatty Acids 73, 163–177 [DOI] [PubMed] [Google Scholar]
- 29. Leedom A. J., Sullivan A. B., Dong B., Lau D., Gronert K. (2010) Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 176, 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biteman B., Hassan I. R., Walker E., Leedom A. J., Dunn M., Seta F., Laniado-Schwartzman M., Gronert K. (2007) Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 21, 2257–2266 [DOI] [PubMed] [Google Scholar]
- 31. Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Laniado Schwartzman M. (2005) A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 280, 15267–15278 [DOI] [PubMed] [Google Scholar]
- 32. Gronert K. (2008) Lipid autacoids in inflammation and injury responses: a matter of privilege. Mol. Interv. 8, 28–35 [DOI] [PubMed] [Google Scholar]
- 33. Gronert K. (2005) Lipoxins in the eye and their role in wound healing. Prostaglan. Leukot. Essent. Fatty Acids 73, 221–229 [DOI] [PubMed] [Google Scholar]
- 34. Jin Y., Arita M., Zhang Q., Saban D. R., Chauhan S. K., Chiang N., Serhan C. N., Dana R. (2009) Novel anti-inflammatory and pro-resolving lipid mediators block inflammatory angiogenesis. Invest. Ophthalmol. Vis. Sci. 50, 4743–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karim M. J., Bhattacherjee P., Biswas S., Paterson C. A. (2009) Anti-inflammatory effects of lipoxins on lipopolysaccharide-induced uveitis in rats. J. Ocul. Pharmacol. Ther. 25, 483–486 [DOI] [PubMed] [Google Scholar]
- 36. Sapieha P., Stahl A., Chen J., Seaward M. R., Willett K. L., Krah N. M., Dennison R. J., Connor K. M., Aderman C. M., Liclican E., Carughi A., Perelman D., Kanaoka Y., Sangiovanni J. P., Gronert K., Smith L. E. (2011) 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci. Transl. Med. 3, 69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calandria J. M., Marcheselli V. L., Mukherjee P. K., Uddin J., Winkler J. W., Petasis N. A., Bazan N. G. (2009) Selective survival rescue in 15-lipoxygenase-1 deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 284, 17877–17882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bazan N. G., Molina M. F., Gordon W. C. (2011) Docosahexaenoic Acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu. Rev. Nutr. 31, 321–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seta F., Bellner L., Rezzani R., Regan R. F., Dunn M. W., Abraham N. G., Gronert K., Laniado-Schwartzman M. (2006) Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am. J. Pathol. 169, 1612–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sator M. O., Joura E. A., Golaszewski T., Gruber D., Frigo P., Metka M., Hommer A., Huber J. C. (1998) Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br. J. Obstet. Gynaecol. 105, 100–102 [DOI] [PubMed] [Google Scholar]
- 41. Liclican E. L., Nguyen V., Sullivan A. B., Gronert K. (2010) Selective activation of the prostaglandin E2 circuit is a key component of chronic injury-induced pathological angiogenesis. Invest. Ophthalmol. Vis. Sci. 51, 6311–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Z., Burns A. R., Rumbaut R. E., Smith C. W. (2007) Gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am. J. Pathol. 171, 838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Z., Rumbaut R. E., Burns A. R., Smith C. W. (2006) Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization. Invest. Ophthalmol. Vis. Sci. 47, 4794–4802 [DOI] [PubMed] [Google Scholar]
- 44. Li Z., Burns A. R., Smith C. W. (2006) Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest. Ophthalmol. Vis. Sci. 47, 1947–1955 [DOI] [PubMed] [Google Scholar]
- 45. Ashcroft G. S., Dodsworth J., van Boxtel E., Tarnuzzer R. W., Horan M. A., Schultz G. S., Ferguson M. W. (1997) Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 3, 1209–1215 [DOI] [PubMed] [Google Scholar]
- 46. Coles N., Lubkin V., Kramer P, Weinstein B, Southren L, Vittek J. (1988) Hormonal analysis of tears, saliva, and serum from normals and postmenopausal dry eyes. Invest. Ophthalmol. Vis. Sci. 29(Suppl.), 48 (abstr.) [Google Scholar]
- 47. Tachibana M., Kasukabe T., Kobayashi Y., Suzuki T., Kinoshita S., Matsushima Y. (2000) Expression of estrogen receptor alpha and beta in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 41, 668–670 [PubMed] [Google Scholar]
- 48. Schirra F., Suzuki T., Dickinson D. P., Townsend D. J., Gipson I. K., Sullivan D. A. (2006) Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea 25, 438–442 [DOI] [PubMed] [Google Scholar]
- 49. Kenchegowda S., Bazan N. G., Bazan H. E. (2011) EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Invest. Ophthalmol. Vis. Sci. 52, 2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma G. D., Ottino P., Bazan N. G., Bazan H. E. (2005) Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Cα translocation to the plasma membrane through 15(S)-hydroxyeicosatetraenoic acid synthesis. J. Biol. Chem. 280, 7917–7924 [DOI] [PubMed] [Google Scholar]
- 51. Suzuki T., Kinoshita Y., Tachibana M., Matsushima Y., Kobayashi Y., Adachi W., Sotozono C., Kinoshita S. (2001) Expression of sex steroid hormone receptors in human cornea. Curr. Eye Res. 22, 28–33 [DOI] [PubMed] [Google Scholar]
- 52. Leitman D. C., Paruthiyil S., Vivar O. I., Saunier E. F., Herber C. B., Cohen I., Tagliaferri M., Speed T. P. (2010) Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr. Opin. Pharmacol. 10, 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krishnan T., Pranja N., Gronert K., Oldenburg E., Ray K., Keenan J. D., Lietman T., Acharya N. (2011) Gender difference in re-epithelialisation time in fungal corneal ulcers. [E-pub ahead of print] Br. J. Ophthalmol. doi: 10.1136/bjophthalmol-2011-300441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vij N., Roberts L., Joyce S., Chakravarti S. (2005) Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Investigat. Ophthalmol. Visual. Sci. 46, 88–95 [DOI] [PubMed] [Google Scholar]
- 55. Russell R., Gori I., Pellegrini C., Kumar R., Achtari C., Canny G. O. (2011) Lipoxin A4 is a novel estrogen receptor modulator. [E-pub ahead of print] FASEB J. doi: 10.1096/fj.11-187658 [DOI] [PubMed] [Google Scholar]
- 56. Kenchegowda S., Bazan H. E. (2010) Significance of lipid mediators in corneal injury and repair. J. Lipid Res. 51, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.